Abstract
The purpose of the present study was to test the hypothesis that the magnitude of hormonal concentration alterations during a prolonged military field exercise with constant energy intake (EI) is influenced by changes in energy deficit (ED) induced by varying the exercise intensity. Basal serum hormone concentrations were measured in a group of healthy young male volunteers (n = 7) during a 20-day field exercise. During the first week of the exercise, the average ED was 4,000 kcal/day (P-I), in the second week only 450 kcal/day (P-II), and in the last week 1,000 kcal/day (P-III). During the first 5 days of the field exercise, significant increases in cortisol (COR, +32%) and growth hormone (GH, +616%) concentrations were observed, while insulin (INS, −70%), total testosterone (TES, −27%), free testosterone (TESfree, −26%) decreased. However, after these initial responses, COR and GH returned to the pre-exercise level by the beginning of P-II. Also TES and TESfree recovered to the pre-exercise level by the beginning of P-III, and INS by the end of P-III. The concentration of TES (+29%) increased above the pre-exercise level by the beginning of P-III. Serum thyroxin (T4) concentration was significantly lesser (−12%) and urine urea concentration significantly higher (+78%) after the field exercise than before it. Therefore, it can be concluded that the lower levels of ED in the second and third phase (ED <1,000 kcal/day) allowed recovery of hormonal changes observed in the first phase with ED much greater than 1000 kcal/day.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In military operations soldiers are often exposed to various stressors, such as prolonged and strenuous physical exercise, energy and fluid deficiency, extreme ambient temperature, and sleep deprivation (Nindl et al. 2002; Opstad 1995). Heavy military field exercises lasting less than 1 week have been shown to induce increases in basal concentrations of circulating cortisol (COR) (Opstad 1991, 1992, 1994) and GH (Opstad 1994), and decreases in serum insulin (INS) (Opstad 1991), testosterone (TES) (Gomes-Merino et al. 2003; Opstad 1992, 1994), thyroid hormone levels (Opstad 1992, 1994) and insulin-like growth factor (Nindl et al. 2003). Similar hormonal alterations for COR, TES and thyroid hormone levels have also been found following stressful military survival training (Morgan et al. 2000).
It has also been shown by Friedl et al. (2000) that during a special 8-week ranger course, the amount of energy deficit (ED), regulated by energy intake (EI), is related to many of these hormonal changes. In that study, the mean total energy expenditure (EE) was slightly over 4,000 kcal/day, and the ED in two groups of subjects was either 1,000 or 1,200 kcal/day. The smaller ED (1,000 kcal/day) was clearly associated with an attenuated decline in thyroid hormone (T3 and T4). In addition, periods of re-feeding (high EI) produced a prompt recovery in T3, TES, luteinizing hormone (LH) and GH (Friedl et al. 2000). However, to the best of our knowledge, the influence of varying ED with constant EI on hormonal responses during prolonged military field exercise has not been reported.
The main purpose of the present study was to investigate the hormonal alterations during a prolonged (20 days) military field course with continuous ED. In particular, we studied whether changes in the degree of ED are reflected in hormonal changes when the EI is kept constant.
Methods
Subjects
Ten healthy male soldiers volunteered as subjects for the present study. Three of them could not complete the entire field exercise due to leg injuries and illness. The data from the remaining seven volunteers (age 24 ± 2 years, height 1.80 ± 0.04 m, body mass 76.2 ± 10.1 kg, body fat 7.4 ± 3.1%) are presented in this paper. All the subjects were fully informed of the procedures and possible risks of the experiment, and they gave their written agreement to participate in this study, which was part of their military training. The Ethical Committee at the University of Jyväskylä and the Surgeon General of the Finnish Defense Forces approved this study.
Procedure and measurements
Four weeks before the field exercise, all the subjects passed a thorough health care examination and performed a maximal exercise test on a motorized treadmill. The entire military training period lasted 26 days, of which 20 days constituted the field exercise. It was divided into three phases. During the first 7 days (Phase I, P-I, Very Heavy), the subjects walked 20–25 km per day in the forest carrying their full combat gear of 49.8 ± 4.7 kg. During the next 6 days (P-II, Easy), the subjects walked only 5–10 km per day with a load of 20–25 kg. The last week, days 14–20 (P-III, Heavy), was again more demanding, and the subjects walked approximately 15 km per day carrying 30 kg of equipment. During the whole field exercise, the amount of sleep averaged 6 h per night according to the sleeping diaries of the subjects.
Blood samples were drawn from the ulnar vein and fingertip one day before the start of the field exercise (PRE), and on days 5 (P-Imid), 8 (P-IIpre), 14 (P-IIIpre), 16 (P-IIImid), and 21 (P-IIIpost). The blood samples were always taken at 7 o’clock in the morning after an overnight fast. In addition, the subjects completed daily food diaries, and the muscle soreness (MS) of leg muscles was evaluated by a questionnaire. The subjects completed this questionnaire daily, with the perceived leg soreness ranging on a five-point scale from 0 (no pain) to 5 (very painful). Heart rate (HR) was continuously recorded with a portable heart rate monitor (Polar S610, Polar Electro, Kempele, Finland). Body height and mass as well as skinfold thickness (Harpenden Skinfold Caliber, British Indicators Ltd., London, UK) and body fat % (Jackson and Pollock 1985) were measured just before and within 24 h after the field exercise, and fat mass and lean body mass (LBM) were calculated. In addition, the body mass was also measured on days 8 and 14 (P-IIpre and P-IIIpre).
Analysis
Determination of VO2max, HRmax and energy expenditure
Maximal oxygen uptake (VO2max) and maximal heart rate (HRmax) were measured during a progressive running test on a motor-driven treadmill. Metabolic measurements were made by indirect calorimetry using a Sensor Medics 2900 computerized metabolic system (Sensor Medics, Yorba Linda, California, USA). Before each test, the O2 and CO2 analyzers were calibrated using gases of known concentrations, and the flow meter was calibrated using a 3-L syringe.
Before the maximal exercise test, the subject warmed up on the treadmill, and the appropriate starting running speed was individually determined. During the test, the treadmill speed or grade was increased after every 3 min, and a fingertip blood sample was taken at the end of each load and analyzed for blood lactate concentration using an automated lactate analyzer (Eppendorff 6600, Hamburg, Germany). VO2max was determined from the highest 1-min average of oxygen uptake. HRmax was determined from the highest 5-s average of the HR recording with a portable heart rate monitor (Polar S610, Polar Electro, Kempele, Finland).
During the 20-day field exercise, HR was continuously measured with a Polar S610 portable heart rate monitor. The estimated EE was determined from the “OwnCal” software (Polar Electro Inc., Kempele, Finland), which is based on subject’s data (age, height, weight, gender, VO2max and HRmax) and exercise HR. Recently, Crouter et al. (2004) have demonstrated that the mean error (± SD) of energy expenditure estimation in males, utilizing the present method, is 4 ± 10% when HR is higher than 90 bpm. To obtain the total energy expenditure, the software was used to estimate the EE only when HR was more than 90 bpm, and for the rest of the time (about 8 h/day), the EE was calculated using the body mass, stature and age of the subjects. Finally, these two EE values were combined to get the total daily EE for each subject.
Energy intake
The daily energy intake rations were kept constant throughout the field exercise. However, the subjects could themselves decide how they consumed their rations during each phase of the field exercise. Different ingredients were calculated from the personal food diaries according to the specifications of food manufacturers. In addition, special nutrition software (Micro Nutrica 3.1, Social Insurance Institution, Turku, Finland) was utilized in the analyses of food articles with no information on ingredients from the manufacturer. The total energy intake as well as the distribution of carbohydrates, fats and proteins was then calculated.
Blood analyses
The percentage change in plasma volume (PV) was calculated from changes in hemoglobin and hematocrit according to the method of Dill and Costill (1974). Blood glucose (GLU) was analyzed by Ascensia Elite XL-analyzer (Bayer; Germany), of which intra-assay variance was 3.5%. Due to technical difficulties, blood GLU could not be measured after day 15. Blood lactate (LA) was analyzed from fingertip blood samples (Lactate Pro, Arkray, Japan). Its coefficient of variance (CV) was 3%. Serum creatine kinase activity (CK) was analyzed photometrically (Hitachi 912, Roche Diagnostics GmbH, Mannheim, Germany) using a commercial test kit (CK NAC, Roche Diagnostics GmbH, Mannheim, Germany). Its intra-assay variance was <6.1%. Urea (U) was analyzed enzymatically (Biochemica Boehringer GmbH, Mannheim, Germany) with intra-assay CV of 3.5%.
Serum total testosterone (TEStot), cortisol (COR), and thyroxine (T4) were analyzed by Vitros ECi (Ortho Clinal Diagnostic, NY, USA) using respective commercial luminoimmunoassays (Ortho-Clinical Diagnostic, Amersham, UK). The sensitivity and intra-assay coefficient of variance for these assays were 0.15 ng/dl and <6.9%, 0.2 μg/dl and 8.4%, and 1.3 pg/ml and 3.3%, respectively. Follicle-stimulating hormone (FSH), luteinizing hormone (LH), and insulin (INS) were analyzed by Immulite 2000 (Diagnostics Products Corporation, Los Angeles, USA). The sensitivity and intra-assay variance for these assays were 0.1 mlU/ml and <5.9%, 0.1 mlU/ml and 5.8%, and 2 μlU/ml and <8.0%, respectively. The growth hormone (GH) was analyzed (1235 Wallac™AutoDelfia, Wallac Oy, Turku, Finland) using time-resolved fluoroimmunoassay (AutoDelfia®hGH, Wallac Oy, Turku, Finland). The sensitivity and intra-assay variance for this assay was up to 0.01 μg/dl and <6.0%. Serum free testosterone (TESfree) was analyzed (1277 GammaMaster GammaCounter, Wallac Oy, Turku, Finland) by radioimmunoassay (Coat-A-Count®Free Testosterone, Diagnostic Product Corporation, Los Angeles, CA, USA). The sensitivity and intra-assay variance for this assay was 0.18 pg/ml and <6.2%.
Statistical analysis
Repeated measures ANOVA and paired samples T-test, or Friedman’s and Wilcoxon’s tests, were used in the statistical analyses depending on the normality of data distribution. Pearson’s and Spearman’s correlation coefficients were calculated accordingly between the changes in several variables. All data are presented as mean ± SD.
Results
Anthropometry
The body mass of the subjects was 76.2 ± 10.1 kg before and 72.0 ± 9.7 kg after the field exercise (P-IIIpost, P < 0.001). The LBM decreased from 70.4 ± 8.1 to 67.9 ± 8.3 kg (P < 0.001) and the body fat% from 7.4 ± 3.1 to 5.5 ± 2.0% (P < 0.01) during the period of 20 days. Thus, the body mass decline of 4.2 ± 0.8 kg (5.6 ± 0.9%) consisted of both fat mass and fat-free tissue loss (1.8 ± 1.1 and 2.4 ± 0.8 kg, respectively)
Energy balance and blood glucose
On average, the total daily EI during the 20-day field exercise was 2,938 ± 454 kcal/day (consisting of carbohydrates 450 ± 75 g/day, fat 100 ± 24 g/day and protein 76 ± 14 g/day). No difference in the total EI between the different phases of the field exercise was observed. EE was, however, significantly different in the three phases of the field exercise (Fig. 1). Therefore, the energy deficits were approximately 4,000, 450 and 1,000 kcal/day in phases PI, PII and PIII, respectively. Blood GLU concentration was significantly lower at the end of P-I when compared to the value of day 1 (Fig. 2).
Hormonal responses
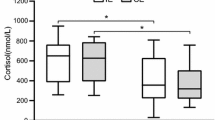
During the first 5 five days of the field exercise, COR and GH concentrations increased and INS concentration decreased. After theses initial responses, COR and GH returned to the pre-exercise level at the beginning of P-II, and INS at the end of P-III (Fig. 3).
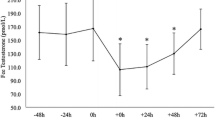
During the first 5 days, TES, TESfree and LH decreased, while FSH remained unchanged. All these hormones had returned to the pre-exercise level by the beginning of P-III. The concentrations of FSH and TES tended to increase above the pre-exercise level by the last part of the 20-day training period (Fig. 4).
Serum T4 concentration was significantly lower and urine urea concentration significantly higher after the field exercise than before it. No changes in T4 and urea values were observed during the first part of the field exercise (Fig. 5). The change in PV was 8.6 ± 7.3% (P < 0.05) on day 5, −0.8 ± 6.3% at the beginning of P-II, −6.6 ± 8.5% at the end of P-II, −2.0 ± 7.3% on day 16 and 3.4 ± 5.3% at the end of P-III.
CK-activity and DOMS
The serum CK activity increased significantly during the first 5 days and returned to the initial level in P-III. At the same time, MS also increased strongly, then decreased in P-II and increased again in P-III (Fig. 6).
Discussion
As expected, during the first days of the field exercise, with very high EE, significant increases in COR and GH concentrations were observed, while TES, TESfree, and LH levels decreased. However, the primary finding of the study was that the easy period in the middle of the field exercise not only attenuated the hormonal concentration changes, but actually recovered many of the hormone concentrations to the pre-exercise level or higher. Furthermore, no significant changes in these hormone concentrations were observed during the last part of the exercise, despite the relatively high EE. Only the T4 concentration behaved as expected, having its lowest value at the end of the 20-day field exercise.
According to Friedl and Hoyt (1997), the sustained EE of soldiers in the field is typically about 4,000 kcal/day, as measured by the highly reliable doubly labeled water (DLW) method. Among Norwegian cadets, however, the estimated mean EE based on DLW has been reported to be as high as 6,360 kcal/day during a 7-day military operation (Hoyt et al. 2006). Therefore, in the present study, the estimated mean EE of 7,011 kcal/day in P-I was clearly higher, and in P-II lower (3,217 kcal/day) than in typical field training exercises, whereas in P-III it was on an average field training level (4,074 kcal/day). The average total daily EI during the field exercise in the present study (2,938 kcal/day) represents well typical field rations (Friedl and Hoyt 1997). However, as the subjects clearly tended to save their daily rations during the first two days in P-I, the EI increased towards the end of this phase. Nevertheless, no significant differences in total EI between the different phases of the field exercise were observed, as planned.
In general, it may be assumed that the estimated EE in the present study represents the amount of energy consumed during the field exercise quite well. The mean error of EE estimation in males, when utilizing the present HR-based method, has been shown to be as low as 4% (±10%) in exercises with HR higher than 90 bpm (Crouter et al. 2004). In the present study, when HR was below 90 bpm, the EE was calculated using the body mass, stature and age of the subjects. These two EE values were then combined to get the total daily EE. Finally, the total estimated ED was about 20,000 kcal during the 20-day field exercise based on the fact that fat and fat free mass provided energy of 9.5 kcal/g fat and 1.1 kcal/g, respectively.
It has been shown earlier that during a 5-day ranger field exercise, the thyroid function is strongly affected by prolonged physical exercise and calorie deficiency (Opstad 1995). In that study, the serum levels of T3 and T4 showed a biphasic pattern during the exercise. Initially, there was increased secretion concomitant with increased deiodination of T4 to T3, mainly due to physical exercise. Then, as the field exercise lasted for several days without a sufficient food supply, the thyroid secretion and the peripheral conversion of T4 to T3 decreased significantly (Opstad 1995). It is well known that when fasting subjects, with decreased serum T3, are given exogenous T3 replacement, muscle protein catabolism is enhanced, as evidenced by increased urinary urea, ammonia and 3-methyhistidine secretion. Therefore, the low T3 levels associated with ED may be beneficial in sparing muscle protein (Griffin 2004).
As already stated, the T4 concentration in the present study decreased towards the end of the 20-day field exercise. This is well in line with earlier findings, where serum thyroid hormone levels have decreased during different types of military field exercises with variable duration and degree of ED (e.g. Friedl et al. 2000; Morgan et al. 2000; Nindl et al. 1997). In the present study, the change in mean serum T4 concentration was most pronounced during the first 5 days (−9%), as hypothesized, but this decline did not reach the level of statistical significance. Thereafter, the T4 level decrease was less significant, being 12% less at the end of the field exercise than before it (P < 0.05). Although the T3 level was not measured, the decrease in T4 may be seen as a consequence of the continuous ED in the present study. At the end of the field exercise, the urine urea concentration also elevated sharply, suggesting that there was some muscle protein catabolism or more likely a sudden influx of food increased the urea excretion.
In previous studies, heavy military field exercises lasting less than 1 week have been shown to induce increases in the basal concentrations of circulating COR (Opstad 1991; 1992, 1994) and GH (Opstad 1991, 1994) and decreases in the level of INS (Opstad 1991). This was also the case in the present study. The changes in COR, GH, and INS concentrations were marked during the first five days of the field exercise. The increases in COR (+32%) and GH (+616%) levels on day 5 may reflect an increased need to catabolise alternate body energy sources and preserve glycogen. Also the decrease in INS level (−70%) can potentially contribute to blood GLU preservation. During this time period, the GLU level was maintained very well. Thereafter, the GLU concentration decreased significantly from the pre-exercise level of 4.5 ± 0.5 mmol·l−1 to 3.9 ± 0.4 mmol·l−1 in the morning of day 7. It is unfortunate that, due to logistic problems, no blood samples for hormone analyses could be obtained at that time point. Nevertheless, it could be assumed that the concentrations of COR and GH were still elevated, and the concentration of INS was reduced on day 7. However, in the morning of day 8, GLU, COR and GH concentrations had all recovered to the pre-exercise level. This is probably due to the fact that the subjects arrived at the destination of P-I on day 7 and consumed the food rations that they had saved during the earlier days, knowing that P-II would be easier in terms of physical exercise. Therefore, the EI on day 7 was actually as high as 3300 kcal. Nevertheless, in the morning of day 8, the level of INS still remained significantly lower than before the exercise.
In the present study, the concentrations of TES (−27%), TESfree (−26%) and LH (−46%) decreased during the first 5 days. This was an expected finding in light of several earlier studies (Gomes-Merino et al. 2003; Opstad 1992, 1994). However, although both TES and LH levels have been shown to be associated with the amount of ED during an 8-week ranger course (Friedl et al. 2000), it was unanticipated that the TES level was actually higher (29%) after P-II, with a low ED, and even higher after P-III (41%), with a somewhat higher ED, than before the exercise. Attenuated testosterone levels have also been reported in wrestlers with dietary restriction, particularly in those individuals with very low body fat content (Roemmich and Sinning 1997). In the present study, the percentage body fat of the subjects was already very low in the beginning (7.4 ± 3.1%) and decreased thereafter to 5.5 ± 2.0%, but the TES level increased at the same time. As the rather moderate plasma volume changes cannot explain this increase in TES level, it is probable that TES secretion and/or its metabolic clearance were influenced by the easy P-II. On the other, most of the present hormones exhibit circadian fluctuations and some are extremely episodic. Although the blood samples were taken at the same in every condition, the results of these one-time point hormonal measures may lead to misinterpretations. Namely, the recent study of Nindl et al. (2006) has clearly shown a pulsatile release of some hormones, of which integral in time may elicit optimal function at the cellular level.
The increase in serum CK activity presumably reflects damage to the exercising muscles, resulting in a loss of cell membrane integrity. In the present study, serum CK activity reached its highest level on day 5 (1,309 ± 377 U/l) and decreased slowly thereafter to the pre-exercise level on day 16. Perceived muscle soreness was also highest in P-I. This result is in good agreement with the study of Ross et al. (1983), who found that in army personnel undergoing a 24-day intensive training course, serum CK was higher in the early than in the latter part of the programme. In a 1,600-km ultramarathon run, lasting 11–16 days, serum CK activity was also found to be higher in the early part of the race (day 4) than after it (Fallon et al. 1999). The authors speculated that the explanation for the early peak and subsequent decrease in CK was multifactorial. A progressive decrease in the daily distance run was likely to be a major factor in decreased CK production, but a change in running technique towards less eccentric muscle contractions may also be important (Fallon et al. 1999).
It has been shown that the muscles adapt very quickly to eccentric muscle actions. Even one eccentric exercise may induce a protective effect that protects the muscles from further damage in similar exercise. This so-called repeated bout effect can last for several months (Clarkson et al. 1992; McHugh et al. 1999; McHugh 2003) In addition to these explanations, increased clearance of CK as an adaptation to continued exercise might explain the decreasing CK during the latter part of the training course in the present study (Noakes 1987). It is of interest that although the CK activity was at the pre-exercise level in P-III, the subjects still reported relatively high muscle soreness ratings. However, the muscle soreness may be different in nature from the so-called delayed onset muscle soreness related to muscle damage.
In summary, the primary finding of the study was that the easy period in the middle of the 20-day field exercise was very beneficial for many of the hormonal concentrations, by actually allowing them to recover to the pre-exercise level or higher, despite the continuous energy deficit. Furthermore, the easy period also seemed to help the body to adapt to the consequent heavy exercise period, so that the hormone concentrations remained very stable during the last part of the exercise, despite the relatively high-energy deficit.
References
Clarkson PM, Nosaka K, Braun B (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24:512–520
Crouter SE, Albright C, Bassett JR (2004) Accuracy of Polar S410 Heart Rate Monitor to Estimate Energy Cost of Exercise. Med Sci Sports Exerc 36:1433–1439
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Fallon KE, Sivyer G, Sivyer K, Dare A (1999) The biochemistry of runners in a 1,600 km ultramarathon. Br J Sports Med 33:264–269
Friedl KE, Hoyt RW (1997) Development and biomedical testing of military operational rations. Ann Rev Nutr 17:51–75
Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW (2000) Endocrine markers of semistarvation in healthy men in a multistressor environment. J Appl Physiol 88:1820–1830
Gomes-Merino D, Chennaoui M, Burnatt P, Drogou C, Guezennec CY (2003) Immune and hormonal changes following intense military training. Mil Med 186:1034
Hoyt RW, Opstad PK, Haugen A-H, DeLany JP, Cymerman A, Friedl KE (2006) Negative energy balance in male and female rangers: effects of 7 d of sustained exercise and food deprivation. Am J Clin Nutr 83:1068–1075
Jackson AS, Pollock ML (1985) Practical assessment of body composition. Phys Sportsmed 13:76–90
Griffin JE (2004) The Thyroid. In: Griffin JE, Ojeda SR (eds) Textbook of endocrine physiology. Oxford University Press, New York p 302
McHugh MP (2003) Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports 13:88–97
McHugh MP, Connolly DAJ, Eston RG, Gleim GW (1999) Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med 27:157–170
Morgan III CA, Wang S, Mason J et al. (2000) Hormone profiles in humans experiencing military survival training. Biol Psychiatry 47:891–901
Nindl BC, Friedl KE, Frykman PN, Marchitelli LJ, Shippee RL, Patton JF (1997) Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med 18:317–24
Nindl BC, Leone CD, Tharion WJ, Johnson RF, Castellani JW, Patton JF, Montain SJ (2002) Physical performance responses during 72 h of military operational stress. Med Sci Sports Exerc 34:1914–1822
Nindl BC, Castellani JW, Young AJ, et al. (2003) Differential responses of IGF-1 molecular complexes to military operational field training. J Appl Physiol 95:1083–1089
Nindl BC, Rarick KR, Castellani JW, Tuckow AP, Patton JF, Young AJ, Montain SJ (2006) Altered secretion of growth hormone and luteinizing hormone after 84 h of sustained physical exerction superimposed on caloric and sleep restriction. J Appl Phys 100:120–128
Noakes TD (1987) Effect of exercise on serum enzyme activities in humans. Sports Med 4:245–267
Opstad P (1991) Alterations in the morning plasma levels of hormones and the endocrine responses to bicycle exercise during prolonged strain: the significance of energy and sleep deprivation. Acta Endocrinol 125:14–22
Opstad P (1992) Androgenic hormones during prolonged physical stress, sleep and energy deficiency. J Clin Endocrinol Metab 74:1176–1183
Opstad P (1994) Circadian rhythm of hormones is extinguished during prolonged physical stress, sleep and energy deficiency in young men. Eur J Endocrinol 131:56–66
Opstad P (1995) Medical consequences in young men of prolonged physical stress with sleep and energy deficiency. NDRE/PUBLICATION-95/05586, Forsvarets Forskningsinstitut
Roemmich JN, Sinning WE (1997) Weight loss, wrestling training: effects on growth-related hormones. J Appl Physiol 82:1760–1764
Ross JH, Attwood EC, Atkin GE et al. (1983) A study of the effects of severe repetitive exercise on myoglobin creatine kinase, transaminases and lactate dehydrogenase. QJM 206:268–79
Acknowledgments
The authors would like to express their gratitude to the Finnish Defense Forces for financially supporting the study. Furthermore, we wish to thank all the subjects who volunteered for the study
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kyröläinen, H., Karinkanta, J., Santtila, M. et al. Hormonal responses during a prolonged military field exercise with variable exercise intensity. Eur J Appl Physiol 102, 539–546 (2008). https://doi.org/10.1007/s00421-007-0619-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0619-0