Abstract
The effect of intensive long-term physical activity on phospholipid fatty acid (FA) composition has not been studied thoroughly. We determined plasma and erythrocyte phospholipid FA status of professional basketball and football players. Our results showed differences in plasma FA profile not only between sportsmen and sedentary subjects, but also between two groups of sportsmen. Plasma FA profile in basketball players showed significantly higher proportion of n-6 FA (20:3, 20:4, and 22:4) and total polyunsaturated FA (PUFA) than controls, while football players had higher palmitoleic acid (16:1) than basketball players and controls. Total PUFA and 22:4 were also higher in basketball than in football players. Erythrocyte FA profile showed no differences between football players and controls. However, basketball players had higher proportion of 18:0 than controls, higher saturated FA and lower 18:2 than two other groups, and higher 22:4 than football players. These findings suggest that long-term intensive exercise and type of sport influence FA profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phospholipids (PL) are major structural components of biological membranes that influence a number of physical properties related to membrane function. Alterations in membrane PL composition have an impact on a broad range of cellular functions from fluidity, transport systems, activity of membrane bound enzymes to cell growth (Helge et al. 1999; Spector and Yorek 1985; Clemens et al. 1987). Furthermore, it has been established that fatty acid (FA) composition of some tissue PL is linked to metabolic disorders, such as obesity, insulin resistance etc. (Nikolaidis and Mougios 2004).
The fatty acid profile in tissues partly reflects not only the dietary fat intake (Nikkari et al. 1995), but also the efficiency of FA metabolism in the body (Nikolaidis and Mougios 2004). The control and regulation of membrane PL composition are not clearly understood; however, dietary FA profile significantly influences the incorporation of fatty acids into the membrane (McMurchie et al. 1996). As a dynamic system, membrane FA composition is constantly changing, different stimuli selectively recruiting fatty acids from precursor pools. Thus, the FA profile of the serum phospholipids is related to the average dietary FA intake during the last 3–6 weeks, while the composition of erythrocyte PL depends on the dietary fat intake during the preceding months (Katan et al. 1997). It has also been established that erythrocytes reflect the general FA metabolism in other organs and tissues, including muscles (Shohet and Nathan 1970).
In addition to dietary fats, physical activity also greatly influences lipid metabolism. It is well known that the regular practice of moderate, aerobic exercise is associated with a healthy plasma lipid profile (Ruiz et al. 2004). Exercise seems to be one of the stimuli to selectively mobilize fatty acids from triglycerides (TG) (Mougios et al. 1995). Studies on humans and animals demonstrated changes in FA composition, particularly in skeletal muscle phospholipids, caused by regular exercise training suggesting that the exercise, independent of fat intake can modify FA profile in different tissues (Helge et al. 1999; Andersson et al. 2000). However, there is no detailed data about changes in fat metabolism caused by intensive exercise over a period of several years, such as in professional sportsmen. It should also be taken into account that sports can be very different in terms of their bioenergetic characteristic and methodology of training, and that intrinsic characteristics of training and competition can also vary accordingly. Metabolic changes vary according to the predominant pathway used, which might be different when a person is competing or training (McArdle et al. 1995).
The aim of this study was to investigate changes in phospholipid fatty acid profile in plasma and in erythrocytes as a result of intensive exercise over a prolonged period of time. For this reason, we have chosen two groups of professional sportsmen—football and basketball players, to investigate the effects of longstanding, intensive exercise on FA metabolism and possible influence of the type of sport on changes in fatty acid profile.
Subjects and methods
We studied two groups of professional sportsmen—basketball players (n = 23) and football players (n = 24), who competed at the national/international level. For comparison, a control group was established, composed of healthy, sedentary individuals (n = 16), comparable to the sportsmen in terms of age, sex, and body mass index (BMI). All subjects were healthy and took no medication or herbal products that might have influenced the lipid profile results. The subjects completed a questionnaire on their health and eating habits under supervision of a trained nutritionist. No significant qualitative differences were recorded among the groups regarding their eating habits. The study protocols were approved by the institutional ethics committee and all subjects gave written informed consent.
Training history of the subjects
All the study subjects completed a questionnaire on their physical activity. The 23 selected basketball players trained for 4.07 ± 0.58 h daily and have played for 9.96 ± 2.01 years. The 24 selected football players trained for 4.17 ± 0.49 h daily and have played for 14.10 ± 2.64 years.
Anthropometric measurements
Participants were asked to remove their shoes and socks for height, weight, and body fat measurements. Standing height was measured to the nearest centimeter using a wall-mounted stadiometer (Perspective Enterprises, Kalamazoo, MI). Weight (in kilograms), BMI and body fat were measured using Tanita body composition analyzer (TBF-300, Tanita Corp., Japan).
Analytical methods
Blood samples were collected in the morning after 12 h fast, 2 weeks after the end of competitive season, when the training intensity was lower than during the season. Glucose, cholesterol, and triglyceride concentrations were measured in serum using the automated enzymatic methods with glucose oxidase, cholesterol oxidase, and glycerol oxidase, respectively (EliTech Diagnostic, Sees, France). Serum high-density lipoprotein (HDL) cholesterol was measured after other classes of lipoproteins had been precipitated with phosphotungstic acid and magnesium chloride (Lopes-Virela et al. 1977). LDL cholesterol was estimated using Friedewald formula (Friedewald et al. 1972).
For the extraction and derivatization of the fatty acid components of erythrocytes’ phospholipids, erythrocytes were washed three times with 0.9% NaCl and separated by centrifugation (1,300×g, 10 min). After homogenization in three mixtures of methanol and chloroform [1:2 v/v with 50 mg/L 2,6-di-tert-butyl-4-methylphenol (BHT)], (2:1v/v with 5% H2O), and (1:1v/v) successively, the total lipid extracts were prepared according to the method of Harth et al (1978). Plasma lipids were extracted with chloroform–methanol mixture (2:1v/v) by the method of Folch et al. (1957), with 10 mg/100 mL BHT added as an antioxidant. The PL fraction was isolated from the extracted lipids by one-dimensional thin-layer chromatography in a neutral solvent system (petrol ether—diethyl ether—acetic acid; 87:12:1v/v) on Silica Gel GF plates (Merck, Darmstadt, Germany). The FA methyl esters were prepared as described previously (Christopherson and Glass 1969) and then analyzed by the gas chromatograph Varian 3400 fitted with a capillary column (Rtx 2330, RESTEC, USA). The individual FA methyl esters were identified from the retention times of authentic standard mixtures (Sigma Chemical Co, St. Louis, MO) and/or polyunsaturated fatty acid (PUFA-2) standard mixture (Supelco, Inc. Belleforte, PA). The results were expressed as the relative percentage of total identified fatty acids.
Statistical analysis
Normality was tested using the Kolmogorov–Smirnov test before statistical analysis. As all variables showed normal distribution, statistical analysis was performed using one-way ANOVA, with training as a fixed factor. Where ANOVA revealed a significant effect, Tukey post hoc test was administered to identify differences between the groups. The differences were considered significant at p ≤ 0.05. All results are expressed as mean ± SD.
Results
Subject characteristics
Table 1 summarizes the characteristics of the study subjects. Age, BMI, serum glucose, triglyceride, cholesterol, and HDL cholesterol were similar in the groups. As a consequence of intensive exercise, percentage of body fat was considerably lower in professional sportsmen, compared to control subjects.
Fatty acid composition in plasma phospholipids
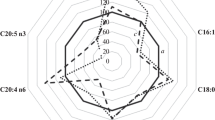
The FA profile of plasma PL in control group and in two groups of sportsmen is shown in Table 2. The percentage of palmitoleic acid (16:1 n-7) was significantly higher in professional football players than in basketball players and control group (p < 0.05). In addition, both groups of sportsmen had significantly higher proportion of dihomo-γ-linolenic acid (20:3 n-6) when compared to sedentary subjects (p < 0.05). The percentage of arachidonic acid (20:4 n-6) was significantly higher in basketball players than in controls (p < 0.05), while the percentage of another n-6 fatty acid, docosatetraenoic acid (22:4 n-6) was significantly higher in basketball players when compared to both football players (p < 0.05) and control group (p < 0.05).
Although there were no significant differences in the percentage of total n-3, n-6 and n-6/n-3 ratio among the three studied groups, the percentage of polyunsaturated fatty acids (PUFA) was significantly higher in basketball players than in football players (p < 0.05) or in control subjects (p < 0.05). No significant differences in the proportion of total saturated fatty acids (SFA), palmitic acid (16:0) and stearic acid (18:0) between sportsmen and sedentary individuals were observed.
Fatty acid composition in erythrocyte phospholipids
The FA profile of erythrocyte PL in control group and two groups of sportsmen is shown in Table 3. One-way ANOVA and Tukey post-hoc test showed no differences between football players and sedentary subjects. Basketball players had significantly higher proportion of stearic acid (p < 0.05) than control group, as well as total SFA than both control group (p < 0.05) and football players (p < 0.05). Basketball players also showed significantly higher percentage of polyunsaturated 22:4 n-6 than football players (p < 0.05). The main difference was found in percentage of linoleic acid (18:2 n-6), that was lower in erythrocyte phospholipids of basketball players than in control group and football players (p < 0.001 and p < 0.01, respectively).
Estimated activity of desaturase and elongase enzymes in plasma and erythrocytes
Table 4 shows that no significant difference was found in the activity of desaturase or elongase enzymes in plasma of sportsmen and sedentary individuals. However, there was a significant main effect of basketball training on the estimated activity of the delta-6-desaturase enzyme [20:3 (n-6)/18:2 (n-6)] and elongase in erythrocytes, when compared to control group (p < 0.001) and football players (p < 0.01). On the contrary, the ratio of 18:1/18:0 in erythrocytes, as a measure of activity of delta-9-desaturase was significantly lower in basketball players than in control group and football players (p < 0.05).
Discussion
In recent years, a wealth of studies has appeared addressing the effect of exercise on the lipid and fatty acid composition of animal and human tissues. FA are components of different lipoproteins, and a small proportion of the FA in tissues are present in free, i.e., nonesterified form (NEFA), representing an important fuel for skeletal muscles (Nikolaidis and Mougios 2004). As NEFA are the most labile lipid class of plasma during exercise, since they receive input from triglycerides hydrolyzed in adipose tissue, they are the most studied class of lipids in terms of the effect of exercise on their profile (Mougios et al. 1995; Borsheim et al. 1999; Hall et al. 1987). However, we wanted to gain a different perspective by studying phospholipids whose FA content is not considerably affected by acute exercise, but changes in chronic exercise, as a result of metabolic changes.
The main finding in the present study was the difference in the phospholipid FA profiles of plasma and erythrocytes, not only between professional sportsmen and sedentary subjects, but also between basketball and football players. The literature data about FA content of plasma PL after regular, intensive exercise is rather poor and inconsistent, and mostly obtained from studies on animals (Quiles et al. 2003; Hashimoto et al. 1999; Hambleton et al. 1980), but contrary to our results the authors reported lower MUFA in the trained group (Quiles et al. 2003; Wirth et al. 1980; Wirth et al. 1979). On the other hand, the higher percentage of total PUFA and the three n-6 FA (20:3, 20:4, and 22:4) in basketball players compared to control found in this study, is in accordance with the paper of Allard et al. (1973). However, two other studies reported no significant effect of chronic exercise on the FA composition of human plasma PL and cholesterol-esters in general (Andersson et al. 2000; Andersson et al. 1998).
Arachidonic acid and eicosapentanoic acid (EPA, 20:5 n-3) are important as precursors for eicosanoid synthesis, which inter alia, modulate the immune functions (Kogteva and Bezuglov 1998). It has been shown that athletes engaged in heavy training programs appear to be more susceptible to infections than sedentary population due to a depression of immune system function (Gleeson and Bishop 1999). Namely, strenuous exercise induces an increase in pro-inflammatory cytokines (TNFα, IL-1β, and specially IL-6), that is balanced by the release of cytokine inhibitors (IL-1ra, sTNF-r1, and sTNF-r2) and the anti-inflammatory cytokine IL-10 (Ostrowski et al. 1999). The PUFA-mediated immune response may be altered by changes in the production of immunologic mediators such as cytokines (James et al. 2000). Therefore, linking FA profile in plasma and eicosanoid production could be responsible for altered immune response in professional sportsmen. However, in our study such changes were detected only in basketball players. One of the possible reasons may be that injuries, followed by an increase in cytokine production, are more common in basketball than in football (Dane et al. 2004).
The main reason for examining the FA profile of erythrocytes is the influence that it exerts on membrane fluidity. Several studies have been published on increased fluidity of red blood cell membranes after chronic exercise (Kamada et al. 1993; Smith et al. 1999). Kamada et al. (1993) found slightly lower MUFA and higher PUFA in erythrocytes in male sprinters and long-distance runners than in sedentary men, though Nakano et al. (2001) reported similar FA profiles of erythrocytes PL in long-distance runners and sedentary individuals. The results in our study showed no difference between football players and control subjects, but we found a higher proportion of stearic acid in erythrocyte PL of basketball players than in controls, and higher total SFA than in controls and football players. Percentage of stearic (and palmitic) acid depends both on dietary intake and on endogenous synthesis. As both groups of athlets had similar dietary intake, we propose that the reason for higher percentage of stearic acid in basketball group is the influence of the type of exercise on increased endogenous synthesis. A higher percentage of stearic acid was also found in muscle PL in endurance trained young men compared with untrained men, after 8 weeks on diet with similar FA composition (Andersson et al. 2000). Moreover, Thomas et al. (1977) showed a positive correlation between the proportion of stearic acid in skeletal muscle membrane with the amount of weekly mileage run in trained individuals. Regarding that erythrocytes reflect FA profile of muscles and other organs, increased percentage of stearic acid probably arrives from muscles. Furthermore, in our study the percentage of linoleic acid in erythrocytes was lower in basketball players than in control group and football players. Linoleic acid content in plasma PL mostly reflects the dietary linoleic acid intake (Okita et al. 1995), but Helge et al. (1999) showed significantly higher relative content of linoleic acid in muscle membranes in rats which exercised regularly, regardless of the dietary fatty acid intake. As there were no significant differences neither in content of linoleic acid in plasma PL nor in eating habits among groups, we think that the type of physical activity affected the proportion of linoleic acid in basketball players. However, we cannot exclude the possibility that other factors, such as subtle differences in diet and/or genetic variability, in addition to sport training may be responsible for the observed differences. Basketball players in our study also had significantly higher percentage of another n-6 PUFA, docosatetraenoic acid (22:4), than football players. This fatty acid is associated with increased risk of diabetes (Kratchler et al. 2008). The level of 18:2 in plasma and erythrocytes correlates inversely, and the level of SFA correlates directly with the incidence of cardiovascular diseases, suggesting that elite basketball players have less favorable FA profile than football players and healthy sedentary subjects.
In addition, several fatty acid indexes reflecting desaturase and elongase activity, were derived from the primary data. The ratio of 20:3 n-6/18:2 n-6 in erythrocytes are significantly higher in basketball players than in controls and football players, showing increased activity of delta-6-desaturase and elongase in basketball group. Significantly lower 18:1 to 18:0 ratio in erythrocyte PL in basketball players than in controls and football group suggests a decrease in delta-9-desaturase activities induced by basketball training, which is in agreement with the results of Nikolaidis et al. (2004) obtained in skeletal muscles of chronically trained rats. Contrary results were reported by Szabo et al. (2002), who found higher delta-9-desaturase activity in muscles of rabbits after chronic treadmill running. The estimated activity of elongase in our study, in contrast to some other authors (Andersson et al. 2000; Turner et al. 2004), remained unchanged in elite sportsmen. Although the possible slight differences in dietary fat intake and other factors could contribute to these changes, according to these findings we suggest that long-term physical activity of professional sportsmen alters the activity of desaturases, but the type of sport could also be responsible for these changes.
The observed differences between FA profile of basketball and football players are currently hard to explain. Because of the game duration, aerobic metabolism dominates the energy delivery during a football game and trainings, about 80% of time. In basketball, players spend even 50–60% of time in anaerobic activities. Aerobic training has beneficial effect on lipid profile, while anaerobic training leads lipid parameters to the opposite direction (Aellen et al. 1993). In addition, both sports are very stressful, with a lot of falls and collisions, but injuries are commoner in basketball (Dane et al. 2004). Although these differences between basketball and football could contribute to the observed alterations in FA profiles, mechanism underlying these changes requires further investigations.
Conclusion
In summary, the findings in the present study suggest that long-term intensive exercise has significant influence on PL fatty acid profile in plasma and in erythrocytes. However, considerable difference between two groups of sportsmen indicates that the type of regular intensive physical activity along with other factors, may play an important role in the changes of fatty acid metabolism.
References
Aellen R, Hollmann W, Boutellier U (1993) Effects of aerobic and anaerobic training on plasma lipoproteins. Int J Sports Med 14:396–400
Allard C, Alteresco M, Ferguson RJ et al (1973) Changes in adipose tissue and increased serum cholesterol of coronary patients following training. Can Med Assoc J 109:194–197
Andersson A, Sjodin A, Hedman A et al (1998) Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol 274:E432–E438
Andersson A, Sjodin A, Hedman A et al (2000) Fatty acid profile of skeletal muscle phospholipids in trained and untrained young men. Am J Physiol Endocrinol Metab 279:E744–E751
Borsheim E, Knardahl S, Hostmark AT (1999) Short-term effects of exercise on plasma very low density lipoproteins and fatty acids. Med Sci Sports Exerc 31:522–530
Christopherson SW, Glass RL (1969) Preparation of milk methyl esters by alcoholysis in an essentially nonalcoholic solution. J Daily Sci 52:1289–1290
Clemens MR, Ruess M, Bursa Z et al (1987) The relationship between lipid composition of red blood cells and their susceptibility to lipid peroxidation. Free Radic Res Commun 3:265–271
Dane S, Can S, Gursoy R et al (2004) Sport injuries: relations to sex, sport, injured body region. Percept Mot Skills 98:519–524
Folch J, Lees M, Stanley-Sloane GH (1957) A simple method for isolation and purification of total lipids from animal tissue. J Biol Chem 226:497–523
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Gleeson M, Bishop NC (1999) Immunology Basic and applied sciences for sports medicine. Oxford, Butterworth Heinemann, pp 199–236
Hall PE, Smith SR, Jack DB et al (1987) The influence of beta-adrenoceptor blockade on the lipolytic response to exercise. J Clin Pharm Ther 12:101–106
Hambleton PL, Slade LM, Hamar DW et al (1980) Dietary fat and exercise conditioning effect on metabolic parameters in the horse. Anim Sci 51:1330–1339
Harth S, Dreyfus H, Urban PF et al (1978) Direct thin-layer chromatography of gangliosides of total lipid extracts. Anal Biochem 86:543–551
Hashimoto M, Shinozuka K, Tanabe Y et al (1999) Hypotension induced by exercise is associated with enhanced release of adenyl purines from aged rat artery. Am J Physiol Heart Circ Physiol 276:H970–H975
Helge JW, Ayre KJ, Hulbert AJ et al (1999) Regular exercise modulates muscle membrane phospholipid profile in rats. J Nutr 129:1636–1642
James MJ, Gibson RA, Cleland LG (2000) Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 71:343S–348S
Kamada T, Tokuda S, Aozaki S et al (1993) Higher levels of erythrocyte membrane fluidity in sprinters and long-distance runners. J Appl Physiol 74:354–358
Katan MB, Deslypere JP, van Birgelen AP et al (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38:2012–2022
Kogteva GS, Bezuglov VV (1998) Unsaturated fatty acids as endogenous bioregulators. Biochemistry 63:4–12
Kratchler B, Norberg M, Eriksson JW et al (2008) Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 18:503–510
Lopes-Virela MF, Stone P, Ellis S et al (1977) Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem 23:882–886
McArdle W, Katch F, Katch V (1995) Fisiologia del ejercicio. Energia, nutricion y rendimiento humano, 2nd edn. Alianza Editorial, Madrid, pp 119–136
McMurchie EJ, Margetts BM, Beilin LJ et al (1996) Dietary-induced changes in the fatty acid compositionof human cheek cell phospholipids: correlation with changes in the dietary polyunsaturated/saturated fat ratio. Am J Clin Nutr 39:975–980
Mougios V, Kotzamanidis C, Koutsari C et al (1995) Exercise induced changes in the concentration of individual fatty acids and triacylglycerols of human plasma. Metabolism 44:681–688
Nakano T, Wada Y, Matsumura S (2001) Membrane lipid components associated with increased filterability of erythrocytes from long-distance runners. Clin Hemorheol Microcirc 24:85–92
Nikkari T, Lukkainen P, Pietinen P et al (1995) Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann Med 27:491–498
Nikolaidis MG, Mougios V (2004) Effects of exercise on the fatty acid composition of blood and tissue lipids. Sports Med 34:1015–1076
Nikolaidis MG, Petridou A, Matsakas A et al (2004) Effect of chronic wheel running on the fatty acid composition of phospholipids and triacylglycerols in rat serum, skeletal muscle and heart. Acta Physiol Scand 181:199–208
Okita M, Yoshida S, Yamoto et al. (1995) n-3 and n-6 fatty acid intake and serum phospholipidfatty acid composition in middle-aged women living in rural and urban areas in Okayama perfecture. J Nutr Sci Vitaminol 41:313–323
Ostrowski K, Rohde T, Asp S et al (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515:287–291
Quiles JL, Huertas JR, Ochoa JJ et al (2003) Dietary fat (virgin olive oil or sunflower oil) and physical training interactions on blood lipids in the rat. Nutrition 19:363–368
Ruiz JR, Mesa LM, Mingorance I et al (2004) Sports requiring stressful physical exertion cause abnormalities in plasma lipid profile. Rev Esp Cardiol 57:499–506
Shohet SB, Nathan DG (1970) Incorporation of phosphatide precursors from serum to erythrocytes. Biochim Biophys Acta 202:202–205
Smith JA, Martin DT, Telford RD et al (1999) Greater erythrocyte deformability in world-class endurance athletes. Am J Physiol Heart Circ Physiol 276:H2188–H2193
Spector AA, Yorek MA (1985) Membrane lipid composition and cellular function. J Lipid Res 26:1015–1035
Szabo A, Romvari R, Febel H et al (2002) Training induced alterations of the fatty acid profile of rabbit muscles. Acta Vet Hung 50:357–364
Thomas TR, Londeree BR, Gerhardt KO et al (1977) Fatty acid profile and cholesterol in skeletal muscle of trained and untrained young men. J Appl Physiol 43:709–713
Turner N, Lee JS, Bruce CR et al (2004) Greater effect of diet than exercise training on the fatty acid profile of rat skeletal muscle. J Appl Physiol 96:974–980
Wirth A, Neermann G, Eckert W et al (1979) Metabolic response to heavy physical exercise before and after a 3-month training period. Eur J Appl Physiol Occup Physiol 41:51–59
Wirth A, Heuck CC, Holm G et al (1980) Changes in the composition of fatty acids of total lipids in various tissues and serum due to physical training and food restriction in the rat. Scand J Clin Lab Investig 40:55–62
Acknowledgments
This work was supported by the Project 145071 financed by the Ministry of Science of the Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tepsic, J., Vucic, V., Arsic, A. et al. Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur J Appl Physiol 107, 359–365 (2009). https://doi.org/10.1007/s00421-009-1131-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1131-5