Abstract
Transcutaneous electrical nerve stimulation (TENS) increases local blood flow. It is not known whether increase in blood flow may be caused by inhibition of sympathetic activity, mediated by muscle metaboreflex activity. The purpose of this study was to evaluate the effect of TENS on metaboreflex activation and heart rate variability (HRV) in young and older individuals. Eleven healthy young (age 25 ± 1.3 years) and 11 healthy older (age 63 ± 4.2 years) were randomized to TENS (30 min, 80 Hz, 150 μs) or placebo (same protocol without electrical output) applied on the ganglion region. Frequency domain indices of HRV and hemodynamic variables were evaluated during the pressor response to static handgrip exercise at 30% of maximal voluntary contraction, followed by recovery with (PECO+) or without (PECO−) circulatory occlusion, in a randomized order. At the peak exercise, the increase in mean blood pressure was attenuated by TENS (P < 0.05), which was sustained during PECO+ and PECO−. TENS promoted a higher calf blood flow and lower calf vascular resistance during exercise and recovery. Likewise, TENS induced a reduction in the estimated muscle metaboreflex control both in young (placebo: 28 ± 4 units vs. TENS: 6 ± 3, P < 0.01) and in older individuals (placebo: 13 ± 3 units vs. TENS: 5 ± 3, P < 0.01). HRV analysis showed similar improvement in sympatho-vagal balance with TENS in young and older individuals. We conclude that application of TENS attenuates blood pressure and vasoconstrictor responses during exercise and metaboreflex activation, associated with improved sympatho-vagal balance in healthy young and older individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies have demonstrated non-analgesic effects of transcutaneous electrical nerve stimulation (TENS), including alterations to the local circulation (Indergand and Morgan 1994; Sandberg et al. 2007; de Vries et al. 2007; Hallen et al. 2010; Kaada 1982), improvement in oxygen supply to the myocardium, and reduction of oxygen demand in cardiac patients (Sanderson et al. 1995; Mannheimer et al. 1990). Despite being influenced by the method of application, TENS at low frequencies elicits decreased levels of epinephrine and norepinephrine (Mannheimer et al. 1990; Emanuelsson et al. 1987), and it has been suggested that TENS could have a favorable impact on the sympathetic nervous system, by mitigating the effects on the pressor reflex during static exercise (Hollman and Morgan 1997).
It is well known that stimulation of metabosensitive nerve endings by chemical products of muscle contraction is associated with reflex increase in blood pressure and sympathetic-mediated vasoconstriction in non-active limbs, a mechanism known as the muscle metaboreflex (Victor et al. 1988; Seals 1989; Sinoway et al. 1989). Among other physiological differences, older subjects present tonic elevations in the basal sympathetic vasoconstrictor outflow to skeletal muscle with a decline in vascular function (Seals and Dinenno 2004; Roseguini et al. 2007) and altered muscle metaboreflex (Markel et al. 2003; Houssiere et al. 2006). Due to its effect on the sympathetic nervous system, it is conceivable that the use of TENS, when applied to the ganglion region (cervical), could attenuate the response of the muscle metaboreflex in young and older individuals and modulate heart rate variability (HRV). The present study was conducted to test this hypothesis.
Methods
Subjects
The study sample consisted of 11 healthy young individuals (5 women and 6 men) and 11 healthy older subjects (5 women and 6 men). All subjects were non-smokers, non-obese and free of any signs or symptoms of disease, as revealed by medical history, physical examination and electrocardiogram at rest and during cardiopulmonary exercise testing. Exclusion criteria were pregnancy, breast-feeding, alcohol or drug abuse, and any medication with potential effects on cardiovascular variables. Subjects were asked not to drink caffeine-containing drinks or exercise for at least 12 and 48 h, respectively, before the experimental protocols. All procedures were approved by the Committee for Ethics in Research of the Hospital de Clínicas de Porto Alegre. Subjects were informed about the study protocol and gave their informed written consent before their participation.
Design and procedures
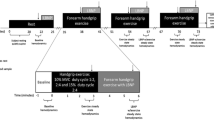
The study was a randomized crossover investigation that involved three visits to the laboratory (Fig. 1). On the first visit, subjects completed a health questionnaire and performed a maximal cardiopulmonary exercise test, as previously described (Roseguini et al. 2008). On the second and third visits, at least 72 h after the last visit, subjects performed the protocol for the evaluation of muscle metaboreflex activity. TENS or placebo intervention was randomized and utilized acutely before the muscle metaboreflex protocol.
Transcutaneous electrical nerve stimulation
Individuals were acutely treated with conventional TENS (TensMed 911 Device, Enraf–Nonius B.V., Rotterdan, Netherlands, GB 3004) or a placebo condition during 30 min. TENS intervention consisted of continuous flow, symmetrical, and rectangular TENS biphasic pulses for 30 min. To evaluate the waveform of the stimulus, the TENS unit was calibrated at a 4-Hz frequency and 200-μs pulse duration before the experiment, using a digital recording oscilloscope (1602 Gould Electronics Ltd., Instruments Systems, UK). The frequency of stimulation was 80 Hz and the pulse duration was 150 μs, with the intensity in milliamperes (mA) adjusted to the point of muscle contraction defined as the sensitivity threshold (Cipriano et al. 2008). The stimulation level of 80 Hz was intended to elicit strong sensations of paresthesia, without pain or contractions of any muscles (Low and Reed 2000; Mannheimer and Lampe 1984). Intensity was then increased from zero until the perceived sensation, set at the maximal level at which subjects did not report pain and no voluntary contraction was observed. Adhesive electrodes (MultiStick®, Axelgaard Manufacturing CO, Ltd., Fallbrook, CA, USA) were placed on each side, about 3 cm to the right and left of midline vertebral process, at C7 (Channel 1) and T4 (Channel 2) (Fig. 2). The same instructions and electrode positions were provided to the placebo, although the equipment did not provide any stimulation current (DeSantana et al. 2008).
Measurements
Muscle metaboreflex study
The muscle metaboreflex was evaluated as described elsewhere (Roseguini et al. 2008). Briefly, maximal voluntary contraction (MVC) of the dominant arm was initially determined with a handgrip dynamometer (Jamar® Hydraulic Hand Dynamometer, Sammons Preston CO, Bolingbrook, Illinois, USA). A static handgrip exercise was performed at 30% of MVC for 3 min, immediately followed by vascular occlusion (PECO+) or no occlusion (PECO−) of the exercising arm to promote selective induction of the metaboreflex. Heart rate (HR) was measured by a heart rate monitor (POLAR model RS800, Kempele, Finland) and mean blood pressure (MBP) was measured in the non-dominant arm using a calibrated oscillometric automatic device (Dinamap 1846SX/P, Critikon, Tampa, Florida, USA). Calf blood flow (CBF) was measured by venous occlusion plethysmography (Hokanson, TL-400, Bellevue, USA). Calf vascular resistance (CVR) was calculated as MBP/CBF (Roseguini et al. 2008; Arnold et al. 1990). All flow recordings were manually traced by an operator who was blinded to the intervention and time. Reproducibility of CBF measurements in our laboratory has been observed to be good with coefficients of variation of 5.7 and 5.9% for intra and inter-day measurements, respectively (Roseguini et al. 2007, 2008).
Heart rate variability
Recordings obtained from the heart rate monitor were analyzed using intervals during the 9-min HRV data acquisition period. HRV in the frequency domain was calculated according to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Power spectral components were reported using Fast Fourier Transform at LF and HF, expressed in normalized units. Briefly, the LF component partially reflects sympathetic modulation, although it is also influenced by parasympathetic components (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996; Malliani et al. 1991), while the HF component is representative of the parasympathetic modulation (Malliani et al. 1991; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996), and the LF/HF ratio indicates sympatho/vagal balance. Analyses were performed with a personal computer using customized software (KUBIOS, Kuopio, Finland) (Tarvainen et al. 2002; Tarvainen and Niskanen 2008). Artifacts were reviewed by visual inspection of the computer display. Only segments with >95% pure sinus beats were included in the final analysis.
Statistical analysis
Values are shown as means ± SE. Hemodynamic responses to exercise and to PECO+/PECO− were compared by two-way ANOVA for repeated measures and Tukey–Kramer’s for post hoc comparisons. Comparisons between the responses of young and older individuals were performed with three-way analysis of variance for repeated measures. Statistical significance was accepted when P < 0.05. Data were analyzed using SigmaPlot® version 11.
Results
Table 1 shows the characteristics of the two groups. Older individuals presented significantly higher age and lower handgrip force as well as peak oxygen uptake, but similar resting hemodynamic measures. No adverse events occurred throughout the protocols. Figure 3 shows the responses of HR, MBP, CBF, and CVR at baseline, during the handgrip exercise, and during the recovery with PECO+ or PECO−, with and without TENS. Young and older individuals presented similar responses. The HR response to exercise showed a smaller increase after the use TENS. However, recovery was not affected by TENS or placebo during PECO+ and PECO−. MBP was reduced at peak exercise and during early recovery in the TENS group, compared to placebo. CBF was increased and CVR was reduced with TENS throughout the entire protocol. PECO+ resulted in a higher MBP and CVR, with reduced CBF during both TENS and placebo conditions.
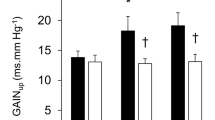
As shown in Fig. 4, the difference in areas under the curves of CVR during the occlusion protocol (PECO+/PECO−), which estimates the metaboreflex activity, was larger in young when compared to older individuals during placebo stimulation. The application of TENS reduced metaboreflex activity in both groups, but with a significantly larger reduction in young individuals.
Mean blood pressure (MBP), heart rate (HR), calf blood flow (CBF), and calf vascular resistance (CVR) in absolute values during the static hangrip exercise, and after exercise with (PECO+) and without (PECO−) circulatory occlusion in healthy young (left panels) and older (right panels) individuals with TENS (continuous lines) and placebo (dotted lines). Asterisk two-way repeated measures ANOVA (P < 0.05): TENS versus placebo, PECO+ (TENS) versus PECO+ (placebo); dagger two-way repeated measures ANOVA (P < 0.05): TENS versus placebo, PECO− (TENS) versus PECO− (placebo)
Estimated muscle metaboreflex control of calf vascular resistance, obtained by the subtraction of the area under the curve during circulatory occlusion from the control period, in young and older individuals during transcutaneous electrical stimulation (TENS) or placebo (PLA). Two-way ANOVA for repeated measures: P < 0.05 for group, intervention, and interaction effects. Multiple comparisons: asterisk significantly (P < 0.01) different from placebo; dagger significantly different from young
Table 2 presents the analytical results for HRV parameters during PECO− and PECO+. Both groups presented similar responses to the protocol. LF component was unchanged in both PECO− and PECO+. However, the HF component increased during the period of occlusion with TENS. The sympatho-vagal balance modulation with TENS reduced significantly during occlusive period, in comparison with the placebo, with no differences when the PECO− condition was analyzed.
Discussion
We hypothesized that the impaired peripheral muscle blood flow in healthy young and older subjects would be at least partially restored by the application of TENS to the ganglion region. To our knowledge, this is the first study to show the effect of TENS on the pressor response to static handgrip exercise in healthy individuals. In this study, TENS was associated with a significant decrease in CVR and increased CBF, resulting in a blunted muscle metaboreflex. Both young and older individuals demonstrated reduced metaboreflex activation with TENS, but the size of the effect was larger in young individuals. In addition, TENS evoked modulation of HRV in young and older individuals, with increased HF component and decreasing LF/HF ratio during PECO+. Furthermore, the use of TENS resulted in significantly lower levels of MBP (vasoconstrictor tone), which were observed even during the blood pressure increases during the handgrip exercise.
Aging has been related to impaired vascular endothelial function (Gates et al. 2007) that results in a limited vasodilatory capacity (Dinenno et al. 1999, 2001a). However, these changes evoke reductions in both limb blood flow and vascular conductance with age, which is mediated by increased sympathetic activity (Dinenno et al. 2001b). Similar to the present findings, previous studies have demonstrated an altered muscle metaboreflex (Houssiere et al. 2006; Markel et al. 2003) and chemoreflex control that could limit muscle contraction by impaired oxygen transport (cardiac output limited by blood pressure elevation) (Parker et al. 2008). Therefore, strategies to attenuate the redistribution of blood flow during exercise appear to be important in this population. In the present study, TENS was found to improve this response together with an effect on sympatho-vagal modulation.
TENS significantly reduced blood pressure at the peak of the handgrip exercise, which was sustained throughout the PECO+. Several studies have reported the favorable effects of TENS on hemodynamic variables (Kaada et al. 1990; Indergand and Morgan 1994; Chauhan et al. 1994; Sandberg et al. 2007). Studies in animal models have suggested an attenuated autonomic activity upon the use of TENS (Collins and DiCarlo 2002). Thus, some studies have suggested that the application of TENS on the stellate ganglion may result in a reduction of blood pressure (Sherry et al. 2001). In contrast, Indergand and Morgan (1994) failed to demonstrate any effect on blood pressure. We also applied TENS on the ganglion region; however, our results showed a significant reduction in blood pressure. In additional, baroreflex buffering is attenuated in older subjects (Jones et al. 2003). These factors may indicate that the elevation in HR during PECO+ in older subjects may be due to reduced baroreflex-mediated increases in parasympathetic activity (Taylor et al. 1995; Roseguini et al. 2007).
Furthermore, in our study, the application of TENS resulted in a significant reduction in blood pressure and HR during the exercise peak and PECO+, which evoked improvements in the sympatho-vagal modulation. Hollman and Morgan (1997) demonstrated that the application of TENS on the forearm resulted in a reduced sympathetically-mediated pressor response. This response is compatible with our findings. It is possible that TENS changed the level of the central command necessary to maintain the required force output. In our study, HR at the peak of exercise increased, but, when performed after TENS, a smaller increase in HR was observed indicating an attenuation of the central command effect by TENS. Additionally, TENS may be able to evoke neurostimulation of the stellate ganglion by higher diameter vessels that supply the upper limbs. This ganglion region at C8 level is responsible for upper limb innervations (Rogers et al. 1973; Janes et al. 1986).
In parallel, blockade of this pathway may help to reduce sympathetic activity (Rogers et al. 1973) and increase HRV (Kim et al. 2010). Indeed, in our experiments, TENS reduced the LF and increased the HF components of HRV during PECO+ in both young and older individuals. These findings indicate an improvement in the sympatho-vagal balance during exercise and PECO+ after the application of TENS, as demonstrated by a reduction in the LF/HF ratio. Pharmacological blockade of the right stellate ganglion has been reported to reduce both sympathetic and parasympathetic modulation (Hilz et al. 2001; Taneyama and Goto 2009). In addition, studies of left stellate ganglion blockage showed no change in any of the HRV components (Rogers et al. 1973; Taneyama and Goto 2009). However, TENS may influence the stellate ganglion directly, resulting in an improvement of the HRV. Thus, this effect may be linked to the reduction of CVR and augmentation of CBF, which are compatible with the attenuation of the muscle metaboreflex.
Study limitations
Our placebo intervention consisted of the application of TENS electrodes at the same dorsal region as that used during TENS in the experimental group; however, the power of the TENS unit was turned off. Thus, no stimulation was provided and we are unaware of any device that could provide a real minor placebo stimulus, without promoting some level of neuromodulation (DeSantana et al. 2008).
Conclusions
Acute application of TENS at the ganglion region attenuates blood pressure and vasoconstrictor responses during exercise and metaboreflex activation, associated with improved sympatho-vagal balance in healthy young and older individuals.
References
Arnold JM, Ribeiro JP, Colucci WS (1990) Muscle blood flow during forearm exercise in patients with severe heart failure. Circulation 82(2):465–472
Chauhan A, Mullins PA, Thuraisingham SI, Taylor G, Petch MC, Schofield PM (1994) Effect of transcutaneous electrical nerve stimulation on coronary blood flow. Circulation 89(2):694–702
Cipriano G Jr, de Camargo Carvalho AC, Bernardelli GF, Tayar Peres PA (2008) Short-term transcutaneous electrical nerve stimulation after cardiac surgery: effect on pain, pulmonary function and electrical muscle activity. Interact Cardiovasc Thorac Surg 7(4):539–543. doi:10.1510/icvts.2007.168542
Collins HL, DiCarlo SE (2002) TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283(4):H1734–H1739. doi:10.1152/ajpheart.00253.2002
de Vries J, Svilaas T, DeJongste MJ, Nieuwland W, Hoekstra-Mars EW, Zijlstra F (2007) Impact of electrical neurostimulation on persistent ST elevation after successful reperfusion by primary percutaneous coronary intervention. J Electrocardiol 40(6):522–526. doi:10.1016/j.jelectrocard.2007.05.014
DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA (2008) Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep 10(6):492–499
Dinenno FA, Jones PP, Seals DR, Tanaka H (1999) Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100(2):164–170
Dinenno FA, Seals DR, DeSouza CA, Tanaka H (2001a) Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531(Pt 2):573–579. pii:PHY_1722
Dinenno FA, Tanaka H, Stauffer BL, Seals DR (2001b) Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536(Pt 3):977–983 pii: PHY_12670
Emanuelsson H, Mannheimer C, Waagstein F, Wilhelmsson C (1987) Catecholamine metabolism during pacing-induced angina pectoris and the effect of transcutaneous electrical nerve stimulation. Am Heart J 114(6):1360–1366
Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR (2007) Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102(1):63–71. doi:10.1152/japplphysiol.00660.2006
Hallen K, Hrafnkelsdottir T, Jern S, Biber B, Mannheimer C, DuttaRoy S (2010) Transcutaneous electrical nerve stimulation induces vasodilation in healthy controls but not in refractory angina patients. J Pain Symptom Manage 40 (1):95–101. doi:10.1016/j.jpainsymman.2009.12.009
Hilz MJ, Dutsch M, Perrine K, Nelson PK, Rauhut U, Devinsky O (2001) Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann Neurol 49(5):575–584
Hollman JE, Morgan BJ (1997) Effect of transcutaneous electrical nerve stimulation on the pressor response to static handgrip exercise. Phys Ther 77(1):28–36
Houssiere A, Najem B, Pathak A, Xhaet O, Naeije R, Van De Borne P (2006) Chemoreflex and metaboreflex responses to static hypoxic exercise in aging humans. Med Sci Sports Exerc 38(2):305–312. doi:10.1249/01.mss.0000187426.93464.81
Indergand HJ, Morgan BJ (1994) Effects of high-frequency transcutaneous electrical nerve stimulation on limb blood flow in healthy humans. Phys Ther 74(4):361–367
Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, Armour JA (1986) Anatomy of human extrinsic cardiac nerves and ganglia. Am J Cardiol 57(4):299–309
Jones PP, Christou DD, Jordan J, Seals DR (2003) Baroreflex buffering is reduced with age in healthy men. Circulation 107(13):1770–1774. doi:10.1161/01.CIR.0000057811.86187.88
Kaada B (1982) Vasodilation induced by transcutaneous nerve stimulation in peripheral ischemia (Raynaud’s phenomenon and diabetic polyneuropathy). Eur Heart J 3(4):303–314
Kaada B, Vik-mo H, Rosland G, Woie L, Opstad PK (1990) Transcutaneous nerve stimulation in patients with coronary arterial disease: haemodynamic and biochemical effects. Eur Heart J 11(5):447–453
Kim JJ, Chung RK, Lee HS, Han JI (2010) The changes of heart rate variability after unilateral stellate ganglion block. Korean J Anesthesiol 58(1):56–60. doi:10.4097/kjae.2010.58.1.56
Low J, Reed A (2000) Electrotherapy explained: principles and practice, 3rd edn. Buttworth-Heinemann, Oxford, pp 54–139
Malliani A, Pagani M, Lombardi F, Cerutti S (1991) Cardiovascular neural regulation explored in the frequency domain. Circulation 84(2):482–492
Mannheimer JS, Lampe GN (1984) Clinical transcutaneous electrical nerve stimulation. F. A. Davis Co., Philadelphia, USA, p 636
Mannheimer C, Emanuelsson H, Waagstein F (1990) The effect of transcutaneous electrical nerve stimulation (TENS) on catecholamine metabolism during pacing-induced angina pectoris and the influence of naloxone. Pain 41(1):27–34 pii: 0304-3959(90)91105-R
Markel TA, Daley JC 3rd, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI (2003) Aging and the exercise pressor reflex in humans. Circulation 107(5):675–678
Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN (2008) Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104(3):655–664. doi:10.1152/japplphysiol.01150.2007
Rogers MC, Abildskov JA, Preston JB (1973) Cardiac effects of stimulation and block of the stellate ganglion. Anesthesiology 39(5):525–533
Roseguini BT, Alves CN, Chiappa GR, Stein R, Ribeiro JP (2007) Muscle metaboreflex contribution to resting limb haemodynamic control is preserved in older subjects. Clin Physiol Funct Imaging 27(5):335–339. doi:10.1111/j.1475-097X.2007.00756.x
Roseguini BT, Alves CN, Chiappa GR, Stein R, Knorst MM, Ribeiro JP (2008) Attenuation of muscle metaboreflex in chronic obstructive pulmonary disease. Med Sci Sports Exerc 40(1):9–14. doi:10.1249/mss.0b013e3181590bd9
Sandberg ML, Sandberg MK, Dahl J (2007) Blood flow changes in the trapezius muscle and overlying skin following transcutaneous electrical nerve stimulation. Phys Ther 87(8):1047–1055. doi:10.2522/ptj.20060178
Sanderson JE, Tomlinson B, Lau MS, So KW, Cheung AH, Critchley JA, Woo KS (1995) The effect of transcutaneous electrical nerve stimulation (TENS) on autonomic cardiovascular reflexes. Clin Auton Res 5(2):81–84
Seals DR (1989) Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol 66(5):2472–2478
Seals DR, Dinenno FA (2004) Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 287(5):H1895–H1905. doi:10.1152/ajpheart.00486.2004
Sherry JE, Oehrlein KM, Hegge KS, Morgan BJ (2001) Effect of burst-mode transcutaneous electrical nerve stimulation on peripheral vascular resistance. Phys Ther 81(6):1183–1191
Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R (1989) Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol 66(1):429–436
Taneyama C, Goto H (2009) Fractal cardiovascular dynamics and baroreflex sensitivity after stellate ganglion block. Anesth Analg 109(4):1335–1340. doi:10.1213/ane.0b013e3181b018d8
Tarvainen MP, Niskanen JP (2008) Kubios HRV version 2.0 User’s Guide. Department of Physics University of Kuopio, Finland
Tarvainen MP, Ranta-Aho PO, Karjalainen PA (2002) An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng 49(2):172–175. doi:10.1109/10.979357
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93 (5):1043–1065
Taylor JA, Hayano J, Seals DR (1995) Lesser vagal withdrawal during isometric exercise with age. J Appl Physiol 79(3):805–811
Victor RG, Bertocci LA, Pryor SL, Nunnally RL (1988) Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82(4):1301–1305. doi:10.1172/JCI113730
Acknowledgments
The authors thank all colleagues from the Exercise Pathophysiology Research Laboratory (Cardiology Division, Federal University of Rio Grande do Sul, Brazil) for their friendly collaboration. This study was supported by a research grant from the Hospital de Clínicas de Porto Alegre Research Fund, Porto Alegre Brazil. Paulo J. C. Vieira is supported by a Doctoral Scholarship and Gaspar R. Chiappa receives a Post-doctoral Fellowship from the Brazilian Research Council (CNPq), Brasilia, Brazil.
Conflict of interest
None of the authors have any potential conflict of interest related to the contents of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dag Linnarsson.
Rights and permissions
About this article
Cite this article
Vieira, P.J.C., Ribeiro, J.P., Cipriano, G. et al. Effect of transcutaneous electrical nerve stimulation on muscle metaboreflex in healthy young and older subjects. Eur J Appl Physiol 112, 1327–1334 (2012). https://doi.org/10.1007/s00421-011-2084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2084-z