Abstract
This study addressed whether O2 delivery during recovery from high-intensity, supra-gas exchange threshold exercise would be matched to O2 utilization at the microvascular level in patients with mitochondrial myopathy (MM). Off-exercise kinetics of (1) pulmonary O2 uptake \( (\dot{V}{\text{O}}_{2} {\text{p}}), \) (2) an index of fractional O2 extraction by near-infrared spectroscopy (Δ[deoxy-Hb + Mb]) in the vastus lateralis and (3) cardiac output (Q ′T ) by impedance cardiography were assessed in 12 patients with biopsy-proven MM (chronic progressive external ophthalmoplegia) and 12 age- and gender-matched controls. Kinetics of \( \dot{V}{\text{O}}_{2} {\text{p}} \) were significantly slower in patients than controls (τ = 53.8 ± 16.5 vs. 38.8 ± 7.6 s, respectively; p < 0.05). Q ′T , however, declined at similar rates (τ = 64.7 ± 18.8 vs. 73.0 ± 21.6 s; p > 0.05) being typically slower than \( \dot{V}{\text{O}}_{2} {\text{p}} \) in both groups. Importantly, Δ[deoxy-Hb + Mb] dynamics (MRT) were equal to, or faster than, \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \) in patients and controls, respectively. In fact, there were no between-group differences in \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \)/MRTΔ[deoxy-Hb + Mb] (1.1 ± 0.4 vs. 1.0 ± 0.2, p > 0.05) thereby indicating similar rates of microvascular O2 delivery. These data indicate that the slower rate of recovery of muscle metabolism after high-intensity exercise is not related to impaired microvascular O2 delivery in patients with MM. This phenomenon, therefore, seems to reflect the intra-myocyte abnormalities that characterize this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In similarity with the onset of exercise, sluggish pulmonary oxygen uptake (\( \dot{V}{\text{O}}_{2} {\text{p}} \)) kinetics at the “off-transient” might be related to the impaired diffusive and/or convective O2 transport to muscle mitochondria (Hughson et al. 2001; MacDonald et al. 2001) and/or abnormal metabolic activity of intra-myocyte machinery (Burnley et al. 2000; Grassi et al. 1996). The final consequence is a slower capability to replenish local phosphocreatine (PCr) stores, thereby retarding the restoration of muscle metabolism (Harris et al. 1976; McMahon and Jenkins 2002).
Near infrared spectroscopy (NIRS) is a non-invasive technique that has been widely used to investigate the (im)balance between microvascular O2 delivery (Q′O2mv) and utilization (Grassi et al. 2003; DeLorey et al. 2003; Koga et al. 2007). Among the variables provided by NIRS, deoxy-hemoglobin (Hb) + myoglobin (Mb) is particularly informative as it provides an index of muscle fractional O2 extraction, i.e., the Q′O2mv-to-\( \dot{V}{\text{O}}_{2} \) relationship. In this context, previous studies have demonstrated that the off-transient kinetics of deoxy-Hb + Mb is faster than \( \dot{V}{\text{O}}_{2} {\text{p}} \) recovery in healthy subjects (Ferreira et al. 2005), thereby lending support to the notion that intra-myocyte metabolic inertia, not O2 availability, is the key limiting step of \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics after dynamic exercise (Harper et al. 2008; Ferreira et al. 2005).
Mitochondrial myopathy (MM) is associated with slower \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics at the onset of exercise, a finding thought to be related to impaired muscle O2 utilization (Grassi et al. 2009). It has been assumed that off-transient \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics would also be slowed, as oxidative phosphorylation would take longer to replenish creatine (Cr), ADP, and inorganic phosphate (Pi) back to pre-exercise levels (Rossiter et al. 2002). If this is the case, deoxy-Hb + Mb would be expected to decrease at faster rates than \( \dot{V}{\text{O}}_{2} {\text{p}} \) thereby indicating that O2 availability is in excess to O2 needs.
There is, however, some intriguing evidence that convective O2 transport to contracting fibres could also be impaired in MM patients thereby predisposing \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics to be delivery-limited. For instance, Jensen et al. (2002) found a decrease in forearm blood flow in MM patients and Trenell et al. (2007) described that O2 supplementation enhanced patients’ ability to regenerate ATP. In fact, considering that O2 utilization per se might be important to spatially adjust muscle blood flow to O2 needs during exercise (Wolin 2009; Ellsworth et al. 2009; Sprague et al. 2010), it is possible that patients with impaired O2 utilization could not maintain Q′O2mv well matched to demand. The usefulness of the deoxy-Hb + Mb analysis during exercise recovery in clinical populations has been demonstrated, for instance, by Kemps et al. (2009) who described slower rates of muscle reoxygenation relative to post-exercise PCr resynthesis in patients with decreased Q′O2mv (chronic heart failure). Despite its potential to elucidate the mechanistic determinants of slower \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics in disease states, this analytical approach has not been previously used in patients with MM.
Our objective, therefore, was to investigate whether Q′O2mv would be commensurate to O2 utilization during recovery from high-intensity, supra-gas exchange threshold exercise in MM patients. Knowledge of the etiological mechanisms leading to the microcirculatory O2 exchange impairment in MM is crucial to understand the mechanisms of exercise intolerance and to plan efficacious therapeutic strategies for this patient population.
Methods
Subjects
The study population comprised of 12 patients (5 males) with biopsy-proven MM and 12 age-matched sedentary healthy controls (8 males). The patients had been followed at the Neuromuscular Division and volunteered for study participation. Consecutive patients who presented with ragged-red fibers pattern in all specimens and abnormally low mitochondrial electron transport chain activity (cytochrome c oxidase) in muscle biopsy associated with symptoms and signs of chronic progressive external ophthalmoplegia (ptosis, exercise intolerance and chronic fatigue) were selected (Moraes et al. 1989). There was no evidence of cardiomyopathy or impaired cardiac conduction in any patient at rest. Subjects with mitochondrial encephalomyopathy, lactic acidosis and strokes episodes (MELAS), myoclonus epilepsy with ragged-red fibers (MERRF), and other MMs were excluded from the study. The patients have not been involved on any regular physical activity in the preceding year and the controls presented with very low daily activity scores according to the Baecke questionnaire (total score <6) (Baecke et al. 1982). Written informed consent to participate was obtained from each subject and the experimental protocol was approved by the Institutional Ethics Committee of the Federal University of Sao Paulo, Brazil.
Study protocol
Subjects performed a ramp-incremental exercise test (5–10 W/min in patients and 15–20 W/min in controls) to determine parameters of aerobic function during exercise (Whipp et al. 1981). The tests were performed on an electronically braked cycle ergometer (Corival 400, Lode, The Netherlands) at 60 rpm and they were preceded by an unloaded baseline pedaling for 3 min. On a separate day, subjects performed a constant work rate exercise test at the same pedalling rate to the limit of tolerance (Tlim) at an intensity of 70% of peak work rate. Tlim was defined as the time point in which the patients signaled to stop exercising or could not maintain the required pedalling rate for 10 s despite being encouraged by the investigators. Subjects also underwent an active 3-min recovery period of unloaded pedalling at the same rate.
Measurements
Cardiopulmonary exercise test (CPET)
\( \dot{V}{\text{O}}_{2} {\text{p}} \) (mL/min), pulmonary carbon dioxide output (\( \dot{V}{\text{CO}}_{2} {\text{p}}, \) mL/min) and minute ventilation (\( \dot{V}{\text{E,}} \) L/min) were measured breath-by-breath using a computer-based system (CardiO2 System™, Medical Graphics, St. Paul, MN, USA). Gas exchange variables measured during the incremental test were averaged every 15 s, and \( \dot{V}{\text{O}}_{2} {\text{peak}} \) was defined as the highest value achieved during the test. Heart rate (HR, bpm) was determined using the R–R interval from a 12-lead electrocardiogram. Subjects were also asked to rate their ‘shortness of breath’ and ‘leg effort’ at exercise cessation using the 0–10 Borg’s category-ratio scale. The \( \dot{V}{\text{O}}_{2} {\text{p}}_{\text{GET}} \) was estimated by the gas-exchange method, inspecting visually the inflection point of \( \dot{V}{\text{CO}}_{2} {\text{p}} \) with regard to \( \dot{V}{\text{O}}_{2} {\text{p}} \) (modified V-Slope) (Beaver et al. 1986) and by the ventilatory method when \( \dot{V}{\text{E}} \)-to-\( \dot{V}{\text{O}}_{2} {\text{p}} \) ratio and end-tidal partial pressure of O2 increased, while \( \dot{V}{\text{E}} \)-to-\( \dot{V}{\text{CO}}_{2} {\text{p}} \) ratio and end-tidal pressure of CO2 remained stable. The reading was performed independently by two experienced observers without knowledge of other results or subject identities.
Skeletal muscle oxygenation
Skeletal muscle oxygenation profiles of the left vastus lateralis were determined by spatially resolved near-infrared spectroscopy (NIRS) (Hamamatsu NIRO 200TM, Hamamatsu Photonics KK, Japan). This technique has been used in several studies to evaluate patients with MM (Bank and Chance 1997; Van Beekvelt et al. 1999a, b; Grassi et al. 2007, 2009). The theory of NIRS has been described in detail elsewhere (Ferrari et al. 2004). Briefly, one fiber optic bundle carried the NIR light produced by the laser diodes to the tissue of interest while a second fiber optic bundle returned the transmitted light from the tissue to a photon detector in the spectrometer. The intensity of incident and transmitted light were recorded continuously and, along with the relevant specific extinction coefficients, used to measure changes in the oxygenation status of Hb and Mb. A set of optodes was placed on the belly of the vastus lateralis muscle midway between the lateral epicondyle and greater trochanter of the femur. To ensure that the position of the optodes, relative to each other, was fixed and invariant, the optodes were housed in an optically dense plastic holder. The optode assembly was secured on the skin surface with tape, and then covered with an optically dense, black vinyl sheet, thus minimizing the intrusion of extraneous light and loss of NIR light.
Among the NIRS variables, several laboratories have adopted the deoxy-Hb + Mb concentration ([deoxy-Hb + Mb]) signal as the preferred indicator of changes in muscle microvascular oxygenation during exercise (Koga et al. 2007; DeLorey et al. 2005; Ferreira et al. 2005; Grassi et al. 2003). The [deoxy-Hb + Mb] response to exercise is then considered as a proxy of O2 extraction in the microcirculation when arterial oxygen content remains constant and has been referred as the balance between O2 delivery and utilization. Before exercise, care was taken that an arterial cuff occlusion (at 300 cmH2O) produced an increase in Δ[deoxy-Hb + Mb] in all patients. The [deoxy-Hb + Mb] values were recorded as a change (Δ) from baseline in μM/cm and data were corrected by the maximal value obtained at peak of exercise and expressed in percentage. The NIRS system was ‘zeroed’ during the unloaded cycling portion of the tests.
Central haemodynamics
Cardiac output (Q′T, L/min) was measured non-invasively throughout the constant work rate test using impedance cardiography (PhysioFlow PF-05™, Manatec Biomedical, France) (Charloux et al. 2000). Before each exercise test, the system was auto-calibrated taking into consideration age, stature, body mass and blood pressure values. Signal quality was verified by visualizing the ECG tracing and its first derivative (dECG/dt) and the impedance waveform (ΔZ) with its first derivative (dZ/dt). In preliminary experiments, the coefficient of variation (CV) for changes in Q ′T during exercise was 3.3%. Although the method is not free from controversies, changes have been previously found to agree well with direct measurements in other disease populations (Borghi-Silva et al. 2008; Kemps et al. 2008; Tonelli et al. 2011).
Kinetics analysis
The breath-by-breath \( \dot{V}{\text{O}}_{2} {\text{p}}, \) Δ[deoxy-Hb + Mb], and Q ′T data were interpolated second-by-second prior to kinetics analysis and expressed as % of total response (SigmaPlot 10.0, Systat Software Inc., San Jose, CA, USA). We opted to analyse data from the last 30 s of exercise to 180 s of recovery, i.e., we analyse only the kinetics of the primary component of response. Using this approach, we assured that the same amount of data was included in the kinetic analysis of \( \dot{V}{\text{O}}_{2} {\text{p}}, \) Δ[deoxy-Hb + Mb] and Q ′T for each subject (controls and patients), minimizing model-dependent effects on our results. The model used for fitting the kinetic response was:
where the subscripts “ss” and “p” refer to stable-state cycling and primary component, respectively; “A”, “TD”, and “τ” are the amplitude, time delay and time constant of the exponential response of interest (~time to reach 63% of the response following the end of exercise). The overall kinetics of Δ[deoxy-Hb + Mb] were determined by the mean response time (MRT = τ + TD).
Statistical analysis
The SPSS version 15.0 statistical software was used for data analysis (SPSS, Chicago, IL, USA). Results were summarized as mean ± SD or median and interquartile ranges when applicable. Between-group differences were evaluated by means of independent-samples t test or Mann–Whitney U test. Wilcoxon test was used to evaluate within-group differences of τQ ′T and \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \). The level of statistical significance was set at p < 0.05 for all tests.
Results
Sample characteristics
Patients presented with lower weight, height and body mass index (BMI) than controls. There were no significant between-group differences in pulmonary function variables with exception of lower MEP in patients (Table 1). As expected, they had reduced maximal work rate, \( \dot{V}{\text{O}}_{2} {\text{peak}}, \) HR, O2 pulse and \( \dot{V}{\text{O}}_{2} {\text{p}}_{\text{GET}} \) as compared to controls (p < 0.05; Table 2). \( \dot{V}{\text{O}}_{2} {\text{p}} \) at the constant work rate test corresponded to 86% and 75% of the \( \Updelta \dot{V}{\text{O}}_{2} {\text{p}}_{{{\text{peak}} - {\text{GET}}}} \) difference in patients and controls, respectively.
\( \dot{V}{\text{O}}_{2} {\text{p}} \) and Q ′T kinetics
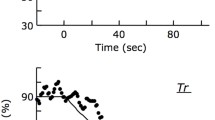
\( \dot{V}{\text{O}}_{2} {\text{p}} \) dynamics were slower in patients than controls (see Fig. 1a for representative subjects, Fig. 2; Table 3). Q ′T , however, declined at similar rates (Fig. 1b for representative subjects, Fig. 2; Table 3). Therefore, Q ′T kinetics were equivalent to that of \( \dot{V}{\text{O}}_{2} {\text{p}} \) in patients and slower in controls (Fig. 2).
Representative examples of the pulmonary oxygen uptake (\( \dot{V}{\text{O}}_{2} {\text{p}}, \) a), cardiac output (Q ′T , b) and change in deoxyhemoglobin + myoglobin concentration (∆[deoxy-Hb + Mb], c) kinetic responses during recovery from a high-intensity constant work rate test in a patient with mitochondrial myopathy (closed circles) and a control subject (open circles). Data are expressed in percent of the maximal obtained value during exercise
Mean (SE) values of τQ ′T , \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \) and MRT (τ + TD) Δ[deoxy-Hb + Mb] at the recovery from a high-intensity constant work rate test in patients with MM (solid bars) and healthy controls (open bars). *p < 0.05 for between-group comparisons; † p < 0.05 for within-group comparisons of Q ′T versus \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \)
Microvascular O2 delivery and utilization
Δ[deoxy-Hb + Mb] amplitude was 33.4 ± 16.5% of the “cuff” value with all patients showing a response larger than 20%. In similarity with \( \dot{V}{\text{O}}_{2} {\text{p}}, \) Δ[deoxy-Hb + Mb] kinetics were slower in patients than controls (Fig. 1c for representative subjects, Fig. 2; Table 3); in fact, MRTΔ[deoxy-Hb + Mb] values >40 s were found in 8/12 MM patients but only in 3/12 controls. Importantly, \( \tau \dot{V}{\text{O}}_{2} \)/MRTΔ[deoxy-Hb + Mb], an index of microvascular O2 delivery dynamics, did not differ between patients and controls (1.1 ± 0.4 vs. 1.0 ± 0.2 s, respectively; p > 0.05). There was a weak, inverse relationship (r = −0.48; p = 0.056) between Δ[deoxy-Hb + Mb] kinetics and \( \dot{V}{\text{O}}_{2} {\text{peak}} \) in patients with MM.
Discussion
This study investigated whether microvascular O2 delivery (Q′O2mv) would be matched to O2 utilization during recovery from high-intensity exercise in patients with MM (chronic progressive external ophthalmoplegia) compared to healthy controls. Q ′T declined at rates which were not faster than \( \dot{V}{\text{O}}_{2} {\text{p}} \) in both groups thereby indicating that “bulk” O2 delivery was sufficient to attend the peripheral demands for O2. In addition, \( \dot{V}{\text{O}}_{2} {\text{p}} \) and Δ[deoxy-Hb + Mb], an index of muscle fractional O2 extraction at the microvascular level, recovered at similar rates. This finding indicates that muscle blood flow was precisely distributed to serve the O2 needs of individual fibers, i.e., Q′O2mv was well matched to O2 utilization. These data, therefore, lend indirect support to the notion that the slower rate of recovery of muscle metabolism after high-intensity exercise is mechanistically linked to intra-myocyte disturbances in MM patients. This study sets the scene for future NIRS-based investigations aimed to investigate the consequences of interventions with a potential to improve post-exercise muscle metabolic abnormalities in this patient population.
Mitochondrial diseases are a heterogeneous group of disorders that shares the same basic pathophysiological feature, i.e., dysfunction of the mitochondrial respiratory chain. Impaired oxidative metabolism, therefore, is a hallmark of these diseases (Tarnopolski 2004; Tarnopolski and Raha 2005). During dynamic exercise, this has been traditionally assessed by the examination of some specific response which is assumed to reflect the adequacy of the aerobic metabolism, such as \( \dot{V}{\text{O}}_{2} \) at peak exercise (Taivassalo et al. 2003) and lactate production (Jensen et al. 2002). In contrast, much less attention has been paid to the rate of change in \( \dot{V}{\text{O}}_{2} {\text{p}} \) at the transition to (“on-kinetics) or from (“off-kinetics”) exercise. In fact, the study of \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics is widely recognized as a fundamental tool to establish the “efficiency” (health) or appropriateness (disease) of the aerobic adjustments to exercise (Hughson et al. 2001; Grassi et al. 2009).
There is little doubt that under normal conditions muscle O2 delivery is unlikely to limit \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics after the cessation of exercise (Ozyener et al. 2001; Perrey et al. 2002; Cleuziou et al. 2004; McDonough et al. 2004; Behnke et al. 2009). In patients with MM, however, we reasoned that this scenario could be different. For instance, Ellsworth et al. (2009) had put forward the hypothesis that the erythrocyte, when exposed to reduced O2 tensions, releases ATP in a controlled manner. This molecule, in turn, has been shown to interact with purinergic receptors on the endothelium producing both local and conducted vasodilation (Sprague et al. 2009) thereby enabling a precise matching of muscle O2 delivery to O2 needs (Sprague et al. 2010). In patients with MM, however, muscle capillary O2 tensions could not decrease to levels sufficiently low to elicit appreciable ATP release and, therefore, adequate vasodilation at the sites of (impaired) O2 utilization. In fact, intra-muscular perfusion heterogeneities might be more extensive in these patients (Sakuta and Nonaka 1989). Moreover, morphological and quantitative analysis of quadriceps muscle fibers in MM patients showed that mitochondrial changes affected predominantly type I fibers (Scelsi 1992). The recovery kinetics of estimated muscle blood flow in proportion to muscle \( \dot{V}{\text{O}}_{2} \) kinetics are faster in type II as compared to type I fibers (McDonough et al. 2004; Ferreira et al. 2005) thereby further increasing the likelihood of \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics to be delivery-limited. Collectively, therefore, it seemed plausible that under conditions of high intra-muscular O2 needs, such as during recovery from high-intensity exercise, impaired Q′O2mv could play a prominent role in slowing off-exercise \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics in this patient population.
Despite these theoretical considerations, however, our main results did not indicate that impaired convective O2 delivery was etiologically linked to the sluggishness of \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics in MM patients. Therefore, the findings of slower rates of decrease in Q ′T as compared to \( \dot{V}{\text{O}}_{2} {\text{p}} \) coupled with the temporal similarity between \( \dot{V}{\text{O}}_{2} {\text{p}} \) and Δ[deoxy-Hb + Mb] dynamics are consistent with the notion that Q′O2mv was preserved in these patients. By exclusion, these data suggest that the defective intra-myocyte machinery set the limit of recovery of aerobic metabolism patients. In other words, assuming that exercise increased adenosine diphosphate (ADP), phosphate (Pi), and Cr in these patients (Kemp et al. 1993; Jeppesen et al. 2007; Trenell et al. 2007), this accumulated pool decreased slowly at exercise cessation, as compared to a normal, rapid recovery rate.
Some few previous studies used NIRS-derived parameters to investigate muscle metabolism in patients with MM (Van Beekvelt et al. 1999a, b, 2002; Grassi et al. 2007). In particular, Grassi et al. 2009 addressed the on-exercise \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics in a heterogeneous group of patients including MM, myophosphorylase deficiency (McArdle’s disease) and patients with non-defined metabolic myopathy. These authors reported slower \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics in patients as compared to controls; of note, \( \dot{V}{\text{O}}_{2} {\text{p}} \) kinetics correlated well with Δ[deoxy(Hb + Mb)] at peak exercise measured in a previous study (Grassi et al. 2007). The present results extend those from Grassi et al. by simultaneously assessing the behavior of \( \dot{V}{\text{O}}_{2} {\text{p}} \) and Δ[deoxy-(Hb + Mb)] after the cessation of exercise. The collective evidence brought about by these studies indicate that the analysis of Δ[deoxy(Hb + Mb)] at peak exercise (Grassi et al. 2007) and during recovery from high-intensity exercise (present study) are likely to give valuable insights in the functioning of muscle metabolism in MM patients.
A fundamental critique to the present study is the assumption that Δ[deoxy-Hb + Mb] reflects fractional O2 extraction in a syndrome where impaired O2 extraction has been considered a key physiopathological marker (Taivassalo et al. 2002, 2003; Grassi et al. 2007). However, the amplitude of the Δ[deoxy-Hb + Mb] response exceeded 20% of the maximum “cuff” value in all patients indicating that this variable can be used as a proxy of fractional O2 extraction in MM patients who are not severely affected by the disease process. In fact, the seminal studies that described a lack of directly determined reductions in PvO2 have valuated patients with more advanced disease performing small muscle mass exercise (Jensen et al. 2002; Taivassalo et al. 2003). Nevertheless, it should be recognized that our confidence in Δ[deoxy-Hb + Mb] as an index of fractional O2 extraction decreases as disease progresses which might limit the practical use of this approach in patients with end-stage disease.
Other potential points of concern might be related to the choice of supra-GET exercise (Ozyener et al. 2001) and the lack of analysis of on-exercise kinetics and the \( \dot{V}{\text{O}}_{2} \) response profile during exercise. As shown in Table 2, however, the GET occurred at very early exercise in these patients. Therefore, the amplitude of the \( \dot{V}{\text{O}}_{2} {\text{p}} \) response would have been too low for a reliable description of the on-exercise kinetic responses had we used a sub-GET work rate. In fact, pilot trials showed that the reproducibility of Δ[deoxy-Hb + Mb] response was rather poor at the onset of exercise. In addition, thought visual inspection of the data did suggest the presence of the “slow” component of the \( \dot{V}{\text{O}}_{2} {\text{p}} \) response in all subjects, the excessive short duration of the tests in some MM had precluded a formal analysis of this important response profile. All of these aspects, therefore, await further investigation.
The adipose tissue thickness (ATT) has been described to influence in spreading the light of NIRS, leading to higher estimated muscle \( \dot{V}{\text{O}}_{2} \) in subjects with lower subcutaneous fat (van Beekvelt et al. 2002). However, Matsushita et al. (1998) concluded that NIR light penetrates shallow regions of muscle under the skin and subcutaneous fat even when the ATT was 1.5 cm. In the present study, patients with MM had lower weight and BMI than the controls and it is therefore unlikely that higher ATT in patients has influenced our results.
This study sets the scene for future NIRS-based investigations aimed to understand mechanisms of exercise intolerance and to determine the degree of muscle metabolic impairment in patients with MM. Furthermore, our results lend support to the assertion that NIRS might be useful to evaluate the effects of interventions with a potential to improve muscle metabolism in this patient population.
Conclusion
The slower rate of recovery of muscle metabolism after high-intensity exercise was not related to a mismatching between Q′O2mv and O2 utilization in patients with MM. These data lend indirect support to the notion that these disturbances are primarily linked to intra-myocyte disturbances being therefore amenable to be assessed by simultaneous analyses of \( \dot{V}{\text{O}}_{2} {\text{p}} \) and NIRS-derived indexes of muscle reoxygenation (Δ[deoxy-Hb + Mb]). Additional studies, however, are warranted to correlate the currently described abnormalities in O2 utilization during recovery with specific genotype and phenotype characteristics of MM patients.
References
Baecke JA, Burema J, Frijters JER (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36(5):936–942
Bank W, Chance B (1997) Diagnosis of defects in oxidative muscle metabolism by non-invasive tissue oximetry. Mol Cell Biochem 174:7–10
Beaver WL, Wasserman K, Whipp BJA (1986) New method for detecting the anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Behnke BJ, Ferreira LF, McDonough PJ, Musch TI, Poole DC (2009) Recovery dynamics of skeletal muscle oxygen uptake during the exercise off-transient. Respir Physiol Neurobiol 168(3):254–260
Borghi-Silva A, Oliveira CC, Carrascosa C, Maia J, Berton DC, Queiroga F Jr, Ferreira EM, Almeida DR, Nery LE, Neder JA (2008) Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax 63(10):910–915
Burnley M, Jones AM, Carter H, Doust JH (2000) Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol 89:1387–1396
Charloux A, Lonsdorfer-Wolf E, Richard R, Lampert E, Oswald-Mammosser M, Mettauer B, Geny B, Lonsdorfer J (2000) A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol 82:313–320
Cleuziou C, Perrey S, Borrani F, Lecoq AM, Candau R, Courteix D, Obert P (2004) Dynamic responses of O2 uptake at the onset and end of exercise in trained subjects. Can J Appl Physiol 28(4):630–641
DeLorey DS, Kowalchuk JM, Paterson DH (2003) Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95(1):113–120
DeLorey DS, Kowalchuk JM, Paterson DH (2005) Adaptation of pulmonary O2 uptake kinetics and muscle deoxygenation at the onset of heavy-intensity exercise in young and older adults. J Appl Physiol 98:1697–1704
Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS (2009) Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology 24:107–116
Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29:463–487
Ferreira LF, Harper AJ, Townsend DK, Lutjemeier BJ, Barstow TJ (2005) Kinetics of estimated human muscle capillary blood flow during recovery from exercise. Exp Physiol 90(5):715–726
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80:988–998
Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P (2003) Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95:149–158
Grassi B, Marzorati M, Lanfranconi F, Ferri A, Longaretti M, Stucchi A, Vago P, Marconi C, Morandi L (2007) Impaired oxygen extraction in metabolic myopathies: detection and quantification by near-infrared spectroscopy. Muscle Nerve 35:510–520
Grassi B, Porcelli S, Marzorati M, Lanfranconi F, Vago P, Marconi C, Morandi L (2009) Metabolic myopathies: functional evaluation by analysis of oxygen uptake kinetics. Med Sci Sports Exerc 41(12):2120–2127
Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ (2008) Matching of blood flow to metabolic rate during recovery from moderate exercise in humans. Exp Physiol 93(10):1118–1125
Harris RC, Edwards RHT, Hultman E, Nordesjo LO, Nylind B, Sahlin K (1976) The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pfluegers Arch 367:137–142
Hughson RL, Tschakovsky ME, Houston ME (2001) Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29:129–133
Jensen TD, Kazemi-Esfarjani P, Skomorowska E, Vissing J (2002) A forearm exercise screening test for mitochondrial myopathy. Neurology 58:1533–1538
Jeppesen TD, Quistorff B, Wibrand F, Vissing J (2007) 31P-MRS of skeletal muscle is not a sensitive diagnostic test for mitochondrial myopathy. J Neurol 254(1):29–37
Kemp GJ, Taylor DJ, Thompson CH, Hauds LJ, Rajagopalan B, Styles P, Radda GK (1993) Quantitative analysis by 31P magnetic resonance spectroscopy of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed 6(5):302–310
Kemps HM, Thijssen EJ, Schep G, Sleutjes BT, De Vries WR, Hoogeveen AR, Wijn PF, Doevendans PA (2008) Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J Appl Physiol 105(6):1822–1829
Kemps HM, Prompers JJ, Wessels B, De Vries WR, Zonderland ML, Thijssen EJ, Nicolay K, Schep G, Doevendans PA (2009) Skeletal muscle metabolic recovery following submaximal exercise in chronic heart failure is limited more by O2 delivery than O2 utilization. Clin Sci 26 118(3):203–210
Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ (2007) Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103:2049–2056
MacDonald MJ, Naylor HL, Tschakovsky ME, Hughson RL (2001) Peripheral circulatory factors limit rate of increase in muscle O2 uptake at onset of heavy exercise. J Appl Physiol 90:83–89
Matsushita K, Homma S, Okada E (1998) Influence of adipose tissue on muscle oxygenation measurement with NIRS instrument. Proc SPIE 3194:151–165
McDonough P, Behnke BJ, Musch TI, Poole DC (2004) Recovery of microvascular PO2 during the exercise off-transient in muscles of different fiber type. J Appl Physiol 96:1039–1044
McMahon S, Jenkins D (2002) Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med 32(12):761–784
Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC, Servidei S, Nonaka I, Koga Y, Spiro AJ, Brownell AKW, Schmidt B, Schotland DL, Zupanc M, DeVivo DC, Schon EA, Rowland LP (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns–Sayre syndrome. N Engl J Med 320:1293–1299
Ozyener F, Rossiter HB, Ward SA, Whipp BJ (2001) Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 533(3):891–902
Perrey S, Candau R, Borrani F, Millet GY, Rouillon JD (2002) Recovery kinetics of oxygen uptake following severe-intensity exercise in runners. J Sports Med Phys Fit 42(4):381–388
Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ (2002) Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate and high-intensity exercise in humans. J Physiol 541(3):991–1002
Sakuta R, Nonaka I (1989) Vascular involvement in mitochondrial myopathy. Ann Neurol 25(6):594–601
Scelsi R (1992) Morphometric analysis of skeletal muscle fibres and capillaries in mitochondrial myopathies. Path Res Pract 188:607–611
Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML (2009) Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 61:183–190
Sprague RS, Bowles EA, Achilleus D, Ellsworth ML (2010) Erythrocytes as controllers of perfusion distribution in the microvasculature of skeletal muscle. Acta Physiol 22. doi: 10.1111/j.1748-1716.2010.02182.x. (Epub ahead of print)
Taivassalo T, Abbott A, Wyrick P, Haller RG (2002) Venous oxygen levels during aerobic forearm exercise: an index of impaired oxidative metabolism in mitochondrial myopathy. Ann Neurol 51:38–44
Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG (2003) The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain 126:413–423
Tarnopolski M (2004) Exercise testing as a diagnostic entity in mitochondrial myopathies. Mitochondrion 4:529–542
Tarnopolski M, Raha S (2005) Mitochondrial myopathies: diagnosis, exercise intolerance, and treatment options. Med Sci Sports Exerc 37:2086–2093
Tonelli AR, Alnuaimat H, Li N, Carrie R, Mubarak KK (2011) Value of Impedance Cardiography in Patients Studied for Pulmonary Hypertension. Lung. doi:10.1007/s00408-011-9299-y
Trenell MI, Sue CM, Thompson CH, Kemp GJ (2007) Supplemental oxygen and muscle metabolism in mitochondrial myopathy patients. Eur J Appl Physiol 99:541–547
Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG (1999a) Quantitative measurement of oxygen consumption and forearm blood flow in patients with mitochondrial myopathies. Adv Exp Med Biol 471:313–319
Van Beekvelt MC, Van Engelen BG, Wevers RA, Colier WN (1999b) Quantitative near-infrared spectroscopy discriminates between mitochondrial myopathies and normal muscle. Ann Neurol 46(4):667–670
Van Beekvelt MC, Van Engelen BG, Wevers RA, Colier WN (2002) Near-infrared spectroscopy in chronic progressive external ophthalmoplegia: adipose tissue thickness confounds decreased muscle oxygen consumption. Ann Neurol 51(2):272–273
Whipp BJ, Davis JA, Torres F, Wasserman K (1981) A test to determine parameters of aerobic function during exercise. J Appl Physiol 50:217–221
Wolin MS (2009) Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296(3):H539–H549
Acknowledgments
The authors would like to thank all colleagues from the Pulmonary Function and Clinical Exercise Physiology Unit [Division of Respiratory Diseases. Department of Medicine, Federal University of Sao Paulo (UNIFESP), Brazil] for their friendly collaboration and support obtained from FAPESP (Fundação de Amparo à Pesquisa do Estado de Sao Paulo, Sao Paulo, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil). Supported by a Research Grant from FAPESP (Fundação de Amparo à Pesquisa do Estado de Sao Paulo, Sao Paulo, Brazil). DMB was a recipient of a Master Scholarship Grant from FAPESP. JAN and LEN are Established Investigators (level II) of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
Rights and permissions
About this article
Cite this article
Bravo, D.M., Gimenes, A.C., Nascimento, R.B. et al. Skeletal muscle reoxygenation after high-intensity exercise in mitochondrial myopathy. Eur J Appl Physiol 112, 1763–1771 (2012). https://doi.org/10.1007/s00421-011-2136-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2136-4