Abstract
We evaluated the effects of an 8-week exercise training program in previously sedentary individuals on whole-body heat balance during exercise at a constant rate of metabolic heat production. Prior to and after 8 weeks of training, ten participants performed 60-min of cycling exercise at a constant rate of heat production (~450 W) followed by 60-min of recovery, at 30°C and 15% relative humidity. Rate of total heat loss was measured directly by whole-body calorimetry, while rate of metabolic heat production was measured simultaneously by indirect calorimetry. Esophageal (T es), skin blood flow (SkBF) and local sweat rate (LSR) were also measured continuously. The 8-week exercise training program elicited a 10% increase in maximal aerobic capacity (P < 0.001). Furthermore, exercise training reduced (P ≤ 0.05) baseline (37.10 ± 0.28 vs. 36.95 ± 0.24°C) and end-exercise (37.85 ± 0.30 vs. 37.55 ± 0.20°C) values for T es as well as onset thresholds for LSR (37.23 ± 0.26 vs. 36.96 ± 0.22°C, P < 0.001) and SkBF (37.16 ± 0.38 vs. 36.83 ± 0.26°C, P < 0.001). However, these improvements in thermoregulatory function did not translate into a greater rate of total heat loss between the pre- and post-training exercise trials (P = 0.762). Furthermore, there were no differences in SkBF (P = 0.546) and LSR (P = 0.475) from pre- to post-training. Although physical training resulted in significant improvements of cardiorespiratory and thermoregulatory functions, these adaptations did not improve whole-body and local heat loss responses during exercise performed at a given rate of metabolic heat production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that exercise training improves cardiorespiratory fitness, body composition, as well as neuromuscular, metabolic, and endocrine functions (Jones and Carter 2000). While long-term exercise training has consistently been related to an improved thermoregulatory function (Baum et al. 1976; Buono and Sjoholm 1988; Fritzsche and Coyle 2000; Gisolfi and Robinson 1969; Piwonka et al. 1965; Selkirk and McLellan 2001), short-tem exercise training has also been linked to improvements in the capacity to dissipate heat. Increases in maximal aerobic capacity \( \left( {\dot{V}{\text{O}}_{2\,\max } } \right) \) of 10–15% associated with short-term exercise training have consistently been shown to enhance thermoregulatory function as evidenced by reductions in the core temperature at which the onset of local sweating and skin vasodilation occurs during exercise (Henane et al. 1977; Ichinose et al. 2009; Johnson 1998; Okazaki et al. 2002; Roberts et al. 1977). While some studies report that these adaptations lead to increases in sweating and skin blood flow (SkBF) during exercise and/or heat exposure (Gisolfi and Robinson 1969; Gisolfi 1973; Henane et al. 1977; Ichinose et al. 2009; Nadel et al. 1974; Okazaki et al. 2002; Shvartz et al. 1974; Strydom et al. 1966), these improvements in thermoregulatory function have not always resulted in greater local heat loss responses after exercise training (Gisolfi 1973; Nielsen et al. 1993; Shields et al. 2004; Shvartz et al. 1973).
The first possibility for this discrepancy may be related to differences in experimental design between studies. Some studies employed a parallel group design, comparing untrained and trained individuals whereas others used a pre- and post-treatment study design (i.e., pre-treatment condition served as the study control). In both cases, erroneous conclusions could result if differences in \({\dot{V}{\text{O}}_{2\,\max } }\) between groups were not considered when determining the exercise work rate. For example, when a pre- and post-treatment design is employed, both experimental sessions are performed at the same percentage of \({\dot{V}{\text{O}}_{2\,\max}}.\) However, the exercise training program performed between both experimental sessions (i.e., pre- and post-training) induces an increase in absolute \({\dot{V}{\text{O}}_{2\,\max } }.\) Therefore, the individual performs the post-training exercise session at a greater rate of absolute oxygen consumption, and therefore a greater rate of metabolic heat production, despite being performed at the same percentage of \({\dot{V}{\text{O}}_{2\,\max } }.\) From a heat balance perspective, a greater rate of metabolic heat production during the post-treatment session necessitates a greater rate of total heat loss to achieve heat balance under compensable heat stress conditions (Jay and Kenny 2007). As such, a greater rate of metabolic heat production will elicit a greater rate of total heat loss and consequently elevated sweating and SkBF responses. Hence, it is unclear if the previously reported improvements in local sweat rate (LSR) and SkBF were due to exercise training per se or a consequence of the greater rates of metabolic heat production during the post-training exercise trials.
The second possibility may be related to the fact that previous studies have only reported changes in core temperature and/or the temporal changes in local heat loss responses at relatively few sites (Baum et al. 1976; Buono and Sjoholm 1988; Fritzsche and Coyle 2000; Piwonka et al. 1965; Selkirk and McLellan 2001). Examining the effects of exercise training on heat loss responses at only one or two sites may be problematic given the growing evidence demonstrating regional variations in local SkBF and sweating response during heat stress (Kondo et al. 1998; Machado-Moreira et al. 2008a, b; Peters et al. 2000). Therefore, if no improvements were observed at one site, it does not exclude the possibility that improvements may have been evidenced elsewhere. Since local heat loss responses do not illustrate how much whole-body heat transfer is altered, and core temperature measurements do not accurately represent the magnitude of residual body heat storage (Jay et al. 2007), we evaluated both local and whole-body heat loss responses during 60 min of exercise performed at a constant rate of metabolic heat production prior to, and following an 8-week exercise training program. It was hypothesized that an 8-week exercise training program would elicit lower absolute resting and exercise core temperatures as well as onset thresholds for sweating and skin vasodilation. However, we hypothesized that rate of whole-body heat loss would not improve due to the constant rate of metabolic heat production employed pre- and post-training.
Methods
Participants
Following approval of the experimental protocol from the University of Ottawa Research Ethics Committee and obtaining written informed consent, 10 healthy, non-smoking normotensive participants (7 males, 3 females) volunteered to participate for the study. All participants were assumed to be non-heat acclimatized as the study took place in the winter months from late October to March. None of the participants were engaged in any sort of training program for more than 30 min of exercise, 2 days per week. To control for hormonal effects, questionnaires were given to each female participant regarding the regularity of their menstrual cycle. Female participants were tested during the early follicular phase (3–5 days after the start of menstruation) of their menstrual cycle (Kenny et al. 2008). Mean (±SD) characteristics of the participants were: age, 20 ± 4 years; body mass, 72.61 ± 15.34 kg; body fat, 24.04 ± 6.43%; body surface area, 1.84 ± 0.22 m2; and \({\dot{V}{\text{O}}_{2\,\max } }\) 47.73 ± 4.74 ml kg−1 min−1.
Experimental design
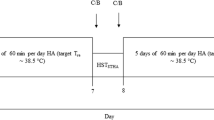
All participants visited the laboratory on four separate occasions, including two screening visits and two experimental sessions. The first screening visit was performed 48–72 h prior to the first experimental session. During this visit, \({\dot{V}{\text{O}}_{2\,\max } }\) was assessed during a progressive treadmill running protocol. The protocol consisted of a 2-min warm-up period during which the appropriate running speed was selected (~6 mph for females and ~7 mph for males). Subsequently, running speed remained constant and the inclination of the treadmill was increased by 2% every 2 min until volitional fatigue (Canadian Society for Exercise Physiology 1986). Body density was also measured using the hydrostatic weighing technique and body fat percentage was then calculated using the Siri equation (Siri 1956). Following this first screening visit, the participants performed a baseline calorimetry experimental exercise test prior to the start of training (pre-training). Subsequently, a second screening visit was undertaken at the end of the 8 weeks of exercise training to determine training-induced changes in \({\dot{V}{\text{O}}_{2\,\max } }\) and body fat percentage. Finally, a second calorimetry exercise test was performed 48–72 h following the second screening visit (post-training).
Experimental protocol
All calorimetry exercise trials were performed at the same time of day. For both sessions, participants were asked to arrive at the laboratory after eating a small breakfast (i.e., dry toast and juice), but not consuming any tea or coffee that morning. Participants were also asked to not drink alcohol or exercise for 24 h prior to the experimentation. Clothing was standardized at ~0.2 Clo (sandals, running shorts and sports bra for females).
Following instrumentation, the participant entered the calorimeter regulated at an ambient air temperature of 30°C and a relative humidity of 15%. The participants were seated in a semi-recumbent position and rested for a 60-min habituation period. Subsequently, participants began cycling on a semi-recumbent constant-load cycle ergometer at a constant rate of metabolic heat production of 450 ± 30 W for 60-min. Following exercise, the participants were required to remain seated for a 60-min post-exercise recovery period. The rate of metabolic heat production was the same for each participant for the first and second calorimetry exercise trials.
Training program
All participants were provided with a gym membership to the University of Ottawa Fitness Centre. The membership fee was provided by the study funding to remove economic barriers to participation. All sessions were supervised by a qualified personal trainer. During the 8-week training program, participants were required to attend 4–5 supervised exercise training sessions per week. The University of Ottawa Fitness Centre was maintained at a temperature between 22–24°C and 40% RH. The training sessions consisted of both aerobic and resistance training. Aerobic training included treadmill, stationary bike or elliptical machine exercise. The duration of the aerobic session increased progressively from 30 min in week 1 to a maximum of 90 min at week 8. The program was designed in a periodized manner to allow for adequate rest in combination with an appropriate stimulus. Each week followed the same design including one long aerobic session per week (60–90 min), one interval workout, one circuit workout, and 1–2 short (30–45 min) aerobic workouts separated by a maximum of 2 days of rest between workouts. Aerobic exercise consisted of both continuous and interval training performed at a moderate and high intensity (i.e., 55–75 and 75–90% of the participant’s pre-determined heart rate reserve respectively). The resistance training was performed in a circuit manner using stationary and free weight equipment progressing from one set of each exercise in week 1 to three sets in week 8 at a weight that participants could lift comfortably 12–15 times. With the exception of heart rate monitoring, no physiological measurements were performed during the 8-week training period.
Instrumentation
The individual components of the heat balance equation were measured by combined direct and indirect calorimetry (Gagge and Gonzales 1996):
where S is the rate of body heat storage, M is the rate of metabolic energy expenditure, W is the rate of external work, R is the rate of radiant heat exchange, C is the rate of convective heat exchange and, E is the rate of evaporative heat exchange.
The modified Snellen direct air calorimeter was employed for the purpose of measuring rate of evaporative (E) and dry (R + C) heat loss, yielding an accuracy of ±2.3 W for the measurement of rate of total heat loss. A full peer-reviewed technical description of the fundamental principles and performance characteristics of the Snellen whole-body calorimeter is available (Reardon et al. 2006). In brief, the calorimeter incorporates a semi-recumbent constant-load eddy current cycle ergometer regulating rate of external work (W) at a pre-determined level. While the pedals of the cycle ergometer are located within the calorimeter, the mechanical unit is linked by chains and located outside of the calorimeter such that the heat generated from the unit does not enter the calorimeter. Differential air temperature and humidity are measured over the calorimeter by sampling the influent and effluent air. The water content is measured using precision dew point thermometry (RH Systems model 373H, Albuquerque, NM, USA), while the air temperature is measured using RTD high precision thermistors (±0.002°C, Black Stack model 1560, Hart Electronics, UT, USA). Air mass flow through the calorimeter is estimated by differential thermometry over a known heat source (2 × 750 W heating elements) placed in the effluent air stream using a third aforementioned high precision thermistor placed down-stream from the heater. Air mass flow rate [(kg air) min−1] is continuously measured during each trial. Data from the calorimeter are collected continuously at 8 s intervals throughout the trials. The real time data were displayed and recorded on a personal computer with LabVIEW software (Version 7.0, National Instruments, TX, USA).
Rate of evaporative heat loss (E) was calculated from the calorimetry data every minute using the following equation:
where mass flow is the rate of flow of air mass [(kg air) s−1]; (Humidityout − Humidityin) is the calorimeter inflow–outflow difference in absolute humidity [(g water) (kg air)−1]; and 2,426 is the latent heat of vaporization of sweat [J (g sweat)−1] at 30°C (Wenger 1972).
Rate of dry heat loss (R + C) from radiation (R), and conduction (C) was calculated from the calorimetry data every minute using the following equation:
where mass flow is the rate of flow of air mass [(kg air) s−1], (Temperatureout − Temperaturein) is the calorimeter inflow–outflow difference in air temperature (°C), and 1,005 is the specific heat of air [J (kg air)−1°C].
A 6 l fluted mixing box housed within the calorimeter was utilized for the concurrent measurement of rate of metabolic energy expenditure (M). Expired gas was analyzed for oxygen (error of ±0.01%) and carbon dioxide (error of ±0.02%) concentrations using electrochemical gas analyzers (AMETEK model S-3A/1 and CD 3A, Applied Electrochemistry, Pittsburgh, PA, USA). Expired air was recycled back into the calorimeter chamber in order to account for respiratory dry and evaporative heat loss. Prior to each session gas mixtures of 4% carbon dioxide, 17% oxygen, balance nitrogen were used to calibrate the gas analyzers and a 3 l syringe was used to calibrate the turbine ventilometer (error ±3%, typically <1%). Rate of metabolic energy expenditure was calculated from minute-average values for oxygen consumption \( \left( {\dot{V}{\text{O}}_{2\,\max } } \right) \) and the respiratory exchange ratio (RER) using the following equation (Nishi 1981):
where e c is the caloric equivalent per liter of oxygen for the oxidation of carbohydrates (21.13 kJ), and e f is the caloric equivalent per liter of oxygen for the oxidation of fat (19.62 kJ).
The calorimeter data was then used to calculate rate of body heat storage (S) and change in body heat content (∆H b) using the following equations:
Esophageal temperature was measured by placing a pediatric thermocouple probe of approximately 2 mm in diameter (Mon-a-therm Nasopharyngeal Temperature Probe, Mallinckrodt Medical, St-Louis, MO, USA) through the participant’s nostril while they were asked to sip water through a straw. The location of the probe tip in the esophagus was estimated to be at the level of the eighth and ninth thoracic vertebrae (Mekjavic and Rempel 1990). Rectal temperature was measured using a pediatric thermocouple probe (Mon-a-therm General Purpose Temperature Probe, Mallinckrodt Medical, St-Louis, MO, USA) inserted to a minimum of 12 cm past the anal sphincter. Aural canal temperature was measured with a tympanic thermocouple. The tip of the probe was inserted into the aural canal until it reached the tympanic membrane and then withdrawn slightly. It was held in this position with cotton, and the ear was covered with tape to minimize any temperature imbalance from the outside environment. Mean skin temperature was calculated using four skin temperatures weighted to the regional proportions as determined by Ramanathan (1964): upper trapezius 20%, chest 30%, quadriceps 30%, and back calf 20%. All temperature data were collected using a HP Agilent data acquisition module (model 3497A) at a sampling rate of 15 s and simultaneously displayed and recorded in spreadsheet format on a personal computer with LabVIEW software (Version 7.0, National Instruments, TX, USA).
LSR was measured using a 5.0 cm2 ventilated capsule placed over the medial inferior aspect of the trapezius muscle. Anhydrous compressed air was passed at a flow rate of 0.7 l min−1 through the capsule and over the skin surface (Brooks 5850, mass flow controller, Emerson electric, Hetfield, PA). The vapor density of the effluent air was calculated from the relative humidity and temperature measured using the Omega HX93 humidity and temperature sensor (Omega Engineering, Stanford, CT). Sweat rate was defined as the product of the difference in water content between effluent and influent air and the flow rate adjusted for the skin surface area under the capsule in and expressed in milligrams per minute per square centimeter.
SkBF was estimated using laser-Doppler velocimetry (PeriFlux System 5000, main control unit; PF5010 LDPM, function unit; Perimed, Stockholm, Sweden) at the right midanterior forearm. The laser-Doppler flow probe (PR 401 angled probe, Perimed) was taped to cleaned skin, in an area that did not appear by visual inspection to be overly vascular and from which consistent readings were noted (Mack 1998). At the end of the experiment, a heating element (PF 5020 temperature unit, Perimed) which houses the laster-Doppler flow probe, was activated to elevate local skin temperature to 44°C until maximum SkBF was measured (~30 min). Maximum SkBF was determined as a sustained elevated plateau in local SkBF and the data are presented as a percentage of maximum.
Heart rate was monitored using a Polar coded transmitter, recorded continuously and stored with a Polar Advantage interface and Polar Precision Performance by (Polar Electro Oy, Finland). Systolic and diastolic blood pressures were determined using an ambulatory blood pressure monitor (ABPM 6100, Welch and Allyn, Skaneateles Falls, NY) and used to calculate mean arterial pressure (diastolic pressure + 1/3 of pulse pressure).
Statistical analysis
Post-training differences (relative to pre-training) in physical fitness and body composition; baseline, end-exercise and end-recovery core temperatures, changes in body heat content, heart rate and mean arterial pressure; as well as onset thresholds for cutaneous vasodilation and sweating were compared using paired sample t tests. The onset threshold for cutaneous vasodilation was determined as the esophageal temperature at which there was an increase in SkBF observed in three consecutive measurements. The onset threshold for sweating was determined as the esophageal temperature at which sweat rate exceeded 0.06 mg min−1 cm−2. Differences in local (SkBF and sweating) and whole-body (calorimetry) heat loss responses were analyzed combined for the exercise and recovery periods using a two-way repeated analysis of variance using the factors of training status (i.e., pre- and post-training) and time (2, 5, 8, 12, 15, 30, 45, 60-min). Paired sample t tests were used to perform pair-wise post hoc comparisons. The level of significance was set at 0.05 and alpha level was adjusted during multiple comparisons so as to maintain the rate of Type I error at 5% during the Holm-Bonferroni post hoc analysis. All analyses were performed using the statistical software package SPSS 17.0 for Windows (SPSS Inc. Chicago, IL, USA).
Results
Physical fitness and body composition
All ten participants completed the full 8 weeks of the training program, which elicited a 10% increase in \({\dot{V}{\text{O}}_{2\,\max } }\) (i.e., 49.08 ± 4.74 pre-training versus 53.75 ± 4.09 ml kg−1 min−1 post-training, P < 0.001). However, there were no differences in body mass (72.6 ± 15.3 pre-training vs. 71.9 ± 14.2 kg post-training, P = 0.149), or percentage of body fat (i.e., 24.0 ± 6.4 pre-training vs. 22.5 ± 7.3% post-training, P = 0.138) following the exercise training program.
Calorimetry data
Due to the experimental protocol employed, there were no differences in the rate of metabolic heat production during exercise or recovery (P = 0.751) (Fig. 1). This resulted in a corresponding reduction of 6% (P < 0.001) in the relative work rate for the post-training exercise test compared to the pre-training level [i.e., 46–40% of \({\dot{V}{\text{O}}_{2\,\max } }\)]. There were no differences from pre- to post-training for rate of total heat loss during exercise and recovery (P = 0.762) (Fig. 1). As such, there were also no differences in the rates of whole-body evaporative (P = 0.838) and dry (P = 0.215) heat loss. Furthermore, there were no differences in the change in body heat content during exercise between pre- (372 ± 71 kJ) and post- (359 ± 85 kJ) training (P = 0.715) or recovery between pre- (−89 ± 85 kJ) and post- (−94 ± 76 kJ) training (P = 0.810). The overall change in body heat content (from baseline rest to end recovery) was similar between exercise sessions (282 ± 81 vs. 339 ± 136 kJ for pre- and post-training, respectively, P = 0.244).
Core and skin temperatures
Esophageal temperature was not significantly lower at baseline rest post-training compared to pre-training (P = 0.140). On the other hand, baseline rectal (P = 0.045) and aural canal (P = 0.051) temperatures were lower post-training relative to pre-training. In addition, there were no differences in resting mean skin temperature (P = 0.512).
At the end of 60-min exercise, the absolute values for esophageal (P = 0.024), rectal (P = 0.002) and aural canal (P = 0.024) temperatures were significantly lower post-training compared to pre-training (Fig. 2). The change from baseline rest in esophageal temperature was significantly lower (P = 0.023) post- (0.56 ± 0.16°C) compared to pre-training (0.68 ± 0.18°C). However, there were no differences between pre- and post-training in the change from baseline rest in rectal (P = 0.408) and aural canal (P = 0.169) temperatures. There were also no differences in absolute (P = 0.452) or relative change (P = 0.757) in mean skin temperature.
Mean (±SE) esophageal (Tes), rectal (Tre), aural canal (Tac) and mean skin (Tsk) temperatures during 60-min of cycling exercise and a subsequent 60-min recovery period performed prior to (Pre), and following (Post) an 8-week exercise training program. A significant difference (P ≤ 0.05) between conditions is denoted by an asterisk (*)
At the end of the 60-min recovery period, absolute values for esophageal (P = 0.335) and aural canal (P = 0.200) temperatures were not significantly different post-training relative to pre-training (Fig. 2). On the other hand, absolute values for rectal (P = 0.006) temperature were significantly lower post-training at the end of the recovery period. However, there were no differences between pre- and post-training in the change from baseline rest in esophageal (P = 0.921), rectal (P = 0.555) and aural canal (P = 0.339) temperatures. There was no difference in absolute (P = 0.410) or relative (P = 0.682) mean skin temperature at the end of the 60-min recovery period. At the end of 60-min recovery, pre- and post-training esophageal, rectal and aural canal temperatures were similarly elevated relative to baseline rest by ~0.13, ~0.34 and ~0.19°C, respectively (Fig. 2).
Local sweat rate
There were no significant differences in LSR either during exercise or recovery (P = 0.475) as a function of the 8-week training program (Fig. 3). However, there was a significant reduction in the esophageal temperature at which onset of sweating occurred during the post-training exercise test relative to pre-training (i.e., 37.23 ± 0.26 vs. 36.96 ± 0.22°C, P < 0.001). When adjusted for the change in esophageal temperature from baseline rest, the onset temperature threshold for sweating was still significantly lower post-training (i.e., 0.34 ± 0.09 vs. 0.20 ± 0.04°C, P = 0.037).
Local skin blood flow
There were no differences between pre- and post-training sessions in the percentage of maximum local SkBF during exercise or recovery (P = 0.546) (Fig. 3). The esophageal temperature onset threshold for cutaneous vasodilation was significantly reduced (P < 0.001) post-training (36.83 ± 0.26°C) relative to pre-training (37.16 ± 0.38°C). However, when adjusted for changes in esophageal temperature from baseline rest, this difference was not significant (i.e., 0.40 ± 0.09 to 0.27 ± 0.06°C, P = 0.106).
Heart rate and mean arterial pressure
Resting heart rate decreased from a pre-training value of 82 ± 6 to 73 ± 9 beats min−1 post-training (P = 0.008). This was paralleled by a significantly lower heart rate at the end of the 60-min exercise (P = 0.008) and 60-min recovery period (P = 0.015). Mean arterial pressure was significantly lower (P = 0.023) during baseline rest post- (81 ± 8 mmHg) compared to pre- (87 ± 12 mmHg) training. However, there were no differences after 60-min exercise (P = 0.286) or 60-min recovery (P = 0.099).
Discussion
The main finding of this study was that an 8-week exercise training program did not increase local and whole-body heat loss responses during exercise despite the fact that the program elicited: (1) a 10% increase in \({\dot{V}{\text{O}}_{2\,\max } };\) (2) a reduction in resting heart rate and mean arterial pressure; (3) lower resting core temperatures, and; (4) lower onset thresholds for sweating and cutaneous vasodilation.
The notion that exercise training itself can improve thermoregulatory function is partly due to the fact that many studies have shown that endurance-trained individuals have elevated sweat rates and levels of skin perfusion during exercise and/or heat exposure and therefore a greater heat tolerance as compared to sedentary individuals (Baum et al. 1976; Buono and Sjoholm 1988; Fritzsche and Coyle 2000; Piwonka et al. 1965; Selkirk and McLellan 2001). The greater thermoregulatory function in endurance-trained individuals is thought to result from both a central downward shift in thermoregulatory set-point temperature (Baum et al. 1976) and peripheral adaptations such as a greater sweat gland sensitivity (Buono and Sjoholm 1988). It is quite likely that the adaptations observed in endurance-trained athletes are the result of the exercise-induced increase in core temperature associated with the repeated exercise training bouts (Piwonka et al. 1965). Although there remains controversy as to whether short-term exercise training itself can improve thermoregulatory function, many studies have reported an increase in heat loss responses (Gisolfi and Robinson 1969; Henane et al. 1977; Nadel et al. 1974; Roberts et al. 1977; Shvartz et al. 1979). Therefore, the question arises as to why we did not observe any improvements in local (i.e., upper back sweat rate and forearm SkBF) and whole-body (evaporative and dry) heat loss responses despite the well characterized reductions in resting core temperatures (Fig. 3) and onset thresholds for sweating and cutaneous vasodilation (Gisolfi and Robinson 1969; Henane et al. 1977; Ichinose et al. 2009; Kampmann et al. 2008; Roberts et al. 1977; Shvartz et al. 1974) which would be indicative of an improvement in system function.
The first possibility is that most of the studies which have reported increased heat loss responses following short-term exercise training employed a pre- and post-training experimental design with both sessions performed at the same percentage of \({\dot{V}{\text{O}}_{2\,\max } }.\) However, such an experimental design may lead to misinterpretations if differences in the rate of metabolic heat production between sessions are not considered. For example, Shvartz et al. (1974) reported a decrease in LSR as a function of exercise training. Although this may seem contradictory, they correctly concluded that the reduction in sweat rate was the result of differences in the rate of metabolic heat production during the pre- and post-exercise test as opposed to a training specific deterioration in the sweating response. Although standardizing rate of metabolic heat production between pre- and post-training exercise sessions may seem obvious, such is not the case as many studies still employ an experimental design based on the same percentage of \({\dot{V}{\text{O}}_{2\,\max } }.\)
Ichinose et al. (2009) have recently reported an increase in chest and thigh sweat rate following a 3-month exercise training program. These improvements were paralleled by a significant increase in \({\dot{V}{\text{O}}_{2\,\max } }\) from a pre-training value of ~1,875 ml min−1 to a post-training value of ~2,130 ml min−1. The pre- and post-training exercise heat stress test was performed at the same relative work rate of 50% \({\dot{V}{\text{O}}_{2\,\max } }.\) Therefore, absolute oxygen consumption during the pre-training exercise trial was ~938 ml min−1, while it increased to ~1,065 ml min−1 during the post-training trial. Assuming a RER of 0.85, the 127 ml min−1 difference in oxygen consumption between the pre- and post-training sessions would result in a ~45 W greater rate of metabolic heat production. From a heat balance perspective, this greater rate of metabolic heat production inevitably requires a greater rate of total heat loss to achieve heat balance (Gagnon et al. 2008). Therefore, the greater rate of total heat loss post-training most likely induced a greater rate of evaporative heat loss, explaining the greater sweat rates observed at the chest and thigh. As such, it is unknown if the greater sweat rates reported by Ichinose et al. (2009) were due to exercise training-induced adaptations, or simply due to the greater rate of metabolic heat production.
In order to avoid the confounding influence of different rates of metabolic heat production between the pre- and post-training exercise session, the rate of metabolic heat production was kept constant in the present study. By exposing the participants to the same rate of metabolic heat production during exercise, it was possible to isolate the effects of exercise training itself on local and whole-body heat loss responses. However, we did not observe any significant differences in either local (i.e., sweating and SkBF) or whole-body (i.e., evaporative and dry heat loss) heat loss responses following an 8-week exercise training program in previously sedentary individuals. An interesting point is that the participants did not attain heat balance within 60 min during the pre-training exercise trial (see Fig. 1). Consequently, the observed improvements in thermoregulatory function, as evidenced by lower onset thresholds for sweating and skin vasodilation, were not paralleled by the attainment of heat balance during the post-training exercise trial (see Fig. 1). These observations reinforce the notion that changes in local heat loss responses and/or core temperature do not necessarily reflect changes in whole-body heat balance. As such, future studies should consider examining multiple sites when examining the effects of short-term exercise training on thermoregulatory function.
Implications
The fact that many studies employ a pre- and post-training design with both exercise sessions performed at the same percentage of \({\dot{V}{\text{O}}_{2\,\max } }\) stems from the early study by Saltin and Hermansen (1966) which suggested that core temperature during exercise is determined by the relative exercise intensity. However, as shown from the present data, differences in the rate of metabolic heat production (i.e., the absolute exercise intensity) must be considered when comparing thermoregulatory responses prior to and following an exercise training program. Our findings have important implications as future studies should compare pre- and post-exercise training responses using similar rates of metabolic heat production. It is important to note that this approach should also be applied to parallel study designs examining populations (e.g., untrained vs. trained) which have different \({\dot{V}{\text{O}}_{2\,\max } }.\) By standardizing the rate of metabolic heat production, it will be possible to clearly delineate or isolate the influence of the exercise training stimulus on thermoregulatory function. Finally, the effects of the duration of the exercise training program remain unclear. Thermoregulatory adaptations have been reported in exercise training programs of only 10 days in duration (Nadel et al. 1974; Roberts et al. 1977), albeit it remains unclear if these responses are indeed training induced or the result of differences in exercise-induced heat load. The present training program was limited to 8 weeks, therefore it is possible that a more intense and/or longer duration training program may provide a greater stimulus and therefore promote increases in local and whole-body heat loss responses consistent with those levels observed in endurance-trained athletes. Nonetheless, one advantage provided by an 8-week exercise training program could be to allow for a longer duration of exercise before reaching levels of core temperature associated with hyperthermia by lowering resting core temperatures (Kampmann et al. 2008).
In conclusion, an 8-week exercise training program in previously sedentary individuals significantly improved overall cardiovascular function. However, despite reductions in resting core temperatures and onset thresholds for sweating and cutaneous vasodilation, such a training program did not improve local and whole-body heat loss responses during exercise performed at a given rate of metabolic heat production. Although previous studies have reported improvements in local heat loss responses as a function of exercise training, differences in oxygen consumption between pre- and post-training exercise trials could have confounded interpretation of the results. Therefore, it is suggested that when examining thermal adaptations after an exercise program, the rate of metabolic heat production should be similar during the pre- and post-training experimental test sessions in order to accurately isolate the effects of exercise training.
References
Baum E, Brück K, Schwennicke HP (1976) Adaptive modifications in the thermoregulatory system of long-distance runners. J Appl Physiol 40:404–410
Buono MJ, Sjoholm NT (1988) Effect of physical training on peripheral sweat production. J Appl Physiol 65:811–814
Canadian Society for Exercise Physiology (1986) Certified fitness appraiser resource manual. CSEP, Gloucester
Fritzsche RG, Coyle EF (2000) Cutaneous blood flow during exercise is higher in endurance-trained humans. J Appl Physiol 88:738–744
Gagge AP, Gonzales RR (1996) Mechanisms of heat exchange. In: Handbook of physiology. Section 4: environmental physiology. Oxford University press, New York, pp 45–84
Gagnon D, Jay O, Lemire B, Kenny GP (2008) Sex-related differences in evaporative heat loss: the importance of metabolic heat production. Eur J Appl Physiol 104:821–829
Gisolfi CV (1973) Work-heat tolerance derived from interval training. J Appl Physiol 35:349–354
Gisolfi C, Robinson S (1969) Relations between physical training, acclimatization, and heat tolerance. J Appl Physiol 26:530–534
Henane R, Flandrois R, Charbonnier JP (1977) Increase in sweating sensitivity by endurance conditioning in man. J Appl Physiol 43:822–828
Ichinose TK, Inoue Y, Hirata M, Shamsuddin AK, Kondo N (2009) Enhanced heat loss responses induced by short-term endurance training in exercising women. Exp Physiol 94:90–102
Jay O, Kenny GP (2007) The determination of changes in body heat content during exercise using calorimetry and thermometry. J Hum Environ Syst 10:19–29
Jay O, Reardon FD, Webb P, Ducharme MB, Ramsay T, Nettlefold L, Kenny GP (2007) Estimating changes in mean body temperature for humans during exercise using core and skin temperatures is inaccurate even with a correction factor. J Appl Physiol 103:443–451
Johnson JM (1998) Physical training and the control of skin blood flow. Med Sci Sports Exerc 30:382–386
Jones AM, Carter H (2000) The effect of endurance training on parameters of aerobic fitness. Sports Med 29:373–386
Kampmann B, Bröde P, Schütte M, Griefahn B (2008) Lowering of resting core temperature during acclimation is influenced by exercise stimulus. Eur J Appl Physiol 104:321–327
Kenny GP, Leclair E, Sigal RJ, Journeay WS, Kilby D, Nettlefold L, Reardon FD, Jay O (2008) Menstrual cycle and oral contraceptive use do not modify postexercise heat loss responses. J Appl Physiol 105:1156–1165
Kondo N, Takano S, Aoki K, Shibasaki M, Tominaga H, Inoue Y (1998) Regional differences in the effect of exercise intensity on thermoregulatory sweating and cutaneous vasodilation. Acta Physiol Scand 164:71–78
Machado-Moreira CA, Smith FM, van den Heuvel AM, Mekjavic IB, Taylor NA (2008a) Sweat secretion from the torso during passively-induced and exercise-related hyperthermia. Eur J Appl Physiol 104:265–270
Machado-Moreira CA, Wilmink F, Meijer A, Mekjavic IB, Taylor NA (2008b) Local differences in sweat secretion from the head during rest and exercise in the heat. Eur J Appl Physiol 104:257–264
Mack GW (1998) Assessment of cutaneous blood flow by using topographical mapping techniques. J Appl Physiol 85:353–359
Mekjavic IB, Rempel ME (1990) Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol 69:376–379
Nadel ER, Pandolf KB, Roberts MF, Stolwijk JA (1974) Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol 37:515–520
Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B (1993) Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 460:467–485
Nishi Y (1981) Measurement of thermal balance in man. In: Cena K, Clark J (eds) Bioengineering, thermal physiology and comfort. Elsevier, New York, pp 29–39
Okazaki K, Kamijo Y, Takeno Y, Okumoto T, Masuki S, Nose H (2002) Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol 93:1630–1637
Peters J, Nishiyasu T, Mack GW (2000) Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol 88:1756–1764
Piwonka RW, Robinson S, Gay VL, Manalis RS (1965) Preacclimatization of men to heat by training. J Appl Physiol 20:379–383
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Reardon FD, Leppik KE, Wegmann R, Webb P, Ducharme MB, Kenny GP (2006) The Snellen human calorimeter revisited, re-engineered and upgraded: design and performance characteristics. Med Biol Eng Comput 44:721–728
Roberts MF, Wenger CB, Stolwijk JA, Nadel ER (1977) Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol 43:133–137
Saltin B, Hermansen L (1966) Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol 21:1757–1762
Selkirk GA, McLellan TM (2001) Influence of aerobic fitness and body fatness on tolerance to uncompensable heat stress. J Appl Physiol 91:2055–2063
Shields CL, Giesbrecht GG, Pierce GN, Ready AE (2004) The effects of a moderate physical activity program on thermoregulatory responses in a warm environment in men. Can J Appl Physiol 29:379–394
Shvartz E, Saar E, Meyerstein N, Benor D (1973) A comparison of three methods of acclimatization to dry heat. J Appl Physiol 34:214–219
Shvartz E, Magazanik A, Glick Z (1974) Thermal responses during training in a temperate climate. J Appl Physiol 36:572–576
Shvartz E, Bhattacharya A, Sperinde SJ, Brock PJ, Sciaraffa D, Van Beaumont W (1979) Sweating responses during heat acclimation and moderate conditioning. J Appl Physiol 46:675–680
Siri WE (1956) Gross composition of the body. In: Advances in biological and medical physics. Academic, New York, pp 239–280
Strydom NB, Wyndham CH, Williams CG, Morrison JF, Bredell GA, Benade AJ, Von Rahden M (1966) Acclimatization to humid heat and the role of physical conditioning. J Appl Physiol 21:636–642
Wenger CB (1972) Heat of evaporation of sweat: thermodynamic considerations. J Appl Physiol 32:456–459
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada (Grant # RGPIN-298159-2004, held by Dr. Glen P. Kenny). Dr. Glen P. Kenny was supported by a University of Ottawa Research Chair Award. The authors wish to thank all the participants who volunteered for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by George Havenith.
Rights and permissions
About this article
Cite this article
Stapleton, J., Gagnon, D. & Kenny, G.P. Short-term exercise training does not improve whole-body heat loss when rate of metabolic heat production is considered. Eur J Appl Physiol 109, 437–446 (2010). https://doi.org/10.1007/s00421-010-1380-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1380-3