Abstract
Recent attention given to the mechanical work of the lower extremity joints, the emerging importance of the stance phase of running, and the lack of consensus regarding the biomechanical correlates to economical running were primary justifications for this study. The purpose of this experiment was to identify the correlations between metabolic power and the positive and negative mechanical work at lower extremity joints during stance. Recreational runners (n = 16) ran on a treadmill at 3.35 m s−1 for physiological measures and overground for biomechanical measures. Inverse dynamics were used to calculate net joint moments and powers at the ankle, knee, and hip. Joint powers were then integrated over the stance phase so that positive and negative joint mechanical work were correlated with metabolic power (r = 0.60–0.69). Positive work at the hip and ankle during stance was positively correlated to metabolic power. In addition to these results, more economical runners (lower metabolic power) exhibited greater negative work at the hip, greater positive work at the knee, and less negative work at the ankle. Between the most and least economical runners, different mechanical strategies were present at the hip and knee, whereas the kinetics of the ankle joint differed only in magnitude.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metabolic power for a given submaximal speed of running (i.e., running economy) varies substantially between individuals. Researchers have reported a 20–30% range in metabolic power among age-, gender-, and performance-matched groups of trained distance runners (e.g., Heise et al. 2008; Morgan and Craib 1992; Williams and Cavanagh 1987). Biomechanists have identified several variables describing structural characteristics and running mechanics that are related to metabolic power. However, many of the relationships are weak and inconsistent between one study and the next regardless of the level of complexity used in biomechanical models (see Saunders et al. 2004 for a review). For example, the comprehensive work of Williams and Cavanagh (1987) identified kinematic and kinetic variables that characterized runners with low, medium, and high aerobic demand, but a study by Kyrolainen et al. (2001) showed that similar measures were not good predictors of metabolic power.
The mechanics during the stance phase of running, which is the time a runner is in contact with the ground, are considered important with respect to metabolic power. A classic study by McMahon et al. (1987) showed that when runners exaggerated their knee flexion during ground contact, so-called Groucho running, effective vertical stiffness decreased while metabolic power increased substantially. This inverse relation between metabolic power and effective vertical stiffness was statistically shown in a homogenous group of trained distance runners (Heise and Martin 1998). A broader statement was made by Kram and Taylor (1990), who formulated a hypothesis stating that the metabolic cost of running was proportional to the cost of supporting one’s mass and the time course of generating force during stance. Data from various animal species and speeds of locomotion supported their claim (see Kram 2000 for a review).
Early attempts at identifying the energetic costs of the stance and swing phases during running suggested that the cost of swing was negligible (e.g., Taylor et al. 1980). More recently, Modica and Kram (2005) concluded that “leg swing requires ~20% of the net energy consumed in running” (p. 2131). Modestly higher estimates were reported by Marsh et al. (2004) when examining leg muscle blood flow in guinea fowl (~26%). Even if researchers do not agree on the exact value of the partitioned cost between stance and swing during running, their findings underscore the large proportion of metabolic power devoted to the stance phase of running. In addition, Kram and Taylor’s broad hypothesis concerning the importance of stance phase during running has not been tested in a more narrow sample (e.g., homogeneous group of trained distance runners). To investigate how muscle groups of the lower extremity produce the energy required for stance, recent work has focused on the joint mechanical work and power associated with the lower extremity (DeVita et al. 2007a, b; DeVita et al. 2008; Umberger and Martin 2007).
DeVita et al. (2007b) reported that the total joint mechanical work for walking and running (i.e., sum of positive and negative joint work at the hip, knee, and ankle joints) was positive over the gait cycle. The muscles crossing the hip and ankle generated more energy (positive joint work) over the gait cycle, while the muscles across the knee showed greater energy dissipation (negative joint work). Furthermore, a greater increase in positive work across the lower extremity joints was found during incline running when compared to the increase in negative work during decline running (DeVita et al. 2008). Umberger and Martin (2007) used a similar joint mechanical work analysis of the lower extremity when investigating deviations from the preferred stride rate of walking. They suggested that changes in the mechanical work demands of the lower extremity or changes in mechanical efficiency were responsible for increases in metabolic power that accompanied deviations from a preferred stride rate.
Taken collectively, the aforementioned research points to the importance of the stance phase of locomotion and the mechanical energy generated and dissipated in the lower extremity as potential determinants of metabolic power. The aim of the present study was to examine the relationships between metabolic power and the positive and negative mechanical work at lower extremity joints during stance. Based on the predominance of positive joint mechanical work during running reported by DeVita et al. (2007b) and the fact that increased positive joint mechanical work results in an increased metabolic power, it was hypothesized that positive joint work during stance, especially at the hip and ankle, would be positively correlated to metabolic power. In addition, it was thought that the joint mechanical work summed across all lower extremity joints would be positively correlated with metabolic power.
Methods
Sixteen well-trained men (mean ± SD: age = 27.3 ± 4.8 years; body mass = 75.0 ± 8.3 kg; body height = 178.7 ± 7.4 cm) with recent 10-km run times between 38 and 45 min volunteered to be participants and attended two test sessions. In the first, procedures were explained and written informed consent was obtained from each participant. Running shoes were provided for each participant to control for possible confounds related to footwear design. After a series of treadmill accommodation trials totalling 30 min (at least 20 min at 3.35 m s−1 for all participants) and a brief rest (minimum of 10 min), \( \dot{V}{\text{O}}_{{2{\text{peak}}}}\) was determined using the open circuit method and a continuous running protocol. Treadmill speed initially was set at 2.69 m s−1 for 3 min of warm-up at level grade. Speed was then increased to 3.14 m s−1 and kept constant for all remaining stages. Every 2 min thereafter, treadmill grade was increased 2.5% until the runner signaled that he had reached exhaustion. The highest value attained for the final workload was considered as \( \dot{V}{\text{O}}_{{2{\text{peak}}}} \). This measure was used for sample description purposes only.
Oxygen consumption data were measured with a VISTA online data acquisition system (Vacumed, Ventura, CA, USA). Inspired ventilation was measured using a Ventilation Measurement Module (VMM; Alpha Technologies, Laguna Hills, CA, USA) and converted to a rate function with units of l min−1. The VMM was calibrated before each test using a 3-l calibration syringe (Vacumed, Ventura, CA, USA). Expired gas was sampled continuously from a mixing chamber, passed through a drying tube, and analyzed for carbon dioxide and oxygen concentration using an Applied Electrochemistry analyzer (model CD-3A, Sunnyvale, CA, USA) and a Beckman analyzer (model OM-11, Fullerton, CA, USA) respectively. The gas analyzers were calibrated before and rechecked after each test using certified commercial gas preparations.
During the second session, participants completed a 5-min warm-up run followed by a 5-min rest period and a 6-min run at 3.35 m s−1 on the treadmill at level grade. Of the 16 participants, 14 completed this second session within 2–3 weeks of the first session; the remaining 2 subjects completed their second session within 4 weeks of the initial session. Submaximal oxygen consumption data were measured continuously during the 6-min run using the VISTA system described previously. Mean rates of oxygen consumption (\( \dot{V}{\text{O}}_{2} \)) and carbon dioxide production (\( \dot{V}{\text{CO}}_{ 2} \)) over the last 2 min of running were used to estimate each participant’s average rate of metabolic energy expenditure (Weir 1949):
where \( \dot{E} \) is the rate of energy expenditure (i.e., rate of heat output) in kcal min−1, and \( \dot{V}{\text{O}}_{2} \) and \( \dot{V}{\text{CO}}_{ 2} \) in l min−1. \( \dot{E} \) was converted to units of watts (W) and then normalized to body mass (i.e., final units were W kg−1). For the remainder of this paper, this measure will be referred to as metabolic power.
Each participant then completed overground running trials across an AMTI force platform (Watertown, MA, USA) positioned in the middle of a 15-m runway. Speed was monitored using two photocells spaced approximately 3 m apart, the first of which triggered a timer and force platform data collection immediately before the runner contacted the force platform and the second of which stopped the timer. Force platform sampling was performed at 480 Hz for 0.65 s, ensuring that the entire contact phase was recorded. Acceptable trials were those in which average velocity was within ±3% of the criterion speed (3.35 m s−1), and there was no visible indication of stride modification within the measurement zone. A single video camcorder, with a sampling rate of 60 Hz was used to record a sagittal plane record of five acceptable trials in which each participant contacted the force platform with his right foot. A scaling factor was imaged in the recording plane for the conversion of video position data into object space data.
Two trials for which average speed most closely matched the criterion speed (3.35 m s−1) were selected for analysis from the five acceptable trials. For each trial, coordinate data for six anatomical landmarks (approximate shoulder joint center, greater trochanter, approximate knee joint center, lateral malleolus, heel, head of the fifth metatarsal) were obtained from the sagittal plane video records for the stance phase of running. A four-segment model that consisted of head–arms–trunk (HAT), thigh, shank, and foot was used to calculate net joint moments and joint powers at the ankle, knee, and hip of the stance leg. The inclusion of the HAT segment was necessary for the definition of the hip joint angular velocity. Coordinate data from ten images before and ten images after ground contact were included to avoid endpoint problems commonly associated with data smoothing procedures and finite difference calculations. The coordinate data were filtered using a fourth order, zero-lag, recursive Butterworth digital filter. Cutoff frequencies ranged from 5 Hz (x-coordinate of greater trochanter) to 7 Hz (y-coordinate of the fifth metatarsal head) and were identified using a procedure described by Jackson (1979).
Standard link segment mechanics using an inverse dynamics approach were used to generate net joint moments for the duration of stance (Winter 1983). Segment masses and center of mass locations were estimated using the procedures of Clauser et al. (1969) and Hinrichs (1990). Moments of inertia about transverse axes were calculated using the approach of Whitsett (1963) as adjusted by Dapena (1978). Linear and angular velocities and accelerations were calculated from the filtered coordinate data using finite difference equations. Ground reaction force and center of pressure data needed during the stance phase were quantified from the force platform recordings. Center of pressure and body coordinate data were translated to a common reference frame based at the origin of the force platform.
Instantaneous mechanical power was calculated for each joint as the product of the net joint moment and the joint angular velocity. Then, for the duration of stance, positive and negative mechanical work values at each joint were determined by numerically integrating positive and negative mechanical power, respectively. Results from the two analyzed trials for each participant were averaged to yield a single expression of each joint mechanical work variable for each participant (normalized to body mass). Positive and negative joint work values at the ankle, knee, and hip, as well as the total joint work across the lower extremity, were correlated with metabolic power. Pearson-product moment correlations were used to test for linear relationships and the probability associated with a Type I error was set at 0.05.
Results
The magnitude and range of physiological measures for the sample (Table 1) were typical of those reported previously (Heise et al. 1996, 2008; Williams and Cavanagh 1987). The difference between the least and most economical runners expressed as a percentage of the mean metabolic power, 28%, also agrees with results of previous studies (Heise et al. 1996; Heise et al. 2008; Williams and Cavanagh 1987). There existed no distinct pattern between \( \dot{V}{\text{O}}_{{2{\text{peak}}}} \) and \( \dot{V}{\text{O}}_{{2{\text{submax}}}} \) as was verified by an insignificant relationship between these two variables (r = −0.08, p = 0.77). The runner with the lowest \( \dot{V}{\text{O}}_{{2{\text{submax}}}} \) used only 59% of \( \dot{V}{\text{O}}_{{2{\text{peak}}}} \), whereas the participant with the highest \( \dot{V}{\text{O}}_{{2{\text{submax}}}} \) used 83%. These relative intensity values, the absence of change in those values over the final 2 min, and the fact that the respiratory quotients of all participants were less than 1.0 suggests that runners were in an aerobic, steady-state condition for the 3.35 m s−1 runs.
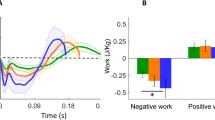
As hypothesized, higher metabolic power was related to greater positive joint work at the hip and ankle (Fig. 1). In addition to these expected results, more economical runners (i.e., those with lower metabolic power) exhibited greater negative joint work at the hip, greater positive joint work at the knee, and lower negative joint work at the ankle. It should be noted that most runners exhibited low negative joint mechanical work at the hip (i.e., less than 0.5 J kg−1) and, therefore, this significant correlation should be interpreted with caution. The statistically significant relationships explain between 36 and 48% of the variability in metabolic power.
Metabolic power also displayed a significant, positive relationship with total joint mechanical work of the lower extremity during stance (Fig. 2), and from that the sample can be considered as two groups of runners based on metabolic power and total joint work. Specifically, all six runners who exhibited a total joint mechanical work less than 0.6 J kg−1 displayed a metabolic power below the mean, whereas eight of the remaining ten runners who produced a total joint work higher than 0.7 J kg−1 displayed a metabolic power above the mean (Fig. 2).
Discussion
During the stance phase of running, the mechanical work of the lower extremity joints, reported in the present study, leads us to suggest that a more economical running profile involves: a greater reliance on the hip for energy dissipation and less reliance for energy generation; greater reliance on the knee for energy generation; and less reliance on the ankle for generation and dissipation (see Fig. 1). It follows from these results that the interindividual variability in lower extremity kinetics during stance is great enough to help explain the variability in metabolic power.
When considering total joint mechanical work, positive work was 65% greater than negative work (Fig. 3). This difference was due to the predominance of positive work done at the hip and ankle, whereas the knee showed similar values of negative work and positive work. This bias toward positive work was much larger than that reported by DeVita et al. (2008), but they calculated lower extremity joint mechanical work of runners over the entire gait cycle, whereas the values in the present study were for stance only. In a similar study that examined the stance phase of walking, DeVita et al. (2007a) showed greater positive work at the hip and ankle and greater negative work at the knee. They also reported 47% more positive total joint mechanical work across the stance phase of walking. DeVita et al. (2007a) hypothesized “that some of the positive work produced by muscle is not used to maintain gait velocity (i.e., kinetic energy) and upright posture (i.e., potential energy) but is wasted through various energy sinks” (p. 3367). They further suggested that compressions and vibrations of various tissue (e.g., adipose tissue, joint cartilage, menisci, bony structures) are possible examples of energy sinks during locomotion. Modestly higher positive work during stance may also be needed during running because of higher air resistance effects experienced during running compared to walking. In the present study, the ratios of positive to negative mechanical work at the hip and ankle were 2.2 and 1.8, respectively, whereas DeVita et al. (2008) reported 3.3 and 0.2 for running. In addition, DeVita et al. (2008) showed negative work to be 2.5 times that of positive work at the knee, whereas we observed nearly identical values (see Fig. 3). When mechanical work was summed across all joints of the lower extremity, it was shown that the total joint mechanical work during stance was positively correlated to metabolic power, which was consistent with our secondary hypothesis and explained 38% of the interindividual variability (see Fig. 2).
Although previous researchers have described mean joint moment and power patterns for running (Novacheck 1998; Winter 1983), it is clear from our data that differences between individuals exist. The lower extremity positive and negative joint mechanical work values presented in Fig. 1 provide summative values for the stance phase of running, but to see where and when the energy is generated or dissipated, it is necessary to examine instantaneous mechanical power.
To help interpret the correlations between metabolic power and joint mechanical work during stance, the joint mechanical power, net joint moment, and angular velocity curves were examined for runners with low and high metabolic power. This approach is similar to that of Williams and Cavanagh (1987). They focused on three groups of runners (low, medium, and high metabolic power) to biomechanically explain the interindividual variability in economy. With regard to mechanical power, they identified a trend that runners with low metabolic power produced lower whole-body net positive power and lower total mechanical power for the entire running cycle. The present study focused on the lower extremity during stance and used a kinetic-based computational model for mechanical work and power, whereas Williams and Cavanagh (1987) reported work and power from a kinematic-based model (Williams and Cavanagh 1983; Winter 1979).
In the present investigation, runners with the lowest and highest metabolic power exhibited a biphasic pattern of mechanical power at the ankle, indicating energy dissipation during the first half of stance followed by energy generation during the second half of stance (see Fig. 4). Data for the two subsets of runners shown in Fig. 4 represent runners at the low and high ends of the metabolic power range. The net joint moment for both subsets was plantar flexor throughout stance, which is consistent with previous reports (e.g., Novacheck 1998; Winter 1983). The magnitudes of the net joint ankle moments throughout stance differ greatly between runners with low and high metabolic power and, given the similar ankle angular velocities, explain the significant correlations between metabolic power and positive and negative joint mechanical work at the ankle joint (see Fig. 1).
Hip (H), knee (K), and ankle (A) instantaneous joint mechanical power curves (top), net joint moments (middle), and joint angular velocities (bottom) during stance for runners with low metabolic power (left panels) and with high metabolic power (right panels). Each curve represents the mean of three trials from three different runners. Percent of stance time is expressed on the horizontal axis and error bars are ±1 SD
The differences in knee and hip mechanical power between runners with low and high metabolic power is more complex and differs from previously reported, stereotypical joint power patterns (Novacheck 1998; Winter 1983). At the knee, runners with low metabolic power exhibit a biphasic pattern similar to ankle joint power; a period of energy dissipation is followed by a period of energy generation (upper left panel of Fig. 4). This biphasic pattern is also consistent with patterns reported by Novacheck (1998) and Winter (1983) and is the result of an extensor moment throughout stance combined with knee flexion during early stance followed by knee extension (see net joint moment and angular velocity profiles of Fig. 4). On the other hand, runners exhibiting high metabolic power showed very low values of joint mechanical power at the knee. In the example presented in the upper right panel of Fig. 4, a short period of energy generation precedes a longer duration of dissipation, contrary to what is displayed by the low metabolic power runners. Unlike the ankle joint where magnitude differences in the joint moment explained joint power differences, the joint moment profiles at the knee show magnitude and pattern differences. In runners with high metabolic power, the knee joint moments are low and become flexor during the second half of stance, which is quite different from the consistent, relatively high extensor moment displayed by runners with low metabolic power. Knee joint angular velocity profiles are similar between the two subsets. The greater energy generation produced by more economical runners explains the significant negative correlation between metabolic power and positive joint work at the knee (see Fig. 1), whereas no relation was found between metabolic power and negative joint work.
Energy generation at the hip is virtually nonexistent in runners with low metabolic power, whereas a period of moderate-level positive joint mechanical power spans the middle of the stance phase in runners with high metabolic power (upper panels of Fig. 4). This difference is explained by the greater magnitude and longer duration of the hip extensor moment and the larger magnitude of hip extensor angular velocity (see right panel of Fig. 4), which helps explain the significant, positive relation between metabolic power and positive joint mechanical work at the hip (Fig. 1). Most researchers have reported energy dissipation at the hip during stance, but in one of the first kinetic analyses of running, Winter (1983) stated that “the hip had relatively low power levels, and when different trials are compared no consistent patterns are seen” (p. 96). It is clear that more economical runners rely on lower energy generation and dissipation at the ankle, higher energy generation at the knee, lower energy generation at the hip, and higher energy dissipation at the hip. With regard to net joint moments, runners with low metabolic power exhibit a lower plantar flexor moment throughout stance when compared to runners with high metabolic power, but the differences at the knee and hip are more complex and suggest different muscle activation strategies.
One such strategy focuses on the duration of rectus femoris–gastrocnemius coactivation during the stance phase of running (Heise et al. 1996, 2008). In these two studies, participants who displayed greater muscle coactivation exhibited lower metabolic power. Consistent with the suggestions of others (Bourdin et al. 1995; Kyrolainen et al. 2001), muscle coactivation increases joint stiffness, and greater joint stiffness allows for efficient use of stored elastic energy (Latash 1993) and the possible redistribution of mechanical power between adjacent joints (Bobbert and van Ingen Schenau 1988). In the present study, all runners generated energy at the ankle during stance, but low energy generation at the ankle and high energy generation at the knee were characteristics of runners exhibiting low metabolic power (see Fig. 1 and upper left panel of Fig. 4). This may represent an energy transfer associated with the action of biarticular muscles gastrocnemius, an ankle plantar flexor, and rectus femoris, a knee extensor. In other words, more coactivation between these muscles during stance may be connected to less positive joint work done at the ankle and more positive joint work done at the knee. Runners exhibiting high metabolic power showed no such connection between the ankle and the knee (see upper right panel of Fig. 4).
The lack of a significant correlation between metabolic power and negative joint work at the knee is inconsistent with the findings of researchers who suggest that eccentric activity in knee extensors during stance explains interindividual variability in metabolic power (Abe et al. 2007; Bourdin et al. 1995). Bourdin et al. tested a group of ten trained distance runners and found that the ratio of eccentric-to-concentric vastus lateralis EMG activity during stance was inversely related to metabolic power; a higher EMG ratio was related to lower metabolic power. They suggested that greater eccentric muscle activity in the knee extensors resulted in a relatively lower cost concentric action or, in the context of the present study, more economical energy generation across the knee joint. The results of the present investigation support the notion that greater energy generation at the knee is associated with lower metabolic power, but no relation was found between energy dissipation at the knee, the mechanical indicator of eccentric muscle action, and metabolic power.
Conjectures about muscle activity and proposed, low-cost strategies for joint power production must be balanced with the limitations inherent in the inverse dynamics analysis used in the present study. Although this analysis is well accepted among biomechanists, net joint moments provide no information about contributions from individual muscles and coactivation of antagonistic muscles. Another limitation is the inability of an inverse dynamics model and subsequent joint mechanical work analysis to account for isometric muscle actions, especially those of biarticular muscles, which may play a prominent role in the stance phase of running (Roberts et al. 1997). Mechanical energy transfers are not accounted for in the traditional joint power analysis used in the present study, and the independence of mechanical power values at the lower extremity joints was also not examined.
The variability in metabolic power, which was not explained in the present investigation, may be due to factors not considered in our research design. One source would be mechanical contributions from outside the sagittal plane. Novacheck (1998), however, pointed out that although joint moments are high in the frontal plane, contributions to joint power are low because of minimal motion. The joint mechanical power that was not considered in the present investigation, namely that produced during swing, may also help explain additional variability in metabolic power. As stated in the introduction, the swing phase of running has a metabolical cost, although the magnitude of that contribution is not well established. Because the stance phase of running includes the metabolic cost of support, we used a gross measure of metabolic power rather than a net measure. This fact may have impacted the relationships. Another methodological limitation was the low number of trials analyzed per subject. Although Bates et al. (1992) concluded that fewer trials can be adequate when using larger subject sample sizes, they suggested a minimum of three trials per subject for studies such as the present one. On the other hand, our use of two trials that most closely matched the criterion speed may have helped control variation in metabolic power associated with variation in running speed. Finally, the data presented and interpreted here are limited to those runners that fit our definition of a well-trained runner.
Conclusion
In the present study, correlations between metabolic power and the positive and negative mechanical work at lower extremity joints during stance were examined. As hypothesized, positive joint work at the hip and ankle during stance was positively correlated to metabolic power. In addition to these results, more economical runners (lower metabolic power) exhibited greater negative work at the hip, greater positive work at the knee, and less negative work at the ankle. Runners with low metabolic power exhibited a lower plantar flexor joint moment throughout stance when compared with runners with high metabolic power, but the differences at the knee and hip were more complex. At the knee, more economical runners relied on greater extensor moments during stance, and less economical runners relied on greater hip flexor moments. These differences suggest altered muscle activation strategies.
References
Abe D, Muraki S, Yanagawa K, Fukuoka Y, Niihata S (2007) Changes in EMG characteristics and metabolic energy cost during 90-min prolonged running. Gait Posture 26:607–610
Bates B, Dufek J, Davis H (1992) The effect of trial size on statistical power. Med Sci Sports Exerc 24:1059–1068
Bobbert M, van Ingen Schenau GJ (1988) Coordination of vertical jumping. J Biomech 21:241–262
Bourdin M, Belli A, Arsac L, Bosco C, Lacour J (1995) Effect of vertical loading on energy cost and kinematics of running in trained male subjects. J Appl Physiol 79:2078–2085
Clauser C, McConville J, Young J (1969) Weight, volume, and center of mass of segments of the human body (AMRL-TR-69–70). Wright-Patterson Air Force Base, Ohio
Dapena J (1978) A method to determine the angular momentum of a human body about three orthogonal axes passing through its center of gravity. J Biomech 11:251–256
DeVita P, Helseth J, Hortobogyi T (2007a) Muscles do more positive than negative work in human locomotion. J Exp Biol 210:3361–3373
DeVita P, Janshen L, Gruber A, et al. (2007b). Muscle function is biased towards positive over negative work in level human gait. In: proceedings of the American Society of Biomechanics Annual Meeting, Palo Alto, CA, USA
DeVita P, Janshen L, Rider P, Solnik S, Hortobagyi T (2008) Mechanical work is biased toward energy generation over dissipation in non-level running. J Biomech 41:3354–3359
Heise G, Martin P (1998) “Leg spring” characteristics and the aerobic demand of running. Med Sci Sports Exerc 30:750–754
Heise G, Morgan D, Hough H, Craib M (1996) Relationships between running economy and the EMG characteristics of bi-articular leg muscles. Int J Sports Med 17:128–133
Heise G, Shinohara M, Binks L (2008) Biarticular leg muscles and links to running economy. Int J Sports Med 29:688–691
Hinrichs R (1990) Regression equations to predict segmental moments of inertia from anthropometric measurements: an extension of the data of Chandler 1975. J Biomech 18:621–624
Jackson K (1979) Fitting of mathematical functions to biomechanical data. IEEE Trans Bio-Med Eng 26:122–124
Kram R (2000) Muscular force or work: what determines the metabolic energy cost of running? Exerc Sports Sci Rev 28:138–143
Kram R, Taylor C (1990) Energetics of running: a new perspective. Nature 346:265–267
Kyrolainen H, Belli A, Komi PV (2001) Biomechanical factors affecting running economy. Med Sci Sports Exerc 33:1330–1337
Latash M (1993) Control of human movement. Human Kinetics, Champaign
Marsh RL, Ellerby DJ, Carr JA, Henry HT, Buchanan CI (2004) Partitioning the energetics of walking and running: swinging the limbs is expensive. Science 303:80–83
McMahon T, Valiant G, Frederick E (1987) Groucho running. J Appl Physiol 62:2326–2337
Modica J, Kram R (2005) Metabolic energy and muscular activity required for leg swing in running. J Appl Physiol 98:2126–2131
Morgan D, Craib M (1992) Physiological aspects of running economy. Med Sci Sports Exerc 24:456–461
Novacheck T (1998) The biomechanics of running. Gait Posture 7:77–95
Roberts T, Marsh R, Weyand P, Taylor C (1997) Muscular force in running turkeys: the economy of minimizing work. Science 275:1113–1115
Saunders P, Pyne D, Telford R, Hawley J (2004) Factors affecting running economy in trained distance runners. Sports Med 34:465–485
Taylor C, Heglund N, McMahon T, Looney T (1980) Energetic cost of generating muscular force during running: a comparison of small and large animals. J Exp Biol 86:9–18
Umberger B, Martin P (2007) Mechanical power and efficiency of level walking with different stride rates. J Exp Biol 210:3255–3265
Weir J (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9
Whitsett C (1963) Some dynamic response characteristics of weightless man (AMRL-TR-63–18). Wright-Patterson Air Force Base, Ohio
Williams K, Cavanagh P (1983) A model for the calculation of mechanical power during distance running. J Biomech 16:115–128
Williams K, Cavanagh P (1987) Relationship between distance running mechanics, running economy, and performance. J Appl Physiol 63:1236–1245
Winter D (1979) A new definition of mechanical work done in human movement. J Appl Physiol 46:79–83
Winter D (1983) Moments of force and mechanical power in jogging. J Biomech 16:91–97
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jean-René Lacour.
Rights and permissions
About this article
Cite this article
Heise, G.D., Smith, J.D. & Martin, P.E. Lower extremity mechanical work during stance phase of running partially explains interindividual variability of metabolic power. Eur J Appl Physiol 111, 1777–1785 (2011). https://doi.org/10.1007/s00421-010-1793-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1793-z