Abstract
The objective was to investigate if whole-hand mechanical stimulation (MSTIM) in the tapping-flutter frequency range induces outlasting post-stimulus changes in the hand region of the primary motor cortex. MSTIM was delivered to 12 healthy subjects for 20 min using a therapeutic stimulation device (Swisswing BMR 2000). Frequencies of 10 and 25 Hz were tested in separate sessions, and for control additionally the foot sole was stimulated at 25 Hz. Motor evoked potentials (MEPs) after single (recruitment curves) and paired-pulse transcranial magnetic stimulation (TMS) were recorded from FDI and APB muscles of the right hand. TMS assessments were carried out at baseline (T0), immediately after (T1), 30 min (T2), 1 h (T3) and 2 h (T4) after end of MSTIM. After MSTIM with 25 Hz, MEP recruitment curves were increased at all post-stimulation assessments in both muscles. The most significant effect was achieved at T3 (1 h). Intracortical inhibition was decreased within the first hour, while intracortical facilitation was increased at all post-stimulation assessments. No significant effects were found following MSTIM with 10 Hz and following foot vibration. We conclude that 20 min MSTIM with a frequency of 25 Hz induces outlasting plastic changes in the primary motor cortex. Paired-pulse stimulation further confirms that intrinsic intracortical mechanisms are involved in these changes. Spinal adaptation could be excluded (F-wave assessments). These results could be of relevance for hemiplegic patients with motor deficits, to improve the rehabilitation outcome with vibration exercise in combination with motor training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The functional organization of the sensorimotor cortex is dynamic; therefore, changes in afferent input have the capacity to alter cortical maps and to modulate corticomotor excitability. Studies on adult humans have shown that a period of pure sensory stimulation increases corticomotor excitability for a period outlasting the stimulation (Kaelin-Lang et al. 2002; Ridding et al. 2001). The anatomical substrate for this cross-systemic plasticity is provided from in vivo experiments by discovering topographically and functionally specific reciprocal connections between primary motor cortex (M1) and primary somatosensory cortex (S1) (Rocco and Brumberg 2007). As a physiological basis for sensory driven outlasting effects, changes in synaptic efficiency through timing-dependent associative neuronal activities have been described (Feldman 2000).
Various modalities of afferent input (mechanical, electrical, magnetic) have been employed in attempt to influence motor controlling structures. Muscle vibration applied at low amplitude preferentially produces Ia afferent input, reaching both S1 and M1. Using transcranial magnetic stimulation (TMS), changes in motor map organization (Forner-Cordero et al. 2008), in corticospinal excitability (Steyvers et al. 2003), in sensorimotor organization (Rosenkranz and Rothwell 2006) and in intracortical inhibition circuits (Rosenkranz and Rothwell 2003) after periods of muscle or tendon vibration have been reported. In all these studies, vibration has been applied locally on muscles or tendons showing that continuous afferent input effectively modulates the excitability of corticomotor projections to the specific muscle. However, controversial results have been reported by these authors concerning the effects on the vibrated versus non-vibrated muscles. Analogous focal neuroplastic effects have been also obtained following chronic nerve stimulation (Charlton et al. 2003) and peripheral magnetic stimulation (Struppler et al. 2007).
For health and therapeutic applications, it seems advantageous to deliver sensory stimulation not to a single muscle or nerve, but rather to a broader area. Therefore, in this study we considered whole hand mechanical stimulation (MSTIM), by utilizing an oscillating device originally made for vibration exercise. The whole hand gives a projection to the wide area of hand corticomotor representation and by touching the device with the palm, afferent inflow from cutaneous afferents (type II fiber group) will be generated projecting to S1, mainly to Brodman area 3B. Further, muscles and joints become forced into cyclic motion during contact with the oscillating device, thus also proprioceptive inflow will be generated to activate motor cortical cells as shown with neurography (Fourment et al. 1966) and with TMS (Siggelkow et al. 1999). Therefore following MSTIM an increase of excitability within the contralateral M1, over the stimulation-exposed muscles is expected.
Generally, for mechanical stimulation different frequencies have been used: “tapping” (frequencies below 20 Hz), “flutter” (frequencies between 20 up to 50 Hz) and vibration (frequencies above 50 Hz). For this study, we focus on mechanical stimuli in the tapping-flutter frequency range (10 and 25 Hz). In this range, spinal reflex excitation (Bongiovanni and Hagbarth 1990) is not expected and also no evoked sensory illusions should occur (Goodwin et al. 1972). Cortical responses evoked by stimulation in the tapping-flutter frequency range have been explored with various techniques. Functional magnetic resonance (fMRI) studies reported brain activation elicited by vibrotactile stimulation within S1 and the secondary somatosensory cortex (Disbrow et al. 2000) and within the motor cortex (Golaszewski et al. 2006). In a study with primates using optical intrinsic signal imaging Simons et al. (2005) showed that an increase in amplitude of a 25 Hz flutter stimulus led to a proportional increase in absorbance within the forelimb representational region of S1. In an EEG study Snyder (1992) applied pulsed vibration with a pulse rate between 2 and 40 Hz to the palmar surface of the hand. The steady-state somatosensory evoked potentials over the contralateral S1 showed the highest amplitude at a pulse rate of 26 Hz. Due to this resonance-like phenomenon in the somatosensory system stimulation frequencies around 25 Hz are expected to achieve effective cortical modulation. Concerning stimulus duration exposure times of 15 min were found effective for muscle vibration (Smith and Brouwer 2005), and for electrical stimulation exposure times of more than 30 min are very common. (Charlton et al. 2003; Golaszewski et al. 2004).
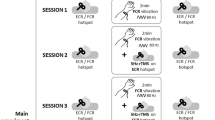
Based on this knowledge the present study examines the hypothesis that continuous MSTIM (20 min) induces outlasting adaptive changes in contralateral M1. Therefore, corticomotor excitability changes were studied at two hand muscles within a post-stimulation period of 2 h by applying single pulse TMS and the paired-pulse paradigm (Kujirai et al. 1993). Two stimulation frequencies with the same vibration amplitude (2 mm) were tested in repeated sessions: 10 and 25 Hz. For control the foot sole was stimulated with a frequency of 25 Hz in a third session (sham stimulation). Further F-wave assessments were performed in order to document spinal adaptive changes.
Methods
Subjects and experimental design
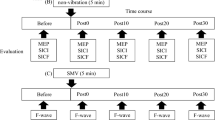
Twelve healthy volunteers (3 males and 9 females, mean age 26.08 ± 4.19 years) gave informed consent to participate in the study approved by the Ethics Committee of the Medical University of Graz. All participants were right-handed according to the Laterality Quotient (LQ) from the Edinburgh Handedness Inventory (Oldfield 1971). During the TMS assessments, subjects were sitting relaxed in a reclining armchair. For MSTIM, both arms were placed relaxed on soft supports beside the body while the palm of the right hand was placed on the surface of the vibration exercise device. For sham stimulation, the left foot sole was placed on the vibration device again at sitting position. During stimulation exposure, subjects were instructed to distract attention from the vibrated site and device. During the resting pauses between the TMS assessments, the subjects stayed in the same relaxed position. The experimental sequence included baseline TMS assessment before mechanical stimulation (T0), stimulation to the hand/foot for 20 min, follow-up TMS assessments immediately after mechanical stimulation (T1), after 30 min (T2), after 1 h (T3), and after 2 h (T4). All participants were exposed to three protocols: MSTIM with 10 Hz (HAND10), MSTIM with 25 Hz (HAND25), and foot mechanical stimulation at 25 Hz (FOOT25). The participants received the protocols on three different days in a random order.
Mechanical stimulation
For mechanical stimulation, a commercial stimulation device (Swisswing BMR 2000, SwissTTP Inc. USA) was utilizied, see Fig. 1. This device consists of a 30 cm diameter barrel forced into eccentric motion by an internal electromotor. The motion amplitude of BMR 2000 device is fixed to ±2 mm (peak amplitude) and the stimulation frequency can be selected between 5 and 35 Hz. Further, this device can be adjusted in position for allowing comfortable stimulation when sitting on a chair while the arm or foot of the investigated subject is stimulated in relaxed position.
For stimulation, the barrel was touched with the palm of the hand or the foot sole in the direction of barrel motion. In this orientation, effective stimulation of muscle fibers is obtained while mechanical stress on bones and joints is rather kept low. Further, a thin sheet of foam rubber covered the barrel in order to avoid discomfort during long stimulation applications. Stimulations at a frequency of 10 Hz are perceived rather as “massage effect” while stimulations at 25 Hz are rather perceived as “vibration.” The stimulation device operates on low noise levels (54 dBA measured at 25 Hz) so that auditory arousals should not occur.
TMS assessments
TMS was performed using two Magstim 200 magnetic stimulators connected via the Bistim module (The Magstim Company, Whitland, Dyfed, UK). Magnetic pulses were delivered through a figure-of-eight-shaped coil (outer loop diameter of 9 cm). The coil was positioned on the scalp over the left motor cortex at the optimal site for stimulating the contralateral right first dorsal interosseous (FDI) and the abductor pollicis brevis (APB) muscle. The intersection of the coil was placed tangentially to the scalp with the handle pointing backward and laterally at a 45° angle away from the midline. Resting motor threshold (rMT) was expressed as percentage of the maximum output of the stimulator. Using suprathreshold intensities, the coil was moved over the scalp in small steps to locate the site with the largest motor evoked potential (MEP). This position was marked on an EEG cap and the coil was fixed at that position.
The following TMS parameters were examined: resting motor threshold (rMT), MEP recruitment curve (RC), short-latency intracortical inhibition (SICI) and intracortical facilitation (ICF). The rMT was defined as the lowest stimulus intensity at which seven out of nine consecutive TMS applications leaded to a MEP of at least 0.05 mV on the relaxed FDI and APB. MEP recruitment curves were obtained with stimulus intensities 100, 110, 120, 130, 140, 150, and 160% of rMT, determined for each subject at T0 and administered in a random order. Eight single pulses were delivered at each stimulus intensity; SICI and ICF were assessed using the paired-pulse paradigm (Kujirai et al. 1993) with interstimulus intervals (ISI) of 3 and 13 ms. Eight conditioned pulses were obtained for each ISI. The conditioning and test stimuli were 80 and 120% of the corresponding rMT at the actual assessment (at T0, T1, T2, T3 and T4).
Electromyographic recordings
MEPs were recorded from the FDI and APB muscles of the right hand using 9 mm diameter Ag–AgCl surface disc electrodes in a tendon-belly manner. Electromyographic (EMG) signals were amplified, band pass filtered (8–2,000 Hz), digitized (sampling rate 10 kHz) and recorded on disc (DasyLab 8.0 software package). The EMG signal during TMS assessments was displayed continuously to ensure the absence of any motor activity.
F-wave assessments
F-waves were employed in separate sessions. The recordings were performed with a Keypoint Portable equipment (Dantec Dynamics Ltd., England) using the F-wave program. Supramaximal electric stimulation on the ulnar nerve at the wrist was delivered transcutaneously using surface electrodes. Stimulus intensity was adjusted to produce a maximal M-wave in the right FDI muscle. F-waves were recorded in relaxed muscles before and after the protocols, HAND25 and HAND10. Peak-to-peak amplitude and persistence of F-waves (average, 20 trials) were determined.
Data analysis and statistics
MEP amplitudes were measured off-line. For rMT, expressed as percentage of the maximum stimulator output (% mean ±SE), a two-factorial repeated measures ANOVA (analysis of variance) was conducted with within-subject factors time (5 levels: T0, T1, T2, T3 and T4) and protocol (3 levels: HAND10, HAND25 and FOOT25). One-way ANOVA was conducted for each protocol separately with within-subject factor time if a significant effect was found.
For the recruitment curves the MEP amplitudes (mean from 8 trials) were normalized to the maximum mean MEP at baseline (T0)— which was usually recorded at 160% MT intensity—for each subject individually. The MEP amplitudes were then analyzed separately for each stimulation intensity (from 100 to 160%) using a repeated measures ANOVA with within-subject factors: time (5 levels: T0, T1, T2, T3 and T4), protocol (3 levels: HAND10, HAND25 and FOOT25) and muscle (2 levels: FDI, APB). In the case of significant effects, follow-up ANOVAs for each level of protocol and for each level of muscle were conducted separately.
For the conditioned MEP responses (SICI and ICF), the amplitudes were calculated as a percentage of the corresponding single pulse MEP for each subject individually. Then a repeated measures ANOVA was used to assess the effects of mechanical stimulation on conditioned MEP amplitudes separately for each ISI (inhibitory/3 ms and facilitatory/13 ms) with within-subject factors time (5 levels: T0, T1, T2, T3 and T4), protocol (3 levels: HAND10, HAND25 and FOOT25) and muscle (2 levels: FDI, APB). In the case of significant effects, follow-up ANOVAs for each level of protocol and muscle were conducted separately.
The F-wave amplitude and persistence were analyzed separately using a two-factorial ANOVA with factors time (2 levels: T0, T1) and protocol (2 levels: HAND25, HAND10). In all statistical tests, a significance level of 0.05 was used. Furthermore, where ANOVA showed significance, Bonferroni-corrected pairwise post-hoc comparisons were conducted.
Results
Valid data were obtained from all 12 participants. Some subjects experienced a slight feeling of itching on the hand palm/foot sole after application of MSTIM; however, none of them reported discomfort.
Effect of protocol on rMT
Mean rMT values measured at baseline (T0) were 38.7 ± 3.0% for protocol HAND10, 39.0 ± 3.4% for protocol HAND25, and 38.8 ± 3.9% for protocol FOOT25. No significant differences were found. Furthermore, the ANOVA revealed no effect of the stimulation protocols on rMT measured at time levels T1, T2, T3, and T4.
Effect of protocol on MEP recruitment curves
Figure 2 represents the MEP recruitment curves, measured at time levels T0, T1, T2, T3 and T4 for each protocol (plots from left to right) and for both muscles (upper/lower plots). For all stimulation intensities, the three-factorial ANOVA revealed a significant interaction effect of protocol × time and significant main effects of muscle, protocol and time. Thus, additional two-factorial ANOVAs were conducted for each level of protocol with factors muscle and time. However, only the protocol HAND25 showed significant effects. Further, for protocol HAND25, a main effect of time was revealed for FDI (Ps < 0.01) and APB (Ps < 0.05). For both the muscles, MEP reached its peak at the time level, T3. HAND10 and FOOT25 failed to induce any significant increase in recruitment curves. No significant differences between the three protocols were revealed at time level, T0.
MEP recruitment curves at baseline (T0), immediately after (T1), 30 min (T2), 1 h (T3) and 2 h (T4) after 20 min hand and foot stimulation (from left to right). The upper plots present results for FDI, the lower plots-for APB muscle. MEPs are normalized to the maximum mean MEP at T0 from each subject individually and then pooled for all subjects for each protocol separately. For each stimulus intensity mean (SEM) of the normalized MEP amplitude is plotted. The significant differences between T0 and the corresponding post-stimulation assessments are presented (*) P < 0.05; (+) P < 0.01
Effect of protocol on SICI and ICF
The results from paired-pulse stimulation are shown in Fig. 3, both for FDI and APB muscles. Separate ANOVAs were conducted for ISI (3, 13 ms) to reveal the effect of the stimulation protocol on SICI and ICF, respectively. Significant interactions muscle × protocol × time were found both for SICI (F (8,88) = 27.9, P < 0.001) and for ICF (F (8,88) = 14.4, P < 0.001), therefore, follow-up ANOVAs were conducted separately for each stimulation protocol (HAND10, HAND25, and FOOT25). Here significance was revealed for HAND25, both for SICI and ICF, further a significant main effect of time was revealed for HAND25 at both muscles. The value of SICI from FDI was increased at T1 (30%), at T2 (26%) and at T3 (26%), equivalent to a reduced effect of intracortical inhibition. Also the value of SICI on APB was increased at T1 (29%), at T2 (47%) and at T3 (43%). At T4 the level of SICI almost returned its baseline level. ICF was increased up to 53% for FDI and up to 47% for APB in all post-stimulation assessments. HAND10 and FOOT25 did not cause any significant changes in SICI and ICF levels. No significant differences between the three protocols were revealed at the time level (T0), both for SICI and ICF.
Normalized paired-pulse responses at baseline (T0), immediately after (T1), 30 min (T2), 1 h (T3) and 2 h (T4) after 20 min hand and foot stimulations. The upper plots present results for ISI of 3 ms (SICI). The lower plots present results for ISI 13 ms (ICF). Values are normalized for each subject to their corresponding values in single pulse stimulation and plotted as mean (SEM). The significant differences between T0 and the corresponding post stimulation assessment are presented (*) P < 0.05; (+) P < 0.01
Effect of protocol on F-waves
The two-factorial ANOVA revealed no significant effect, neither on protocol nor on time both for F-wave amplitude (Fs (1,11) < 1.13, Ps > 0.31) and persistence (Fs (1,11) < 2.39, Ps > 0.15).
Discussion
The goal of this TMS-study was to examine if whole hand mechanical stimulation in the tapping-flutter frequency range induces outlasting neuromodulatory effects in the motor cortex. A main finding is that whole hand stimulation with a vibration frequency of 25 Hz (flutter) induced post-effects up to 2 h, whereas stimulation with 10 Hz (tapping) induced no effects. Also 25 Hz sham stimulation induced no effects in the hand region of the motor cortex.
For the effectiveness of 25 versus 10 Hz stimulation peripheral and central factors may count for. One peripheral factor is that according to Newton’s second law the force acting on a rigid body depends on its acceleration, the second derivative of displacement. Under consideration of a sinusoidal waveform, the acceleration amplitude increases with the square of frequency in the case the body is driven by constant displacement amplitude. For a frequency increase by a factor of 2.5 (from 10 to 25 Hz), accordingly the acceleration amplitude increases by a factor of 6.25. From this point of view 10 Hz stimulation (tapping) can be considered as “low intensity stimulation” and 25 Hz stimulation (flutter) as “high intensity stimulation”. And although the hand is not a rigid body, but rather consists of viscoelastic coupled elements, it seems conceivable that the acceleration amplitude is an important factor. At least also joint resonance may affect the stimulation intensity (Gallasch et al. 1996).
A second peripheral factor is the response characteristics of the mechanoreceptive structures. For whole hand stimulation we have to consider both cutaneous receptors and proprioceptors. Cutaneous receptors responding to stimuli in the tapping-flutter frequency range are the slowly adapting Merkel corpuscles and the rapid adapting Meissner corpuscles. Merkel corpuscles respond to skin deformations and have been found to be most sensitive in a frequency range below 16 Hz (Johansson et al. 1982), while Meissner corpuscles respond to velocity with a best frequency around 30 Hz (Talbot et al. 1968). As with increasing stimulation frequency velocity sensitive receptors and possibly also acceleration sensitive receptors (Pacinian corpuscles) become recruited, a higher afferent inflow to cortical structures will be generated. The same applies for proprioceptors, namely muscle spindles, whose afferents also show a high pass frequency–response characteristic (Matthews and Stein 1969).
Possible central factors that may account for the effectiveness of 25 Hz stimulation are the cortical responsiveness and the existence of a threshold to induce synaptic effects. For hand stimulation steady-state evoked potentials showed a maximal response in the frequency range between 21 and 26 Hz (Snyder 1992; Tobimatsu et al. 1999). Thus the somatosensory cortex appears to be selectively tuned to stimulation frequencies in the flutter range. If such sensory driven potentials have an impact to influence the membrane potential and to evoke neuroplastic changes remains unclear. However some evidence is provided by a recent in vitro and in vivo study where it has been shown that field potentials indeed can have an influence on the membrane potential and the neuronal dynamics (Fröhlich and McCormick 2010).
The existence of a threshold to induce lasting synaptic effects is described by the BCM rule (Bienenstock et al. 1982). According to this rule active synapses become facilitated when the total synaptic response exceeds a critical value, or become inhibited if the response remains beyond this value. Experimental confirmation for this rule came from many sides, for example Pitcher et al. (2003) reported that peripheral electrical stimulation paired with TMS at frequencies of 3 Hz induced depression while higher frequencies (30 Hz) induced facilitation. These findings give evidence that cortical synapses are indeed bi-directionally modifiable and their plasticity depends on input frequency. However if the observed effects in protocol HAND25 are related to such frequency-dependent plasticity remains an open question.
In this study the effects of protocol HAND25 were described by measures of rMT, MEP amplitude, SICI, ICF and F-waves. The results revealed no post effect on rMT; however, stable MEP amplitude increases were found in most post-stimulation assessments and for both muscles. While rMT reflects neuronal membrane excitability, being not changed by drugs influencing neuronal synaptic transmission (Ziemann et al. 1996), MEP amplitude reflects global excitability of the corticospinal pathways transsynaptically (Devanne et al. 1997). Particularly the recruitment curve assesses neurons that are intrinsically less excitable or more far from the center of activation by TMS (Hallett 1999). Since these parameters depend on the excitability of synaptic relays at cortical and spinal level, additional F-wave assessments have been carried out. However, they revealed unchanged spinal excitability, which proves that the observed excitability changes involve cortical rather than spinal networks.
The results on SICI and ICF revealed that protocol HAND25 had a direct effect on intracortical circuits. The effect of SICI was decreased for a period of 1 h post stimulation. It is known that SICI is determined by the activity of the inhibitory GABAa circuits (Ziemann et al. 1996) which are important for the maintenance and adjustment of motor cortical representations (Jacobs and Donoghue1991) and are found to facilitate short term plasticity (Ziemann et al. 2001). Previous studies, applying muscle vibration at higher frequencies, showed decreased SICI in the vibrated muscle (Rosenkranz and Rothwell 2003) and increased SICI in the non-vibrated muscle (Rosenkranz et al. 2003), suggesting that disinhibition is selectively achieved for the projections to the stimulated site. In our study where both muscles were stimulated, the effect of SICI was decreased for both muscles as expected.
ICF is a glutamate-dependent process (Liepert et al. 1997), the underlying circuit is more complex, and the effect of single muscle vibration on ICF was found less pronounced (Rosenkranz and Rothwell 2003). However in our study an outlasting effect appeared as shown by increased ICF values over the observation time of 2 h. Similar long-lasting effects have been reported in studies employing muscle vibration during contraction (Marconi et al. 2008; Christova et al. 2010) and whole hand electrical stimulation (Golaszewski et al. 2010). Here we propose that this effect comes from the excitation of both cutaneous and proprioceptive structures, such producing massive afferent input to circuits in cortical layers II and III which have excitatory effects on the corticofugal cells in layers V and VI (Porter et al. 1990; Kaneko et al. 1994a, b). To generate outlasting effects, changes in synaptic efficiency as described by long-term potentation (LTP) must come in play. LTP involves a cascade of synaptic processes ranging from minutes to hours (Shaw et al. 1994). From this point of view it is likely that LTP is a main factor in the genesis of the observed outlasting effects.
In addition to the right hand, the left foot sole was stimulated with 25 Hz to study the existence of a general arousal that may lead to increased cortical excitability. However this was not the case, rather a slight decrease (not significant) in MEP amplitude is observed over the hand areal, see RC curves in Fig. 2. A limitation of the present study is that proximal muscles outside of the stimulated areal were not examined. But as sensory and motor representations are regulated by activity-driven competitive processes (Donoghue 1995), the MEP amplitude over non-stimulated proximal muscles is expected rather to decrease. A further limitation is that only constant frequency stimulation was tested. In a recent fMRI study it was shown that the evoked focal activity in S1 degraded with the ongoing constant stimulation, while with random stimulation such effect was not observed (Gallasch et al. 2010). However whether random stimulation is effective to boost excitability changes in M1, needs further investigation.
Conclusion
In summary the study shows that prolonged whole hand mechanical stimulation in the flutter frequency range induces outlasting neuroplastic effects in the contralateral motor cortex. These effects were maintained over a period of 2 h, indicating that LTP-like mechanisms were triggered. The present results have an impact on health applications. Recently vibration exercise has become an important tool for prevention against osteoporosis (Cardinale and Rittweger 2006), and further, vibration training was established in sports to improve muscle function (Rittweger 2010). The present TMS-study shows that in addition to musculoskeletal structures, the central sensomotoric structures become modulated too. In combination with health applications, this could be beneficial for improving movement and coordination. Further, the results could be potentially important for the rehabilitation practice. The sensory-induced neuroplastic effects achieved with mechanical stimulation could be paired with subsequent motor practice which may lead to better rehabilitation outcomes. However, such clinical perspectives need further research, performed on patient groups.
References
Bienenstock EL, Cooper LN, Munro PW (1982) Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 2(1):42–48
Bongiovanni LG, Hagbarth KE (1990) Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J Physiol 423:1–14
Cardinale M, Rittweger J (2006) Vibration exercise makes your muscles and bones stronger: fact or fiction? J Br Menopause Soc 12(1):12–18
Charlton S, Ridding M, Thompson P, Miles T (2003) Prolonged peripheral nerve stimulation induces persistent changes in excitability of human motor cortex. J Neurol Sci 208(1–2):79–85
Christova M, Rafolt D, Mayr W, Wilfling B, Gallasch E (2010) Vibration stimulation during non-fatiguing tonic contraction induces outlasting neuroplastic effects. J Electromyogr Kinesiol 20(4):627–635
Devanne H, Lavoie BA, Capaday C (1997) Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Disbrow E, Roberts T, Krubitzer L (2000) Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol 418(1):1–21
Donoghue LP (1995) Plasticity of adult sensorimotor representations. Curr Opin Neurobiol 5:749–754
Feldman DE (2000) Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells. Neuron 27:45–56
Forner-Cordero A, Steyvers M, Levin O, Alaerts K, Swinnen SP (2008) Changes in corticomotor excitability following prolonged muscle tendon vibration. Behav Brain Res 190:41–49
Fourment A, Chennevelle JM, Belhaj-Saif A, Maton B (1966) Responses of motor cortical cells to short trains of vibration. Exp Brain Res 111(2):208–214
Fröhlich F, McCormick DA (2010) Endogenous electric fields may guide neocortical network activity. Neuron 67(1):129–143
Gallasch E, Moser M, Kozlovskaya IB, Kenner T, Noordergraaf A (1996) Effects of an eight day space flight on microvibration and tremor. Am J Physiol 273:R86–R92
Gallasch E, Fend M, Rafolt D, Nardone R, Kunz A, Kronbichler M, Beisteiner R, Golaszewski S (2010) Cuff-type pneumatic stimulator for studying somatosensory evoked responses with fMRI. Neuroimage 50(3):1067–1073
Golaszewski SM, Siedentopf CM, Koppelstaetter F, Rhomberg P, Guendisch GM, Schlager A, Gallasch E, Eisner W, Felber SR, Mottaghy FM (2004) Modulatory effects on human sensorimotor cortex by whole-hand afferent electrical stimulation. Neurology 12:2262–2269
Golaszewski SM, Siedentopf M, Koppelstaetter F, Fend M, Ischebeck A, Gonzalez-Felipe V, Haala I, Struhal W, Mottaghy FM, Gallasch E, Felber SR, Gerstenbrand F (2006) Human brain structures related to plantar vibrotactile stimulation: a functional magnetic resonance imaging study. NeuroImage 29(3):923–929
Golaszewski SM, Bergmann J, Christova M, Nardone R, Kronbichler M, Rafolt D, Gallasch E, Staffen W, Ladurner G, Beisteiner B (2010) Increased motor cortical excitability after whole-hand electrical stimulation: a TMS study. Clin Neurophys 121:248–254
Goodwin GM, McCloskey DI, Matthews PB (1972) Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science 175:1382–1384
Hallett M (1999) Motor cortex plasticity. Electroencephalogr Clin Neurophysiol 50:85–91
Jacobs KM, Donoghue JP (1991) Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251:944–1947
Johansson RS, Landström U, Lundström R (1982) Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244:17–25
Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG (2002) Modulation of human corticomotor excitability by somatosensory input. J Physiol 540:623–633
Kaneko T, Caria MA, Asanuma H (1994a) Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol 345(2):172–178
Kaneko T, Caria MA, Asanuma H, Asanuma H (1994b) Information processing within the motor cortex. I. Responses of morphologically identified motor cortical cells to stimulation of the somatosensory cortex. J Comp Neurol 345(2):161–171
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Liepert J, Schwenkreis P, Tegenthoff M, Malin JP (1997) The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm 104:1207–1214
Marconi B, Filippi GM, Koch G, Pecchioli C, Salerno S, Don R, Camerota F, Saraceni VM, Caltagirone C (2008) Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci 275:51–59
Matthews PB, Stein RB (1969) The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol 200(3):723–743
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Pitcher JB, Ridding MC, Miles TS (2003) Frequency-dependent, bi-directional plasticity in motor cortex of human adults. Clin Neurophysiol 114(7):1265–1271
Porter LL, Sakamoto T, Asanuma H (1990) Morphological and physiological identification of neurons in the cat motor cortex which receive direct input from the somatic sensory cortex. Exp Brain Res 80(1):209–212
Ridding MC, McKay DR, Thompson PD, Miles TS (2001) Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol 112(8):1461–1469
Rittweger J (2010) Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol 108(5):811–904
Rocco MM, Brumberg JC (2007) The sensorimotor slice. J Neurosci Methods 162(1–2):139–147
Rosenkranz K, Rothwell JC (2003) Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol 551(Pt 2):649–660
Rosenkranz K, Rothwell JC (2006) Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci 23:822–829
Rosenkranz K, Pesenti A, Paulus W, Tergau F (2003) Focal reduction of intracortical inhibition in the motor cortex by selective proprioceptive stimulation. Exp Brain Res 149(1):9–16
Shaw CA, Lanius RA, van den Doel K (1994) The origin of synaptic neuroplasticity: crucial molecules or a dynamical cascade? Brain Res Brain Res Rev 19(3):241–263
Siggelkow S, Kossev K, Schubert M, Kappels H, Wolf W, Dengler R (1999) Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle Nerve 22:1544–1548
Simons SB, Tannan V, Chiu J, Favorov OV, Whitzel BL, Tommerdahl M (2005) Amplitude-dependency of response of SI cortex to flutter stimulation. BMC Neurosci 6:43
Smith L, Brouwer B (2005) Effectiveness of muscle vibration in modulating corticospinal excitability. J Rehab Res Develop 42(6):787–794
Snyder AZ (1992) Steady-state vibration evoked potentials: descriptions of technique and characterization of responses. Electroencephalogr Clin Neurophysiol 84(3):257–268
Steyvers M, Levin O, Van Baelen M, Swinnen SP (2003) Corticospinal excitability changes following prolonged muscle tendon vibration. Neuroreport 14:1901–1905
Struppler A, Binkofski F, Angerer B, Bernhardt M, Spiegel S, Drzezga A, Bartenstein P (2007) A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: a PET-H2O15 study. Neuroimage 36:174–186
Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB (1968) The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31:301–334
Tobimatsu S, Zhang Y, Kato M (1999) Steady-state vibration somatosensory evoked potentials: physiological characteristics and tuning function. Clin Neurophysiol 110:1953–1958
Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W (1996) Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40(3):367–370
Ziemann U, Muellbacher W, Hallett M, Cohen LG (2001) Modulation of practice-dependent plasticity in human motor cortex. Brain 124:1171–1181
Acknowledgments
We thank Dr. med. Jörg Waldert from the Sigmund Freud Clinic in Graz for assistance in the F-wave assessments. This work was supported by the Austrian National Bank, Grant Nr. 13221.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dick F. Stegeman.
Rights and permissions
About this article
Cite this article
Christova, M., Rafolt, D., Golaszewski, S. et al. Outlasting corticomotor excitability changes induced by 25 Hz whole-hand mechanical stimulation. Eur J Appl Physiol 111, 3051–3059 (2011). https://doi.org/10.1007/s00421-011-1933-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1933-0