Abstract
To investigate how differing moderate sodium chloride concentrations affect rehydration after exercise and subsequent exercise capacity, eight males lost 1.98 ± 0.1% body mass exercising in the heat, then consumed one of four drinks in a volume equivalent to 150% of mass loss. Drinks were identical except for sodium chloride content (1 ± 1, 31 ± 1, 40 ± 1, 50 ± 1 mmol/l). After 4 h recovery subjects cycled for 5 min at 70% \( \dot{V} {\text{O}}_2 \)peak then at 95% \( \dot{V} {\text{O}}_2 \)peak until volitional fatigue. Urine output was inversely related to sodium intake: more was produced with the 1 than the 40 and 50 mmol/l drinks (P < 0.01). Time to exhaustion in the exercise capacity test was not different between treatments (P = 0.883). The addition of 40 or 50 mmol/l of sodium chloride to a rehydration beverage reduced subsequent urine output, thereby providing more effective rehydration than a sodium-free drink. This did not, however, result in improved performance 4 h after the end of the rehydration period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans tend not to replace enough fluid to offset sweat losses either during, or immediately following exercise, resulting in some degree of hypohydration (Pitts et al. 1944; Greenleaf and Sargent 1965; Greenleaf 1992; Maughan et al. 2004; Passe 2001). If this water deficit is not replaced before subsequent exercise, exercise performance may be impaired (Burge et al. 1993). Athletes often train or compete on consecutive days or even more than once per day, and there may be a need to consume sufficient fluid to restore euhydration—or at least to partially replace losses—before the next exercise bout. When sweat losses are high and the time available is short, this can pose particular challenges.
A number of studies have investigated methods of oral rehydration following exercise-induced dehydration in humans (Nielsen et al. 1986; Gonzalez-Alonso et al. 1992; Burge et al. 1993; Maughan and Leiper 1993; Maughan et al. 1994; Mitchell et al. 1994; Takamata et al. 1994; Maughan et al. 1996; Shirreffs et al. 1996, 2007 ; Shirreffs and Maughan 1997a, 1998; Mitchell et al. 2000; Wong et al. 2000). The consensus opinion is that it is necessary to consume an amount of fluid greater than the volume lost as sweat to ensure restoration of fluid balance, due to ongoing water losses and to the diuresis that normally follows the ingestion of large volumes of fluid even when hypohydrated (Shirreffs et al. 2004). Simply replacing the volume of fluid lost with an equal volume through drinking results in a negative fluid balance within a very few hours (Gonzalez-Alonso et al. 1992; Lambert et al. 1992; Maughan and Leiper 1995; Mitchell et al. 1994; Shirreffs et al. 1996). Shirreffs et al. (1996) showed that a volume of fluid equal to 150 or 200% of the fluid ingested was sufficient to restore fluid balance, whereas 50 or 100% was insufficient. The same study also showed the importance of replacing the sodium lost as sweat. The authors suggested that the sodium lost in sweat must be replaced for complete restoration of fluid balance: if sodium losses are not replaced, a substantial fraction of the ingested fluid is lost as urine. The importance of replacing sodium as part of the rehydration and recovery process has been supported by the studies of Shirreffs et al. (1996), Mitchell et al. (1994) and Takamata et al. (1994). Indeed Mitchell et al. (1994) found that ingestion of 150% of fluid lost resulted in only 68% restoration of fluid lost, during a 3-h rehydration period, when the sodium concentration of the drink consumed was 14 mmol/l. These papers suggest that for optimum rehydration both the volume and sodium concentration of the drink should be high. High in this context means that the amount of sodium should be sufficient to match the sweat loss. Typical sweat sodium concentration in healthy adult humans is about 30–50 mmol/l, though a much wider range exists and some individuals may secrete sweat containing less than 10 mmol/l or more than 80 mmol/l sodium (Shirreffs and Maughan 1997b).
In a practical setting it is important to ensure that the drink is palatable to ensure that large volumes can be consumed willingly, and very high sodium concentrations can render drinks unpalatable. Several other variables can affect the liking for a particular drink, such as drink flavour, temperature, mouth feel, and level of carbonation (Passe 2001), and individuals will tend to drink more of drinks that they find more palatable when rehydrating after exercise (Maughan and Leiper 1993; Passe et al. 2000). Wemple et al. (1997) showed that during ad libitum rehydration following exercise-induced dehydration, subjects drank significantly more of a 25 mmol/l sodium drink, than a 0 or 50 mmol/l sodium drink. These results suggest that in a practical setting where palatability is of importance, drinks with a sodium concentration of less than 50 mmol/l may be more effective rehydration aids by encouraging more fluid to be consumed.
The present study therefore compared the efficacy in promoting rehydration of four drinks with sodium concentrations of 1, 31, 40 and 50 mmol/l sodium chloride. This allowed for the investigation of the rehydration effectiveness of beverages where the sodium concentration is in a range where palatability should be maintained. We hypothesised that increasing the sodium concentration of the rehydration beverage would result in more effective restoration of fluid balance.
A major rationale for ensuring adequate rehydration is to ensure that subsequent exercise performance is not impaired. Most previous studies of post-exercise rehydration have assumed that greater fluid retention will result in improved exercise performance, but have not measured this directly. In order to determine whether any advantages in fluid restoration conferred by increasing the sodium concentration of the rehydration drink results in an increased capacity to perform aerobic exercise, subjects in the presents study were asked to undertake an exercise capacity test by cycling to exhaustion 4 h following the end of the rehydration period.
Methods
Eight healthy men (aged 25 ± 5 years, body mass 75.53 ± 9.23 kg, height 176 ± 5 cm, \( \dot{V} {\text{O}}_2 \) peak 52.4 ± 7.3 ml/kg body mass/min) with no history of cardiovascular or renal diseases were recruited by word of mouth to participate in this study. Local ethics committees approval was obtained and subjects provided written consent after testing procedures had been fully explained and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Subjects attended the laboratory for an initial visit when peak oxygen uptake was measured on an electrically-braked cycle ergometer using a discontinuous protocol. The subjects completed a number of bouts or stages (usually 4–6) of increasing workload until volitional exhaustion. Expired air was collected using a nose clip, mouthpiece and Douglas bag during the final 2 min of the first stage and the final minute of each subsequent stage. The initial stage was a 5-min bout of cycling at 100 W and served as a warm up; in all trials, subjects were asked to keep the cycling cadence at about 60 rpm. Workload in subsequent stages was increased in increments of 25 or 50 W, in consultation with the subject, depending on the performance in the previous stage. Subjects performed stages at increasing workloads until they were unable to complete a stage, or felt unable to attempt to perform at a higher workload. Maximum effort was verified by examining heart rate and RER during the final exercise stage to ensure that \( \dot{V} {\text{O}}_2 \) peak was attained. The peak oxygen uptake was defined as the value obtained during the last full expired air collection period. A Harvard dry gas meter (Harvard Apparatus Ltd, Kent, USA) was used to determine expired air volume and temperature (Comark, Sussex, UK). Expired air was analysed for oxygen and carbon dioxide concentration by use of a Servomex paramagnetic O2 analyser (Sussex, UK) and an infra-red CO2 analyser (Beckman LB-2, Illinois, USA). These values were used to determine oxygen uptake (VO2), carbon dioxide production (VCO2), ventilation rate (V E), and respiratory exchange ratio (RER), and were corrected to STPD. Peak oxygen uptake was 3.93 ± 0.54 l/min and was used to determine the exercise intensities used for subsequent testing procedures.
Experimental design
Subjects performed four experimental trials, in a randomised Latin square design, with trials carried out on the same day of the week and separated from each other by at least 1 week. Subjects arrived at the laboratory between 0800am and 0900am having fasted overnight except for 500 ml of water consumed 2 h before arrival to ensure adequate hydration following the overnight fast. On entering the laboratory subjects were seated for 15 min to allow postural effects on blood parameters to take effect. After 15 min, a 21-g venous cannula was inserted into a superficial forearm vein and a 5-ml blood sample was obtained. Following collection of the blood sample, subjects were asked to provide a urine sample by emptying the bladder completely into an appropriate container.
Subjects then showered, towelled dry and changed into lightweight shorts. Subjects entered an environmental chamber maintained at a temperature of 34–36°C and 60–80% relative humidity. Subjects were weighed nude to the nearest 10 g on a beam balance (Marsdens, London, UK). Subjects then cycled for a 10-min period in the environmental chamber, wearing only the lightweight shorts, at an intensity equal to 55% of the measured peak oxygen uptake. After 10 min of exercise, subjects ceased cycling and towelled dry. Subjects were again weighed nude and the decrease in body mass (g) was assumed to be equal to the loss of body water (ml). The error introduced by not correcting for substrate oxidation is small (Maughan et al. 2007). Subjects repeated the intermittent 10 min exercise bouts and were weighed between bouts until approximately 1.9% of initial body mass had been lost. After the final exercise bout subjects showered and towelled dry before a final nude body mass was measured. At this point, subjects were hypohydrated by 1.98 ± 0.10% of initial body mass.
Subjects then sat in a comfortable chair in a room maintained at about 22°C. A blood sample was collected after 15 min of seated rest and subjects were then asked to provide a urine sample. During the next hour, beginning 30 min after the end of the prior exercise, subjects were provided with equal portions of one of the test drinks every 15 min so that after 1 h subjects had consumed a volume of the drink equivalent to 150% of the fluid lost as sweat. The composition of the drinks provided was identical (2% CHO, 10% low sodium flavouring, distilled water) apart from the sodium chloride concentration. Sodium chloride was added to provide concentrations of 0, 30, 40 and 50 mmol/l: the measured concentrations were 1 ± 1, 31 ± 1, 40 ± 1 or 50 ± 1 mmol/l as shown in Table 1.
Subjects were not informed of the sodium concentration of the drink provided and the drinks were provided in a randomised order. Further blood and urine samples were collected 1, 2, 3 and 4 h after the end of the rehydration period. Throughout the study subjects completed subjective feeling questionnaires (10 cm visual analogue scale) during the 15 min of seated rest before each blood sample collection. Subjects were asked to rate their feelings of thirst, hunger, bloatedness, refreshed, tiredness and head soreness at these times.
After the final blood and urine samples had been obtained, subjects were once again weighed nude, changed into shorts, T-shirt and shoes and a heart rate monitor was positioned. Subjects then performed an exercise capacity test on an electrically braked cycle ergometer. Subjects cycled for 5 min at an intensity equal to 70% \( \dot{V} {\text{O}}_2 \)peak after which the workload was immediately increased to 95% of \( \dot{V} {\text{O}}_2 \)peak and subjects cycled until voluntary exhaustion. During the test, heart rate was recorded, RPE was determined after 4½ min and at exhaustion. Verbal encouragement was given throughout the test in an attempt to ensure maximal effort. Following the performance test, subjects showered, towelled dry and a final nude body mass measurement was made.
The protocol was repeated on three further occasions, when the rehydration drink provided differed in sodium concentration.
Blood and urine analysis
For each blood sample, 2.5 ml was immediately added to a tube containing EDTA and the remainder was added to a plain tube. Serum was subsequently removed from the sample added to the plain tube after centrifugation. The EDTA-treated blood sample was used for measurement of haemoglobin concentration (cyanmethaemoglobin method) and packed cell volume (by spun haematocrit). The values so obtained were used to calculate changes in blood, red cell and plasma volumes using the equations described by Dill and Costill (1974): all calculations were made relative to the post-exercise values. Serum sodium and potassium concentrations were measured by flame photometry (Corning Clinical Flame Photometer 410C; Corning Ltd., Halstead, Essex, UK) and serum chloride concentration by coulometric titration (Jenway Chloride Meter; Jenway Ltd., Dunmow, Essex, UK). Serum osmolality was measured by freezing point depression (Gonotec Osmomat 030 Cryoscopic Osmometer; Gonotec, Berlin, Germany). Urine electrolyte concentrations and osmolality were measured by the same methods as used for the serum samples.
Statistical analysis
Data were first subjected to a Ryan–Joiner test to determine the normality of distribution. A 2 factor repeated measures ANOVA was used to determine if data differed significantly. If data were normally distributed, a one-way ANOVA combined with a Tukey post-hoc test was used to determine the positions of significant differences. If data were found not to be normally distributed, a Kruskal–Wallis test combined with Mann–Whitney tests was used. For data sets that were normally distributed, figures are presented as mean ± standard deviation; data not normally distributed are presented as median (range). Differences between and within trials were accepted as significant when P ≤ 0.05.
Results
As shown in Table 2, pre-exercise body mass was not different between trials. Body mass decreased by a similar amount during the dehydration process in each trial. Body mass decreased by 1.48 ± 0.20, 1.46 ± 0.21, 1.54 ± 0.23 and 1.53 ± 0.22 kg during the 1, 31, 40 and 50 trials (P > 0.8). This corresponded to a loss of 1.50 ± 0.21 kg or 1.98 ± 0.10% of initial body mass over all trials. Subjects were provided with a volume of the test drink equal to 150% of the body mass loss, resulting in a mean test drink volume of 2,253 ± 311 ml over all trials. As drink volume was determined by the mass loss in each trial during the dehydration process there was no difference in the volume of drink consumed between trials (P > 0.8).
Fluid balance
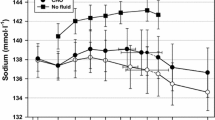
There was no difference between trials in the osmolality of the urine produced when subjects first arrived at the laboratory (P > 0.8) suggesting that subjects were in a similar state of hydration at the start of each trial. Initial urine osmolality was 454 ± 427, 480 ± 359, 367 ± 374 and 393 ± 389 mOsm/kg in the 1, 31, 40 and 50 trials, respectively. The median volume of urine produced at each collection point during each trial is shown in Fig. 1. As expected, there was no difference in the volume of urine produced at any time point prior to ingestion of the drink (P > 0.4). Urine volume was greatly increased following consumption of all drinks 1 h after the end of the drinking period (P < 0.01), the largest urine volume being produced at this time point on all trials. Two hours after the end of the drinking period urine volume was significantly lower than at the previous time point in all trials except the 1 mmol/l trial (P < 0.05). At this timepoint the urine volume produced on the 1 mmol/l trial was not significantly lower than at the 1-h post-ingestion time point (P > 0.15). This resulted in there being a significant difference between trials at this time point: a greater urine volume was produced on the 1 mmol/l trial than on the 40 and 50 mmol/l trials (P < 0.02). The volume of urine produced 3 h following the end of the drinking period was lower in all trials relative to the volume produced 2 h after drinking (P < 0.04). There was no difference in the volume of urine produced 4 h after the end of drinking in comparison to 3 h after drinking (P > 0.15), and there was no difference between trials at 3 h (P > 0.35) or 4 h (P > 0.95) after the end of the drinking period.
The cumulative volume of urine produced throughout each trial is shown in Fig. 2. Cumulative urine output tended to decrease as the sodium concentration of the test drink increased. Three and 4 h after the end of the rehydration period cumulative urine was greater on the 0 trial than on the 40 and 50 trials (P < 0.01).
By comparing the amount of fluid ingested during the rehydration period and the amount of urine produced in the following hours, the fraction of the test drink retained in the body at the end of the experimental period was calculated. Subjects retained more of the test drink as the sodium concentration of the drink increased: 39 ± 14, 50 ± 13, 60 ± 14 and 64 ± 11% of the test drink ingested was retained on the 1, 31, 40 and 50 trials, respectively. Significantly more fluid was retained on the 40 and 50 trials than on the 0 trial (P < 0.01).
The differing urine volumes produced in the trials resulted in differences in the net fluid balance on each trial. Figure 3 shows the net fluid balance on each trial, calculated by subtracting fluid losses due to sweating and urinary output and adding fluid gains through drinking. Assuming euhydration at the start of the trial, subjects were in significant negative fluid balance following the dehydration protocol and were then in significant positive fluid balance immediately after finishing drinking (P < 0.01). From this point on, there was a trend for less fluid to be lost as urine with increasing sodium concentration of the test drink and therefore subjects tended to be in a more negative fluid balance the lower the test drink sodium concentration. Two hours after finishing drinking, subjects were significantly closer to their pre-exercise fluid balance status on the 50 trial than the 0 trial (P < 0.02), and 3 and 4 h after drinking both the 40 and 50 mmol/l sodium drinks resulted in net fluid balance closer to the pre-exercise level than the 0 mmol/l sodium drink (P < 0.01). Four hours after finishing drinking, subjects were in negative mean net fluid balance on all trials but tended to be in greater net negative fluid balance as the sodium concentration of the test drink decreased: 650 ± 298, 394 ± 264, 223 ± 326 and 101 ± 268 ml negative balance on the 1, 31, 40 and 50 trials, respectively.
Net fluid balance calculated from sweat losses, fluid ingested and urine output throughout the experiment. Values are mean ± SD. a denotes a significant difference between the 1 and 50 mmol/l trials (P < 0.02); b denotes a significant difference between the 1 mmol/l trial and both the 40 and 50 mmol/l trials (P < 0.01)
Net sodium balance
Sweat sodium was not measured in the present study, and therefore it is impossible to determine net sodium balance. An estimation of the net sodium balance can be calculated by assuming a sweat sodium concentration in the middle of the normal range for all subjects (Maughan and Leiper 1995). Therefore net sodium balance at the end of the test period was estimated by assuming that sweat sodium concentration was 50 mmol/l for all subjects, and subtracting the amount of sodium lost in sweat and urine from the amount ingested in the rehydration beverage. Estimated net sodium balance 4 h following the end of the rehydration period was −93 ± 15, −35 ± 13, −21 ± 12 and 7 ± 6 mmol, in the 1, 31, 40 and 50 mmol/l trials, respectively.
The relationship between net water balance and estimated net sodium balance is shown in Fig. 4. Net fluid balance and estimated net sodium balance were shown to be significantly correlated (P < 0.01; r = 0.60).
Blood and serum measurements
Following 15 min of seated rest on arrival at the laboratory, haemoglobin concentration was 154 ± 13, 157 ± 14, 151 ± 12 and 155 ± 12 g/l, and haematocrit was 45 ± 2, 46 ± 3, 45 ± 3 and 45 ± 2% on the 1, 31, 40 and 50 mmol/l trials, respectively. There was no difference between trials in either initial haemoglobin concentration (P > 0.8) or haematocrit (P > 0.7). Changes in blood parameters were calculated relative to the post-exercise value. Blood volume decreased by 3.9 ± 5.0, 2.3 ± 2.3, 4.3 ± 1.7 and 3.5 ± 3.9% on the 1, 31, 40 and 50 mmol/l trials, respectively, following the dehydration protocol and this decrease was not different between trials (P > 0.6). Following the 1-h drinking period, blood volume increased on all trials (P < 0.01) relative to the immediate post-exercise value, which was accepted as the baseline. After drinking, blood volume was higher than at baseline on the 31, 40 and 50 mmol/l trials, but not on the 1 mmol/l trial, where blood volume was not significantly different from baseline at the end of the drinking period. From 1 h after finishing drinking onwards, blood volumes were not significantly different from baseline. Blood volume tended to be higher 1, 2 and 3 h following the end of drinking on the 50 mmol/l trial than on the other trials, and was significantly higher 2 h after drinking on the 50 mmol/l trial than on the 1 and 31 mmol/l trials (P < 0.03). At the end of the rehydration period, immediately prior to the exercise capacity test, blood volume was not different from baseline on any trial, and was not different between trials (P > 0.5).
Plasma volume changes followed a similar pattern to the blood volume changes (Fig. 5). Plasma volume tended to be higher after drinking on the 50 mmol/l trial than on the other trials, but there were no significant differences between trials (P > 0.08). After drinking plasma volume was higher in comparison to baseline on the 31, 40 and 50 mmol/l trials but not on the 1 mmol/l trial. The 1 mmol/l trial was not different from baseline at this time point. During the rest of the testing period the 1, 31 and 40 mmol/l trials were not significantly different from the baseline, but at 1 and 2 h after the end of drinking, the plasma volume on the 50 mmol/l trial remained higher than the baseline value. At the end of the rehydration period, immediately prior to the exercise capacity test, plasma volume on all trials was not different from baseline and there was no difference between trials (P > 0.7). Red cell volume decreased by a similar amount in all trials (P > 0.8). After drinking, red cell volume increased on all trials, but this increase was statistically significant only on the 40 (P < 0.02) and 50 (P < 0.05) mmol/l trials. Red cell volume was increased on the 50 mmol/l trial 2 h after finishing drinking, but there were no other differences between trials.
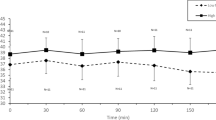
Serum osmolality tended to increase after exercise on all trials, but this was significant only on the 40 mmol/l trial (P < 0.02). Following rehydration, serum osmolality decreased (P < 0.05), by a mean value of 7 mOsm/kg over all trials. Serum osmolality tended to be lowest on the water trial at this time point, but no differences were located between trials (P > 0.08). Following drinking, serum osmolality tended to remain relatively stable in a similar manner to the plasma volume.
Exercise capacity test
There was no effect of drink sodium content on time to exhaustion in the exercise capacity test. Subjects exercised for 521 ± 86, 499 ± 86, 515 ± 84 and 532 ± 81 s on the 1, 31, 40 and 50 mmol/l trials, respectively. There was no difference between these values (P > 0.8). The time to exhaustion in the exercise capacity test was not affected by the order the trials were performed (P > 0.7). Time to exhaustion was 507 ± 77, 512 ± 88, 516 ± 60 and 533 ± 106 s in the first, second third and fourth trials performed, respectively.
The sodium chloride concentration of the rehydration drink had no effect on the subjects’ perception of effort as there was no difference between trials in the rating of perceived exertion after 4½ min of the test, nor at exhaustion (Table 2). Heart rate was measured throughout the performance test and was not different between trials during the section of the test at 70% of peak oxygen uptake (P > 0.75), after 1 min of the higher intensity 95% of peak oxygen uptake section, in the final minute before exhaustion, or at exhaustion.
Subjective feelings
There was no difference between trials at any time in any of the ratings of subjective feeling and no subject had any difficulty consuming any of the drinks.
Discussion
The fluid balance data in this study provide further evidence that replacement of sodium following exercise-induced dehydration is necessary for effective restoration of fluid balance. The cumulative volume of urine produced following rehydration was inversely related to the sodium concentration of the drink consumed. The fraction of fluid retained 4 h following the end of the drinking period was 39, 50, 60 and 64% of the volume of drink ingested on the 1, 31, 40 and 50 mmol/l trials, respectively. Previous studies have shown that the greater the sodium concentration of the rehydration beverage the greater the restoration of fluid balance (Maughan and Leiper 1995; Shirreffs et al. 1996; Shirreffs and Maughan 1998). The study of Shirreffs et al. (1996) compared drinks which were deemed low (23 mmol/l) or high (61 mmol/l) in sodium content and established that better rehydration resulted from consuming the high sodium drink. The studies of Maughan and Leiper (1995) and Shirreffs and Maughan (1998) clearly demonstrated that the sodium concentration of a rehydration beverage influenced net fluid balance 5.5 h after the end of the rehydration period. The study of Shirreffs and Maughan (1998) employed a dehydration/rehydration protocol similar to that of the present study except that the drinks provided had sodium concentrations of 1, 25, 50 and 102 mmol/l. The results obtained in that study were similar to those of the present study; 37 and 59% of the drink ingested was retained at 5.5 h after drinking on the 1 and 50 mmol/l trials, respectively, compared to 39 and 64% at 4 h after drinking on the 0 and 50 mmol/l trials in the present study.
While the results of the present study agree with much of the previous literature, not all studies report similar findings. Mitchell et al. (2000) reported that whole body fluid balance was the same at the end of their study period when subjects ingested water or drinks containing 25 or 50 mmol/l sodium in the period after exercise. Mitchell et al. (2000) employed a different rehydration protocol where drinks were provided over a 2 h and 30 min period and the final urine sample was collected only 30 min following the final bolus. It is likely that this was not sufficient time for any differences in urine production caused by the sodium content of these drinks to become apparent. In the study by Mitchell et al. (2000) the largest volume of urine was obtained at the final timepoint. As shown by the urine volume data in the present study, differences in urine production become apparent only after the rate of urine formation has peaked i.e. 2 h after finishing drinking.
As discussed earlier, one of the most important considerations when formulating a sports drink is to ensure that the drink is palatable. Whenever there is free access to drinks, individuals are likely to consume larger amounts of a fluid that they find more palatable (Hubbard et al. 1990; Maughan and Leiper 1993; Wemple et al. 1997; Passe 2001). This is an important consideration in situations where the extent of rehydration will depend to a large extent on the volume of fluid consumed. A sodium concentration of 50 mmol/l may reduce ad libitum fluid intake following exercise in comparison to a 25 mmol/l sodium drink (Wemple et al. 1997). In addition Passe (2001) quotes unpublished data that suggest that an increase in sodium concentration of ingested fluids from 40 to 60 mmol/l increases the perceived saltiness and decreases the overall liking for the drink. The sodium concentrations of drinks used in the present study may have been in the range where palatability becomes a critical factor. This is not an issue in an experimental situation where a fixed volume is prescribed and consumed, regardless of taste. This presents a problem, however, in a practical setting in that efficacy of the rehydration drink must balance the conflicting requirements for a high sodium concentration to aid retention of the ingested fluid and moderate sodium concentration in order to encourage voluntary consumption.
The present study shows that whole body rehydration is related to the sodium content of the beverage consumed following exercise, as shown by the net fluid balance at the end of the experimental period being closer to 0 (less negative) with increasing drink sodium concentration. The data demonstrate that a sodium concentration of 40 mmol/l or higher in drinks ingested after exercise-induced sweat loss will result in less urine formation and therefore a more effective restoration of fluid balance than when the same volume of a sodium-free drink is consumed.
Exercise capacity
The capacity test at the end of the rehydration period was employed to determine whether the changes in body water content that resulted from varying the sodium content of the drink had any effect on subsequent exercise capacity. In the present study, the more effective rehydration observed with the higher sodium drinks did not confer any advantage during the exercise capacity test. Subjects were not able to extend the cycling time to exhaustion 4 h after consuming the 40 or 50 mmol/l drinks in comparison to the 1 mmol/l drink despite significantly more effective rehydration with these drinks.
The effect of dehydration on exercise performance has been the subject of numerous reviews (Sawka and Pandolf 1990; Sawka 1992; Barr 1999; Coyle 2004). It is generally accepted that hypohydration decreases the capacity to perform aerobic exercise and that this effect may be exacerbated when exercise is performed in a hot environment (Buskirk et al. 1958; Saltin 1964a, b; Craig and Cummings 1966; Caldwell et al. 1984; Burge et al. 1993). In the present study, subjects were essentially euhydrated (net fluid balance −101 ml) in the 50 trial and in net negative fluid balance of 650 ml in the 0 trial. Therefore net fluid balance was reduced by 549 ml further in the 0 trial than in the 50 trial in comparison to the pre-dehydration value. This equates to a deficit in hydration of 0.7% of body mass. Though small, this volume is not insignificant in relation to the amount of fluid that athletes often consume during exercise.
In a review of the available data, Coyle (2004) discussed the interaction of hydration status and environmental conditions on exercise performance. He concluded that hypohydration of 2% or greater may be tolerated without significant effects on performance when environmental conditions are moderate (<25°C) (Robinson et al. 1995; McConell et al. 1997; Backx et al. 2003), but that hypohydration of approximately 2% or more of body mass impairs performance and increases the risk of heat injury when environmental temperatures are high (>30°C) (Walsh et al. 1994; Below et al. 1995; Yoshida et al. 2002). The critical point at which a performance reduction occurs is likely to be influenced by many factors, including the rate at which hypohydration is induced, the subject’s core temperature and the exercise mode: there may be some advantage of a small reduction in body mass when this must be supported or carried, as in running (Sawka and Noakes 2007).
The magnitude of the increase in core temperature and heart rate (McConell et al. 1997) and the decline in stroke volume (Montain and Coyle 1992) noted during exercise has been shown to be related to the degree of dehydration incurred during exercise. Several studies have used an exercise performance test subsequent to exercise-induced dehydration to study the effects of dehydration on exercise performance, but the results are somewhat conflicting. Some of the variation in the results is probably explained by the fact that most studies of this type (including the present study) use change in body mass as a marker of changes in hydration status. Change in body mass is a convenient and non-invasive method of determining changes in hydration status at the level of the whole body, but may be rather simplistic when considering the effective body water loss, as recently discussed by Maughan et al. (2007). For example, in a rehydration study, at the time when body mass is measured, some ingested fluid may still be in the gut and therefore effectively outside the body, whilst the rest of the fluid will be distributed between the plasma, the interstitium and the intracellular compartment. This study did not allow determination of the distribution of fluid among these various compartments, but physiological responses will be determined to some extent by the distribution of the ingested fluid throughout the body. Therefore some of the variability in the results of studies investigating physiological responses to rehydration may arise from differences in the distribution of ingested drinks throughout the fluid compartments of the body, and this cannot be detected by change in body mass measurements.
Costill and Sparks (1973) investigated rehydration after exercise that had resulted in dehydration of 4% body mass. Heart rates were found to be normalised after only 62% of the fluid had been replaced and before plasma volume had returned to pre-dehydration levels. Mitchell et al. (2000) also found that cardiovascular function was restored with rehydration despite fluid and plasma volume not being completely restored. In contrast, Nielsen et al. (1986) reported that performance in a supramaximal exercise test (105% VO2 max) was reduced by 20% after rehydration following a 3% exercise-induced reduction in body mass. Heart rates were also shown to be higher in a submaximal exercise test following rehydration than before dehydration despite the fact that plasma volume had returned to levels higher than before dehydration. In addition, Heaps et al. (1994) found that heart rate during exercise after 65% rehydration was increased despite the fact that blood volume had been fully restored.
The time to complete a set amount of endurance exercise is known to be related to the magnitude of the decrease in plasma volume caused by previous dehydration (Burge et al. 1993). Subjects in that study were dehydrated by 5% of body weight through mild exercise in the heat and fluid restriction and then partially rehydrated by consuming 1.5 l of water. Subjects then performed a fixed amount of exercise as fast as possible: they took significantly longer to complete the exercise after the dehydration/rehydration protocol than after a euhydration control. The increase in time to complete the exercise task was shown to be related to the magnitude of the deficit in plasma volume remaining following the partial rehydration. In the present study, blood and plasma volume tended to increase in proportion to the concentration of sodium in the rehydration drink. At the final blood sample immediately prior to the exercise capacity test, blood and plasma volume had been restored to levels not different from baseline on all trials. Heart rate was not different between trials during the submaximal portion of the performance test, during the higher intensity period, or at exhaustion.
The exercise capacity test in the present study was performed in moderate environmental temperatures (<25°C). Coyle (2004) asserted that in moderate environmental temperatures hypohydration of greater than 2% of body mass can be tolerated without significant impairment of performance, though the emphasis of his review was on more prolonged exercise. In the present study, the largest deficit in fluid balance was 650 ± 298 ml or 0.9% of body mass. The difference in hydration status between the 1 mmol/l drink, which was the least effective at rehydrating, and the 50 mmol/l drink, which was the most effective, was 549 ml or 0.7% of body mass. The difference in hydration status between the trials in the present study did not result in an effect on exercise performance. Burge et al. (1993) reported that impaired exercise performance following partial rehydration after exercise-induced dehydration may relate to a decreased plasma volume. In the present study blood and plasma volume were restored to pre-hypohydration levels on all trials. It is perhaps not surprising, therefore, that there was no difference in exercise time to exhaustion in the exercise performance test due to the relatively small differences in hydration status and the complete restoration of blood and plasma volume.
In conclusion, manipulation of the sodium chloride content of drinks in a range likely to be used in a practical setting can allow significantly better rehydration following exercise-induced dehydration. The addition of 40 or 50 mmol/l of sodium chloride to a beverage reduces subsequent urine output and thereby provides more effective rehydration than a drink containing no sodium. This did not, however, result in improved time to exhaustion in the exercise test performed 4 h after the end of the rehydration period.
References
Backx K, van Someren KA, Palmer GS (2003) One hour cycling performance is not affected by ingested fluid volume. Int J Sport Nutr 13:333–342
Barr SI (1999) Effects of dehydration on exercise performance. Can J Appl Physiol 24:164–172
Below PR, Mora-Rodriguez R, Gonzalez-Alonso J, Coyle EF (1995) Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc 27:200–210
Burge CM, Carey MF, Payne WR (1993) Rowing performance, fluid balance, and metabolic function following dehydration and rehydration. Med Sci Sports Exerc 25:1358–1364
Buskirk ER, Iampietro PF, Bass DE (1958) Work performance after dehydration: effects of physical conditioning and heat acclimatization. J Appl Physiol 12:189–194
Caldwell JE, Ahonen E, Nousiainen U (1984) Differential effects of sauna-, diuretic-, and exercise-induced hypohydration. J Appl Physiol 57:1018–1023
Costill DL, Sparks KE (1973) Rapid fluid replacement following thermal dehydration. J Appl Physiol 34:299–303
Craig EN, Cummings EG (1966) Dehydration and muscular work. J Appl Physiol 21:670–674
Coyle EF (2004) Fluid and fuel intake during exercise. J Sports Sci 22:39–55
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Gonzalez-Alonso J, Heaps CL, Coyle EF (1992) Rehydration after exercise with common beverages and water. Int J Sports Med 13:399–406
Greenleaf JE (1992) Problem: thirst, drinking behavior, and involuntary dehydration. Med Sci Sports Exerc 24:645–656
Greenleaf JE, Sargent F (1965) Voluntary dehydration in man. J Appl Physiol 20:719–724
Heaps CL, Gonzalez-Alonso J, Coyle EF (1994) Hypohydration causes cardiovascular drift without reducing blood volume. Int J Sports Med 15:74–79
Hubbard RW, Szlyk PC, Armstrong LE (1990) Influence of thirst and fluid palatability on fluid ingestion during exercise. In: Lamb DR, Gisolfi CV (eds) Perspectives in exercise science and sports medicine. Benchmark Press, Carmel, pp 39–95
Lambert CP, Costill DL, McConell GK, Benedict MA, Lambert GP, Robergs RA, Fink WJ (1992) Fluid replacement after dehydration: influence of beverage carbonation and carbohydrate content. Int J Sports Med 13:285–292
Maughan RJ, Leiper JB (1993) Post exercise rehydration in man: effects of voluntary intake of four different beverages. Med Sci Sports Exerc 25:1358–1364
Maughan RJ, Leiper JB (1995) Sodium intake and post-exercise rehydration in man. Eur J Appl Physiol 71:311–319
Maughan RJ, Owen JH, Shirreffs SM, Leiper JB (1994) Post-exercise rehydration in man: effects of electrolyte addition to ingested fluids. Eur J of Appl Physiol 69:209–215
Maughan RJ, Leiper JB, Shirreffs SM (1996) Restoration of fluid balance after exercise-induced dehydration: effects of food and fluid intake. Eur J Appl Physiol 73:317–325
Maughan RJ, Merson SJ, Broad NP, Shirreffs SM (2004) Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab 14:333–346
Maughan RJ, Shirreffs SM, Leiper JB (2007) Errors in the estimation of hydration status from changes in body mass. J Sports Sci 25:797–804
McConell GK, Burge CM, Skinner SL, Hargreaves M (1997) Influence of ingested fluid volume on physiological responses during prolonged exercise. Acta Physiol Scand 160:149–156
Mitchell JB, Grandjean PW, Pizza FX, Starling RD, Holtz RW (1994) The effect of volume ingested on rehydration and gastric emptying following exercise-induced dehydration. Med Sci Sports Exerc 26:1135–1143
Mitchell JB, Phillips MD, Mercer SP, Baylies HL, Pizza FX (2000) Postexercise rehydration: effect of Na(+) and volume on restoration of fluid spaces and cardiovascular function. J Appl Physiol 89:1302–1309
Montain SJ, Coyle EF (1992) Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol 73:1340–1350
Nielsen B, Sjogaard G, Ugelvig J, Knudsen B, Dohlmann B (1986) Fluid balance in exercise dehydration and rehydration with different glucose-electrolyte drinks. Eur J Appl Physiol 55:318–325
Passe DH (2001) Physiological and psychological determinants of fluid intake. In: Maughan RJ, Murray R (eds) Sports drinks: basic science and practical aspects. CRC Press, Boca Raton, pp 45–87
Passe DH, Horn M, Murray R. (2000) Impact of beverage acceptability on fluid intake during exercise. Appetite 35:219–229
Pitts GC, Johnson RE, Consolazio FC (1944) Work in the heat as affected by intake of water, salt and glucose. Am J Physiol 142:253–259
Robinson TA, Hawley JA, Palmer GS, Wilson GR, Gray DA, Noakes TD, Dennis SC (1995) Water ingestion does not improve 1-h cycling performance in moderate ambient temperatures. Eur J Appl Physiol Occup Physiol 71:153–160
Saltin B (1964a) Aerobic and anaerobic work capacity after dehydration. J Appl Physiol 19:1114–1118
Saltin B (1964b) Circulatory response to submaximal and maximal exercise after thermal dehydration. J Appl Physiol 19:1125–1132
Sawka MN, Pandolf KB (1990) Effects of body water loss on exercise performance and physiological functions. In: Gisolf CV, Lamb DR (eds) Perspectives in exercise science and sports medicine. Benchmark Press, Indianapolis, pp 1–38
Sawka MN (1992) Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc 24:657–670
Sawka MN, Noakes TD (2007) Does dehydration impair exercise performance? Med Sci Sports Exerc 39:1209–1217
Shirreffs SM, Maughan RJ (1997a) Restoration of fluid balance after exercise-induced dehydration: effects of alcohol consumption. J Appl Physiol 83:1152–1158
Shirreffs SM, Maughan RJ (1997b) Whole body sweat collection in man: an improved method with some preliminary data on electrolyte composition. J Appl Physiol 82:336–341
Shirreffs SM, Maughan RJ (1998) Volume repletion after exercise-induced volume depletion in humans: replacement of water and sodium losses. Am J Physiol 274:F868–F875
Shirreffs SM, Taylor AJ, Leiper JB, Maughan RJ (1996) Post-exercise rehydration in man: effects of volume consumed and drink sodium content. Med Sci Sports Exerc 28:1260–1271
Shirreffs SM, Armstrong LE, Cheuvront SN (2004) Fluid and electrolyte needs for preparation and recovery from training and competition. J Sports Sci 22:57–63
Shirreffs SM, Watson P, Maughan RJ (2007) Milk as an effective rehydration drink. Br J Nutr 98:173–180
Takamata A, Mack GW, Gillen CM, Nadel ER. (1994) Sodium appetite, thirst, and body fluid regulation in humans during rehydration without sodium replacement. Am J Physiol 266:R1493–R1502
Walsh RM, Noakes TD, Hawley JA, Dennis SC (1994) Impaired high-intensity cycling performance time at low levels of dehydration. Int J Sports Med 15:392–398
Wemple RD, Morocco TS, Mack GW (1997) Influence of sodium replacement on fluid ingestion following exercise-induced dehydration. Int J Sport Nutr 7:104–116
Wong SH, Williams C, Adams N (2000) Effects of ingesting a large volume of carbohydrate-electrolyte solution on rehydration during recovery and subsequent exercise capacity. Int J Sport Nutr 10:375–393
Yoshida T, Takanishi T, Nakai S, Yorimoto A, Morimoto T. (2002) The critical level of water deficit causing a decrease in human exercise performance: a practical field study. Eur J Appl Physiol 87:529–534
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merson, S.J., Maughan, R.J. & Shirreffs, S.M. Rehydration with drinks differing in sodium concentration and recovery from moderate exercise-induced hypohydration in man. Eur J Appl Physiol 103, 585–594 (2008). https://doi.org/10.1007/s00421-008-0748-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0748-0