Abstract
The purposes of this study were to investigate the effects of strenuous exercise on apoptosis of the gastrocnemius and soleus muscle fibers and clarify the role of oxidative metabolism in the strenuous exercise-induced apoptosis. The experiment was designed with 49 (n = 49) male, 24-week-old, L. Wistar albino rats. Strenuous exercise model was applied to 42 (n = 42) rats and seven (n = 7) rats served as rested controls. All rats were randomly assigned to one of the following groups (n = 7): rested control (C), immediately after exercise (0 h) and 3, 6, 12, 24, and 48 h after exercise. Apoptotic nuclei were shown by single stranded DNA (ssDNA) determination. Oxidative damage in mitochondrial fractions of the muscle tissues was evaluated by malondialdehyde (MDA) levels and reduced/oxidized glutathione (GSH/GSSG) ratios. Caspase-9, -8 and -3 activities and the level of cytochrome c (Cyt c) were measured in the cytosolic fractions of muscle tissues to follow mitochondrial-dependent (intrinsic) or ligand-mediated death receptor (extrinsic) pathways of apoptosis. Plasma interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels were also determined. Based on our results, apoptosis is significantly triggered in muscle fibers by strenuous exercise (P < 0.05). Apoptosis in the soleus muscle tissues mostly depends on the intrinsic pathway and may be triggered by increased oxidative stress. In contrast, extrinsic pathway of apoptosis was predominant in the gastrocnemius muscle and increases of TNF-α and IL-6 may play a significant role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis, programmed cell death, is characterized by several specific biochemical and molecular events. In mature animals, apoptosis balances cell division, maintaining the constancy of tissue mass. Removal of cells injured by genetic defects, aging, disease or exposure to noxious agents is made possible by apoptosis (Duke et al. 1996; Sastre et al. 2000). Recently, apoptosis has gained interest among exercise researchers due to evidence suggesting that exercise, especially strenuous exercise, can lead to apoptotic cell death in addition to necrotic cell death (Sandri et al. 1997; Podhorska-Okolow et al. 1998, 1999; Sharon and Leeuwenburg 2001; Phaneuf and Leewenburgh 2001; Primeau et al. 2002).
Strenuous exercise modulates several factors that may alter apoptosis. For instance, glucocorticoids, reactive oxygen species (ROS), a rise in intracellular Ca2+ levels, and TNF-α are some signals that can induce apoptosis (Ji 1995; Bejma et al. 1999; Carraro et al. 1997; Leeuwenburg et al. 1999; Kannan et al. 2000; Fumarola et al. 2004; Pistilli et al. 2006). Some of these factors originate from the extracellular milieu (glucocorticoids, TNF-α) and will interact with intracellular or extracellular proteins that may trigger cell death.
Several apoptotic pathways have been shown to be involved in the apoptotic cascades, depending on the stimulus. These include intrinsic (Green and Reed 1998; Phaneuf and Leeuwenburgh 2002; Kroemer 2003; Garrido et al. 2006; Van Gurp et al. 2003), extrinsic (Li et al. 1998), and an endoplasmic reticulum stress-induced pathway (Nakagawa et al. 2000). A family of protease proteins called caspase (cysteine-dependent aspartate protease) is crucial in executing apoptosis. Specific initiator caspases (e.g., caspase-8, -9, and -12) are activated when the cell is exposed to the corresponding apoptotic stimuli. For example, TNF-α or FasL can initiate apoptosis through the extrinsic pathway by activating caspase-8 (Li et al. 1998; Sun et al. 1999). In addition, caspase-9 has been shown to mediate the intrinsic apoptosis through the interaction of procaspase-9 with apoptotic protease activating factor-1 (Apaf-1), dATP, and mitochondrial-released cytochrome c (Phaneuf and Leeuwenburgh 2001, 2002). Although, different initiator caspases are recruited in different apoptotic pathways, it appears that the apoptotic signals finally converge on the activation of the common effector caspases (e.g., caspase-3, -6, and -7), which cause eventual destruction of the cell.
Although, it remains unclear as to how and why apoptosis is induced in adult skeletal muscle after exercise, there are many plausible hypotheses that warrant further investigation. One of the leading hypotheses is that during exercise, muscle metabolism is increased, which leads to an increased production of ROS (Bejma et. al. 1999; Cooper et al. 2002; Vasilaki et al. 2006). Significant amounts of ROS can produce oxidative damage and thereby induce apoptosis via the intrinsic pathway (Di Meo et al. 2001; Primeau et al. 2002; Phaneuf and Leeuwenburg 2001). The other hypothesis is based on the observation that the stress of the exercise increases catecholamine levels, which promote the induction of apoptosis (Stevenson et al. 2001; Shephard 2003). The local inflammatory response in skeletal muscle to strenuous exercise is followed by an influx of leukocytes and a systemic acute-phase response (Pedersen et. al. 2000; Suzuki et al. 2000; Peake et al. 2005a). The various elements of acute-phase response are mediated by cytokines, including TNF-α and IL-6 (Suzuki et al. 1999; Pedersen et al. 2001; Dalla et al. 2001; Peake et al. 2005b). Studies have shown that bounding of these cytokines to the receptors found on the plasma membrane leads to activation of apoptosis via an extrinsic pathway (Fumarola et al. 2004; Pistilli et al. 2006). However, no consensus has been achieved regarding the extent and mechanisms of apoptosis in skeletal muscle. Whether, in strenuous exercise an apoptotic mechanism (intrinsic/extrinsic) is predominant or whether there is any difference in the apoptotic response between gastrocnemius and soleus muscle fibers, has not been explained yet with sufficient clarity.
Therefore, the purposes of this study were to address the difference between different muscle groups in terms of apoptotic response to the strenuous exercise and to clarify the significance of the muscular capacity for oxidative metabolism in apoptotic response following strenuous exercise. All experiments were carried out in rat gastrocnemius and soleus muscle fibers. The muscle fibers were separately analyzed due to differences in their oxidative metabolism rates (Amstrong et al. 1984). The existence and mechanism of apoptosis were evaluated by determination of single stranded DNA (ssDNA), cytosolic caspase-8, -9 and -3 activities and cytochrome c (Cyt c) levels. Oxidative stress levels of mitochondrial fractions of muscle fibers were evaluated by MDA levels, a lipid peroxidation marker, and GSH/GSSG status. Plasma IL-6 and TNF-α levels were used as markers and ligands of inflammation causing extrinsic triggering of apoptosis.
Methods
Animal care
The experiments were carried out with 49 male L. Wistar albino rats, with 87.5% homogeneity, and with a mean body weight of 204 ± 23 g and age of 24 weeks. All animals were fed with a standard pelleted diet for rodents supplemented with microelements and vitamins. Wistar rats were obtained from the Experimental Animal Laboratory of the Dokuz Eylül University, School of Medicine. All experiments were performed in accordance with the guidelines provided by the Experimental Animal Laboratory and approved by the Animal Care and Use Committee of the Dokuz Eylül University, School of Medicine. The animals were housed in individual cages in a temperature-controlled room (22°C) with a 12 h reverse light–dark cycle. After a week of acclimation period, all rats were randomly assigned to one of the following seven groups and each group was formed of seven rats (n = 7): control-rested group, immediately after exercise (0 h) and 3, 6, 12, 24, and 48 h after exercise. All rats were placed back in their cages with free access to food and water after exercise program.
Exercise protocol
Running exercises were performed on three small animal treadmills. All procedures were carried out between 9:00 a.m. and 12:00 a.m. They were run on a treadmill for 10 min/session at a speed of 10 m/min and a slope of 5°, once a day for 1 week to adapt them to running before the experiment. Control animals were run on a treadmill for 10 min/session at a speed of 10 m/min and a slope of 5°, once a day for 1 week in order to stress them. Electric shocks were used sparingly to motivate the animals to run. Two days after the last familiarization run, except for the control group, the rats were forced to run at a speed of 25 m/min and a slope of 5° until exhaustion, which is defined as the animal touching the electrified grid at the rear of the treadmill five times in 2 min, and the time to exhaustion was recorded (Liu et al. 2000). Animals were run until they failed to respond to the electrical grid, and the exhaustion time was recorded. Average exhaustion time of the rats in exercised groups was 52.2 ± 3.0 min and did not differ from each other in exercise groups (Willis et al. 1988).
Blood and tissue sampling and isolation of mitochondrial/cytosolic fractions
Under light ether anesthesia, the chest was opened and blood samples were obtained from the heart of rats with a syringe anticoagulated with sodium heparin. Blood samples were centrifuged at 3,500 rpm for 5 min. and plasma samples were stored at −80°C until cytokine analysis was achieved. After collecting the blood samples, the animals were killed by cervical dislocation, immediately as 0, 3, 6, 12, 24, and 48 h after the exercise programme. Control animals were sacrificed without performing the running exercise. Their gastrocnemius and soleus muscle tissues were quickly removed from both legs. A piece of tissue was dissected for pathological analysis from the middle sections of every gastrocnemius and soleus muscles. The rest of the fresh tissues were used immediately for isolation of cytosolic and mitochondrial fractions and obtained fractions were frozen at −80°C until analysis.
Cytosolic and mitochondrial fractions were prepared as described by Leeuwenburg et al. (1999). Briefly, all tissues were washed in ice-cold antioxidant buffer at first (100 μM diethylene triamine pentaacetic acid (DTPA), 1 mM butylated hydoxytoluene (BHT), 1% (v/v) ethanol, 10 mM 3-aminotriazole, 50 mM NaHPO4, pH 7.4). This step helps to remove the blood from tissues and inhibits oxidation reactions ex vivo. Subsequent procedures were carried out at 4°C. Tissues were minced in 10 volumes of isolation buffer A (0.225 M mannitol, 75 mM sucrose, 0.2% fatty acid free albumine, 13 U/ml collagenase, 10 μM BHT, 100 μM DTPA, pH 7.4). Tissues were homogenized with motor-driven Potter–Elveljem glass homogenizer at 0–4°C at low speed and centrifuged for 7 min at 480g. The resulting supernatant was stored on ice, the pellet was resuspended by homogenization in 0.5 ml of isolation buffer A, and the suspension was centrifuged for 7 min at 480g. The combined supernatants were centrifuged at 7,700g for 7 min. An aliquot of the resulting supernatant was used for analysis of the cytosolic protein fraction. The mitochondrial pellet was washed once with 10 ml of buffer B (0.225 M mannitol, 75 mM sucrose, 1 mM ethyleneglycoltetraaceticacid (EGTA), 10 μM BHT, 100 μM DTPA, pH 7.4) and centrifuged at 7,700g for 7 min. The final mitochondrial pellet was resuspended in 0.5 ml of buffer C (0.25 M sucrose, 2 mM ethylenediaminotetraaceticacid (EDTA), 1 mM BHT, 100 μM DTPA, pH 7.4). Preparation period of cytosolic and mitochondrial fractions was nearly 55 min after the animals’ death. Protein levels in mitochondrial and cytoplasmic fractions were determined by a modification of the Lowry procedure as described by Markwell et al. (1978).
Determination of apoptotic nuclei
Apoptotic nuclei were determined by using anti-ssDNA monoclonal antibody (Chemicon©). Muscular tissues were fixed in 10% neutral buffered formalin at 4°C for 24 h and embedded in paraffin. The paraffin blocks were cut to 4 μm sections and heated in an oven at 60°C for 1 h. After deparaffinization sequentially in xylene, 100, 95, and 70% ethanol, the sections were treated at room temperature with 0.1 mg/ml saponin for 20 min and 20 μg/ml proteinase K for 20 min. The slides were incubated in a coplin jar containing 50 ml of 50% formamide (preheated to 60°C) for 20 min in a water bath. After treatment with 3% H2O2 in methanol for 5 min and 3% non-fat dry milk for 15 min, 100 μl of monoclonal antibody F7-26 (dilution 1:10) were applied to the slides. Further incubation with peroxidase-conjugated anti-mouse IgM (dilution 1:100) was performed for 15 min. The sections were stained with diaminobenzidine solution for 10 min at room temperature and then counterstained with hematoxylin. Positive nuclei were counted among at least 400 nuclei and expressed as percentage of apoptotic nuclei. To assess muscle necrosis, the sections were also stained with hematoxylin and eosin (H&E).
Determination of lipid peroxidation and glutathione status
To assess mitochondrial lipid peroxidation, the concentration of malondialdehyde, dithiobarbituric acid (TBA) adducts, were measured in mitochondrial fractions of muscle tissues. Mitochondrial fractions were processed with TBA and the levels of mitochondrial thiobarbituric acid-reactive substances (TBARS) were determined on a HPLC system consisting fluorescence detector (Lykkesfeldt 2001). Calibration curves were constructed using tetramethoxypropane for mitochondrial fractions. Measurements were performed in duplicate and quantification was carried out by comparing peak areas to the area of standard curves obtained with tetramethoxypropane solutions.
Reduced glutathione (GSH) and total glutathione (GSHt) were determined by HPLC (Cereser 2001). Briefly, samples were treated with ortho-phthalaldehyde (OPA) for pre-column derivatization. OPA derivatives were separated on a reversed-phase HPLC column (Shimadzu C-18 ODS-2 silica column 250 × 4.6 mm, 5 μm particle size) with acetonitrile–sodium acetate gradient system and detected fluorimetrically. All measurements were performed in duplicate and quantification was carried out by comparing peak areas to the area of standard curves obtained with the different concentrations of GSH solutions. Total glutathione is determined after reduction of disulfide groups with dithiothreitol; oxidized glutathione (GSSG) concentration was calculated by subtraction of GSH level from the GSHt level.
Determination of cytosolic markers of apoptosis
Caspase-3, caspase-8 and caspase-9 activities and the level of Cyt c were used to follow apoptosis in the isolated cytosolic fractions. Activities of caspase-3, caspase-8 and caspase-9 in the cytosolic fractions were measured by colorimetric assay kit of MBL©. Caspase-3, caspase-8 and caspase-9 assays were based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrates of caspases DEVD-pNA, IETD-pNA, and LEHD-pNA, respectively. The light emission of pNA was quantified using a microtiter plate reader at 405 nm. Caspase activities in the samples were calculated from the standard curves. Standard curves were prepared for each enzyme between known amounts (unit, U) of active caspase-3 (Biovision©), caspase-8 (Alexis©) and caspase-9 (Alexis©) and the obtained pNA absorbance. Obtained results from the curves were divided by the protein values of each sample to reach the specific activity per microgram protein (SA/μg protein).
Cyt c levels of cytosolic fractions were measured by using one-step solid phase sandwich enzyme-linked immunosorbent assay (ELISA) system (MBL©). Microwell strips of ELISA, coated with anti-cytochrome c polyclonal antibody that is specific for human beings, mouse and rat, respectively, were used to perform the assay. All measurements were performed in duplicate according to the manufacturers’ instructions.
Determination of plasma TNF-α and IL-6 concentrations
For assay of IL-6 and TNF-α plasma levels, ELISA was performed with commercially available kits according to the manufacturers’ instructions. The rat interleukin-6 (rail-6) and rat tumor necrosis factor-alpha (raTNF-α) concentrations were measured using ELISA kits developed by BioSource International (Camarillo, CA). The absorbance was measured spectrophotometrically using a microplate reader (BioTek Instruments©, US) and the concentration of each cytokine was calculated by comparison with its calibration curve.
Statistical analysis
All results are presented as mean ± standard deviation (mean ± SD). The differences among all groups’ were evaluated by one-way Kruskal–Wallis variance analysis. For the comparison of the soleus and the gastrocnemius groups between themselves and with the control group, Mann–Whitney U test was performed. The statistical analysis was performed with the SPSS software statistical program. Differences between groups were considered statistically significant for P < 0.05.
Results
Apoptotic nuclei
No apoptotic nuclei of muscle fibers were detected in the control group (Fig. 1a, b). The percentage of apoptotic nuclei of the soleus muscle fibers showed increases rapidly after exercise compared to the control group whereas, in the gastrocnemius muscle fibers, the percentages showed increases gradually in a time dependent manner (Fig. 2). The highest percentage was found at 0 h in the soleus muscle and at 6 h in the gastrocnemius muscle fibers (Figs. 1c, d, and 2). All data in the gastrocnemius and soleus muscle fibers were significantly different from that of the control group (P < 0.01) (Fig. 2). The percentage of apoptotic nuclei in endothelial cells of the soleus muscle tissue was found at a significantly high level compared to the control group (P < 0.01) (Figs. 3a, b, and 4). Interestingly, the percentage of apoptosis of endothelial cells in the soleus muscle tissue was found at a significantly higher level compared to the control group and the gastrocnemius muscle tissue (P < 0.01) and apoptotic percentages tended to rise after exercise in a time dependent manner (Fig. 4). There was no evidence of muscle necrosis in H&E sections.
Photomicrograph sections of the soleus and gastrocnemius muscles from each experimental group determined by anti-ssDNA monoclonal antibody. Photomicrographs representative of the soleus and gastrocnemius muscle fibers in control (a, b) and exercised groups (c, d) respectively. The nuclei stained dark indicate apoptotic myonuclei in the soleus muscle section at 0 h (c) and in the gastrocnemius muscle section at 6 h (d) after exercise
The percentage of apoptotic nuclei (%) in the soleus, and gastrocnemius muscle tissues. C control (without exercise) and muscle tissues removed after exercise programme: 0, 3, 6, 12, 24, and 48 h. Each bar represents the percentage of apoptotic nuclei. * P < 0.01 indicates significant difference between exercised and the control group
The percentage of apoptotic nuclei (%) in endothelial cells of the soleus and gastrocnemius muscles. C control and muscle tissues, removed after exercise programme: 0, 3, 6, 12, 24, and 48 h. Each bar represents the percentage of apoptotic nuclei. * P < 0.01 indicates significant difference between exercised and the control group
Lipid peroxidation and glutathione status
Mitochondrial MDA, as a lipid peroxidation marker, was found to be significantly different from that in the control group (P < 0.05) in all the groups except for the groups of 48 h (P > 0.05) (Fig. 5). Mitochondrial MDA levels were found significantly higher in the soleus muscle than in the gastrocnemius muscle fibers between all the groups (P < 0.05). Compared to the control group, the highest values were found at 0 h of the soleus muscle fibers and at 3 h of the gastrocnemius muscle fibers (P < 0.01) (Fig. 5). Glutathione is the major intracellular thiol compound in muscle and dropping total/oxidized ratio of it does provide indirect measures of the oxidative stress in the cell (Meister and Anderson 1983). Mitochondrial GSH/GSSG ratios were found to be significantly different from that in the control group, in all of the groups of soleus muscle (P < 0.05), except for the groups of 48 h (P > 0.05) (Fig. 6). Lower ratios were found at 3 h (P < 0.01) and 6 h (P < 0.01) after exercise. In the groups of gastrocnemius muscle, GSH/GSSG ratios were found to be significantly different from the control group only at 6 h (P < 0.01) and at 12 h (P < 0.05) after exercise. All values of GSH/GSSG in the soleus muscle were found to be lower than in the groups of gastrocnemius muscle after exercise but more significant differences between the two groups were obtained at 0 h (P < 0.01), 3 h (P < 0.01), and 6 h (P < 0.05) (Fig. 6).
MDA levels (μmol/l) in mitochondrial fractions of the soleus and gastrocnemius muscles. C control (without exercise) and at specified times after strenuous exercise. MDA levels are presented (means ± SD, n = 7 animals) as a bar for each time point. * P < 0.05 indicates significant difference between exercised group (for the soleus/gastrocnemius muscle) and the control group; cross P < 0.05 indicates significant difference between the soleus and gastrocnemius muscle
GSH/GSSG levels (μmol/mg protein) in mitochondrial fractions of the soleus and gastrocnemius muscles. C control (without exercise) and at specified times after strenuous exercise. GSH/GSSG ratios are presented (means ± SD, n = 7 animals) as bar for each time point. * P < 0.05 indicates significant difference between exercised group (for the soleus/gastrocnemius muscle) and the control group; cross P < 0.05 indicates significant difference between the soleus and gastrocnemius muscle
The level of cytochrome c and activities of caspase-9, -8 and -3
Cytosolic Cyt c levels were found to differ significantly from that in the control group at all points in time (P < 0.05) but the highest value was found at 6 h in the soleus and gastrocnemius muscle tissues after exercise (Fig. 7). All Cyt c values were higher in the groups of soleus than the groups of gastrocnemius but a significant difference between the two types of muscle fibers was determined only at 6 h (P < 0.01) (Fig. 7). Caspase activities of the soleus muscle indicate that the increase of caspase-9, activated by releasing of Cyt c from the mitochondria, was significantly different from the control group (P < 0.05) at all the time points (Fig. 8). While activation of caspase-8 was found at 3 h after exercise, first significant activation for caspase-9 and caspase-3 were found at 0 h (P < 0.01). The highest values for all of the caspases in the all time points were determined at 3 h after exercise (Fig. 8). However, in gastrocnemius muscle, significant activations of caspase-8, activated by extrinsic pathway, were found at 3 and 6 h after exercise (P < 0.01) (Fig. 9). Caspase-3 activation, activated by effects of both caspase-9 and caspase-8, were found significantly different from the control group in all time points in gastrocnemius muscle tissue (Fig. 9). Comparing all the caspase activities in two kinds of muscle fibers, no remarkable differences were obtained for caspase-8 and -3, (P > 0.05) but significant differences (P < 0.01) were found for caspase-9. In soleus muscle tissues, higher values of caspase-9 were determined at the early time points (0, 3 h) (Fig. 8); however, in gastrocnemius muscle tissues, its activation appeared at the later time points (6, 12 h) (Fig. 9).
Cytosolic Cyt c levels (ng/mg protein) in the soleus and gastrocnemius muscles. C control (without exercise) and at specified times after strenuous exercise. Cyt c levels are presented (means ± SD, n = 7 animals) as solid bar for each time point. * P < 0.05 indicates significant difference between exercised group (for the soleus/gastrocnemius muscle) and the control group; cross P < 0.05 indicates significant difference between the soleus and gastrocnemius muscle
Cytosolic caspase activities (SA/μg protein, specific activity per μg protein) in the soleus muscle. C control (without exercise) and at specified times after strenuous exercise Caspase activities are presented (means ± SD, n = 7 animals) as bar for each time point. * P < 0.05 indicates significant difference between control group and at specified times after strenuous exercise
Cytosolic caspase activities (SA/μg protein, specific activity per μg protein) in the gastrocnemius muscle. C control (without exercise) and at specified times after strenuous exercise. Caspase activities are presented (means ± SD, n = 7 animals) as bar for each time point. * P < 0.05 indicates significant difference between control group and at specified times after strenuous exercise
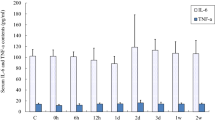
Plasma TNF-α and IL-6 concentrations
Plasma concentrations of TNF-α, a marker of inflammation and IL-6, a marker of muscle contraction, tend to rise immediately after exercise but the highest level was found at 3 h (P < 0.01) (Fig. 10). Plasma concentrations of IL-6 increased at 0 and 3 h after exercise and then gradually decreased with time. Higher levels of TNF-α were found at 3 and 6 h after exercise (P < 0.01).
Discussion
The principle aim of this study was to address the difference between different muscle groups in terms of apoptotic response to the strenuous exercise. We also examined the relation between oxidative metabolism and strenuous exercise-induced apoptosis. The soleus muscle is composed of predominantly type I fibers and has a larger volume of mitochondria, whereas the gastrocnemius is composed primarily of type II fibers and has a lower volume of mitochondria. Therefore, these muscles represent two major muscle fiber types with different mitochondria volumes and approximately a 3- to 4-fold difference in the capacity for oxidative metabolism (Amstrong et al. 1984; Powers et al. 1994).
In the present study, we confirm that muscle apoptosis is triggered by strenuous exercise as shown in recent studies (Podhorska et al. 1998; Sandri et al. 1997). In addition, to our knowledge, this is the first study both addressing the apoptotic difference between the gastrocnemius and soleus muscles against strenuous exercise, and showing the existence of apoptotic nuclei by using anti-ssDNA monoclonal antibody which is a powerful and specific marker of apoptotic nuclei. We showed that soleus muscle nuclei were more prone to apoptosis than the myonuclei of gastrocnemius muscle. On the other hand, apoptotic nuclei were also seen in the endothelial cells of intramuscular capillaries immediately after exercise and apoptosis was enhanced in a time dependent manner. Exercise-induced muscle damage is a process, which usually starts with a mechanical overload of the myofibers; however, in fact, it is a consequence of a complex reaction implicating myofibers, basal lamina and intramuscular vessels. Its persistence may be mediated by inflammatory and non-inflammatory events, like oxidative stress related to ischemia/reperfusion or sustained hyperemia after exercise. The free radical burst may be one of the mechanisms that triggers the apoptosis. It is well known that such events induce apoptosis (Kannan et al. 2000; McArdle et al. 2001). Our evidence of endothelial cell apoptosis close to the capillary wall, increase of MDA levels and reduction of GSH/GSSG ratios after the strenuous exercise, strongly support this hypothesis. Although, the precise mechanism by which oxidative stress can promote endothelial cell apoptosis cannot be elucidated in this study, evidence from in vitro systems suggest that changes in mitochondrial integrity may mediate ROS-induced apoptosis endothelial cells (Kadenbach et al. 2004; Hermann et al. 1997; Rossig et al. 2000).
It has also been hypothesized that the important source and target of ROS during exercise is mitochondria (Goldenthal and Garcia 2004; Di Meo and Venditti 2001). Since mitochondria consume more than 90% of the cellular oxygen, a critical by-end product of mitochondrial bioenergetic activity is the generation of ROS. Exercise-induced ROS generation may cause elevated lipid peroxidation and reduce redox status of the mitochondrial matrix as shown by decreased ratio of GSH/GSSG (Rajguru et al. 1994; McLennan and Degli Esposti 2000; Goldenthal and Garcia 2004; Cooper et al. 2002). Li et al. (1999) reported that exercise decreased membrane fluidity and increased lipid peroxidation in rat skeletal muscle mitochondria. McArdle et al. (2004) also suggested that skeletal muscle has additional sources of generation of superoxide. Our results support that the strenuous exercise causes increase of MDA level and reduction of GSH/GSSG ratio in the mitochondria. In addition, our results also indicate that increase in lipid peroxidation and decrease in redox status occur earlier and predominantly in mitochondrial fractions of the soleus muscle (e.g., at 0 h). These results imply that the different oxidative response to strenuous exercise may be related to difference in the capacity for oxidative metabolism of the muscles.
The effect of strenuous exercise on apoptosis in the soleus and gastrocnemius muscles still remains unknown and few data exist to evaluate apoptosis in these muscle fibers separately. It has been shown that increased oxidative stress is related to the activation of apoptosis (Buttke et al. 1994; Pollack and Leeuwenburgh 2001; Yuan et al. 2003). The present study provides evidence that the soleus and gastrocnemius muscles have different apoptotic response to the strenuous exercise. Our results emphasize the importance of oxidative damage on release of Cyt c in the strenuous exercise. Higher levels of Cyt c in the cytoplasmic fractions suggest that the mitochondria play a significant role in apoptotic signaling in the soleus muscle in contrast to the gastrocnemius muscle. On the other hand, caspase activities are evaluated in a time dependent manner; the highest activity of caspase-9 was found earlier (at 3 h) in the soleus muscle, whereas the highest activity of caspase-8 was demonstrated earlier (at 3 h) in the gastrocnemius muscle. Earlier caspase-9 activation implies that apoptosis in soleus muscle fibers depends primarily on the intrinsic mechanism, but the extrinsic mechanism, activated by caspase-8, supports all the apoptotic effects afterwards. Taken together, these results imply the priority of the intrinsic pathway of apoptosis for the soleus muscle in contrast to the extrinsic pathway for gastrocnemius muscle. Although, the mechanism of exercise-induced apoptosis in the muscle fibers still remains unexplained, our Cyt c and caspase activity results show that classical apoptotic pathways, as observed in the mononucleated cells, may not be effective in the muscle fibers because, we showed that release of Cyt c occurs later (at 6 h) than the activation of caspase-9 and -3 (at 3 h). However, our results may adjust to the hypothesis of biphasic kinetics of Cyt c. This model of biphasic Cyt c release has been first proposed by Scorrano et al. (2002). Recently, Garrido et al. (2006) suggested that vast panels of distinct proapoptotic stimuli (ROS, pro-apoptotic proteins, cardiolipin oxidation, caspase activities and Ca) converge on mitochondria to induce mitochondrial outer membrane permeabilization (MOMP) and Cyt c release. At the first level, an incomplete MOMP is induced and only a small fraction of Cyt c exits in the cytosol. Thereafter, a total, irreversible MOMP may take place, leading to sustained Cyt c release and eventually, apoptosis. The initial release of Cyt c induces the activation of caspases that, in turn, may be implicated in the second wave of MOMP. Moreover, during the first stages, a slight increase in ROS results in the peroxidation of cardiolipin (CL), which is a necessary phospholipid for the insertion of Cyt c into mitochondrial membrane. Oxidized CL, together with the structural alterations of the mitochondrial cristae in which most Cyt c is sequestered, allows for the complete release of Cyt c from mitochondria. These hypotheses may support the early activation of caspases and late elevation of Cyt c in our study. Furthermore, it is worth noting that the muscle samples examined in the present study were obtained after the exhaustion time (52.2 ± 3.0 min); therefore, ROS formed in this period may cause the initial release of Cyt c and activation of caspase-9 and caspase-3 at earlier times.
Contracting skeletal muscle has the capacity to express catabolic cytokines like TNF-α and IL-6. Recent studies show that TNF-α and IL-6 can be detected in plasma during and after strenuous exercise and increase of their levels can be explained by the intensity of exercise (Ostrowski et al. 1998; Pedersen et al. 2000, 2001). On the other hand, it has been shown that TNF-α and IL-6 increases in plasma may mediate apoptosis by activating caspase-3 directly via extrinsic pathway (Reid and Li 2001; Steward et al. 2004; Dirks and Leeuwenburgh 2006). These studies suggest that the resultant rise in circulating TNF-α levels leads to increased ligand binding to TNF-α receptor on the skeletal muscle sarcolemma and its receptor activation also stimulates intracellular production of mitochondrial ROS and induces apoptosis by extrinsic pathway. In the present study, the plasma TNF-α and IL-6 levels showed the same time pattern with the activations of caspase-8 and -3 in the gastrocnemius muscle. The increases of TNF-α and IL-6 levels at 3 h indicate that exercise causes an inflammatory response and auto-oxidation, which may also lead to the production of free radicals. All these effects may cause the activation of caspase-8 and caspase-3 via an extrinsic pathway. Furthermore, the other potential relation between oxidative stress and apoptosis is the control of caspase-3 activity. The control of caspase-3 activity is a complex process and involves several interconnected signaling pathways. It has been shown that caspase-3 is activated by activation of caspase-12 via calcium release pathway (Nakagawa et al. 2000) or by activation of caspase-9 in the case of disuse-induced muscle atrophy (Primeau et al. 2002) and increase of TNF-α in the serum (Dirks and Leeuwenburgh 2006). A key interaction among these caspase-3 activation pathways is that all of these corridors can be activated by ROS (Pistilli et al. 2006).
In conclusion, our study aimed to compare the apoptotic response of the gastrocnemius and soleus muscle groups. Our results show that these two types of muscle fibers react differently to the strenuous exercise. Triggering of apoptosis in the soleus muscle tissue primarily depends on the intrinsic pathway and is activated by oxidative stress, whereas in the gastrocnemius muscle tissue, an extrinsic pathway and cytokine increases are more likely to cause augmentation of apoptotic effect. We also suggest that generation of ROS in the muscle tissues may depend on the oxidative metabolism of the fibers and release of TNF-α and IL-6 after exercise improves the apoptotic effect in a time dependent manner. We conclude that strenuous exercise may cause increased oxidative stress and release of TNF-α and IL-6 in soleus and gastrocnemius muscles. However, apoptotic response of the gastrocnemius and soleus muscle fibers or priority of the mechanisms of apoptosis (intrinsic/extrinsic) show differences related to the muscle types or their capacity for oxidative metabolism.
References
Armstrong R, Phelps R (1984) Muscle fiber type composition of rat hindlimb. Am J Anat 171:259-272
Bejma J, Ji LL (1999) Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87:465-470
Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15(1):7-10
Carraro U, Franceschi C (1997) Apoptosis of skeletal and cardiac muscles and physical exercise. Aging Clin Exp Res 9:19–34
Cereser C, Guichard J, Drai J, Bannier E, Garcia I, Parvaz P, Revol A (2001) Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with flourescence detection: application to red blood cells and cultured fibroblasts. J Chromatogr B 752:123–132
Cooper CE, Vollaard NBJ, Choueiri, Wilson MT (2002) Exercise, free radicals and oxidative stress. Biochem Soc Trans 30:280–285
Dalla LL, Sabbadini R, Renken C, Ravara B, Sandri M, Betto R, Angelini A, Vescovo G (2001) Apoptosis in the skeletal muscle of rats with heart failure is associated with increased serum levels of TNF-alpha and sphingosine. J Mol Cell Cardiol 33(10):1871–1878
Di Meo S, Venditti P (2001) Mitochondria in exercise-induced oxidative stress. Biol Signals Recept 10:125–140
Dirks AJ, Leeuwenburgh C (2006) Tumor necrosis factor a signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem 17:501–508
Duke RC, Ojcius DM, Young JD (1996) Cell suicide in health and disease. Sci Am 275:80–87
Fumarola C, Guidotti G (2004) Stress-induced apoptosis: toward symmetry with receptor-mediated cell death. Apoptosis 9:77–82
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–1433
Goldenthal MJ, Garcia JM (2004) Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem 262:1–16
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Hermann C, Zeiher AM, Dimmeler S (1997) Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol 17:3588–3592
Ji LL (1995) Exercise and oxidative stress: role of cellular antioxidant systems. In: Hollozy JO (ed) Exercise and sport sciences reviews. Williams & Wilkins, Baltimore, pp 135–139
Kadenbach B, Arnold S, Lee I, Huttemann M (2004) The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta 1655:400–408
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7(27):153–163
Kroemer G (2003) Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun 304:433–435
Leeuwenburg C, Hansen PA, Hollosyz JO, Heinecke JW (1999) Hydoxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic Biol Med 27:186–192
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Li JX, Tong CW, Xu DQ, Chan KM (1999) Changes in membrane fluidity and lipid peroxidation of skeletal muscle mitochondria after exhausting exercise in rats. Eur J Appl Physiol Occup Physiol 80:113–117
Liu OJ, Yeo H, Overvik-Douki E, Hagen T, Doniger SJ, Chyu DW, Brooks GA, Ames BN (2000) Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 89:21–28
Lykkesfeldt J (2001) Determination of malondialdehyde as dithiobarbituric acid adducts in biological samples by HPLC with fluorescence detection: comparison with ultraviolent-visible spectrophotometry. Clin Chem 47:1725–1727
Markwell MAK, Hass SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in the membrane and lipoprotein samples. Anal Chem 87:206–210
McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jacson MJ (2001) Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280:C621–C627
McArdle A, Van Der Meulen J, Close GL, Patwell D, Van Remmen H, Huang TT, Richardson AG, Epstein CJ, Faulkner JA, Jacson MJ (2004) The role of mitochondrial superoxide dismutase in contraction-induced generation of reactive oxygen species in skeletal muscle extracellular space. Am J Physiol Cell Physiol 286:C1152–C1158
McLennan HR, Degli Esposti M (2000) The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J Bioenerg Biomembr 32:153–162
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloidbeta. Nature 403:98–103
Ostrowski K, Rohde T, Zacho M et al (1998) Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508:949–953
Peake JM, Suzuki K, Wilson G, Hordern M, Yamaya K, Nosaka K, Mackinnon L, Coombes JS (2005a) Exercise-induced muscle damage, plasma cytokines and markers of neutrophil activation. Med Sci Sports Exerc 37:737–745
Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS (2005b) Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol 95(5-6):514–521
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration and adaptation. Physiol Rev 80:1055–1081
Pedersen BK, Steensberg A, Schjerling P (2001) Exercise and interleukin-6. Curr Opin Hematol 8:137–141
Phaneuf S, Leewenburgh C (2001) Apoptosis and exercise. Med Sci Sports Exerc 33(3):393–396
Phaneuf S, Leeuwenburgh C (2002) Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282:R423–R430
Pistilli EE, Jackson JR, Alway SE (2006) Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis 11:2115–2126
Podhorska-Okolow M, Sandri M, Zampieri S, Brun B, Rossini K, Carraro U (1998) Apoptosis of myofibers and satellite cells: exercise-induced damage in skeletal muscle of the mouse. Neuropath Appl Neurobiol 24:518–531
Podhorska-Okolow M, Krajewski B, Carraro U, Zabel M (1999) Apoptosis in mouse skeletal muscles after physical exercise. Folia Histochem Cytobiol 24:127–128
Pollack M, Leeuwenburgh C (2001) Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci 56(11):B475–B482
Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol 266(35):R375–R380
Primeau AJ, Adhihetty PJ, Hood DA (2002) Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27(4):349–395
Rajguru SU, Yeargans GS, Seidler NW (1994) Exercise causes oxidative damage to rat skeletal muscle microsomes while increasing cellular sulfhdryls. Life Sci 54:149–157
Reid MB, Li YP (2001) Cytokines and oxidative signaling in skeletal muscle. Acta Physiol Scand 171:225–232
Rossig L, Haendeler J, Hermann C, Malchow P, Urbich C, Zeiher AM, Dimmeler S (2000) Nitric oxide down-regulates MKP-3 mRNA levels: involvement in endothelial cell protection from apoptosis. J Biol Chem 275:25502–25507
Sandri M, Podhorska-Okolow M, Geromel V (1997) Exercise-induced myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol 56:45-57
Sastre J, Pallardo VF, Vina J (2000) Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 49:427–435
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ (2002) A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell 2:55–67
Sharon P, Leeuwenburgh C (2001) Apoptosis and exercise. Med Sci Sports Exerc 33:393–396
Shephard RJ (2003) Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med 33(4):261–284
Stevenson JR, Westermann J, Liebmann PM, Hortner M, Rinner I, Felsner P, Wolfler A, Schauenstein K (2001) Prolonged alphaadrenergic stimulation causes changes in leukocyte distribution and lymphocyte apoptosis in the rat. J Neuroimmunol 120:50–57
Stewart CE, Newcomb PV, Holly JM (2004) Multifaceted roles of TNF-alpha in myoblast destruction: a multitude of signal transduction pathways. J Cell Physiol 198:237–247
Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM (1999) Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem 274:5053–5060
Suzuki A, Tsutomi Y, Shimizu M, Matsuzawa A (1999) Another cell death induction system: TNF-alpha acts as a ligand for Fas in vaginal cells. Cell Death Differ 6(7):638–643
Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu O, Kudoh S, Kowatari K, Nakaji S, Sugawara K (2000) Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise endurance exercise in humans. Eur J Appl Physiol 81:281–287
Van Gurp M, Festjens N, Van Loo G, Saelens X, Vandenabeele P (2003) Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun 304:487–497
Vasilaki A, Mansouri A, Remmen HV, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ (2006) Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5:109–117
Willis TW, Dallman P, Brooks GA (1988) Physiological and biochemical correlates of increased work in trained iron-deficient rats. J Appl Physiol 65(1):256-263
Yuan J, Murrell GA, Trickett A, Wang MX (2003) Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta 1641(1):35–41
Acknowledgments
This work was supported by Dokuz Eylul University Research Foundation Grant no: 036.01.01.05. The authors thank Dr. Memduh Bulbul for his excellent technical assistance on the HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koçtürk, S., Kayatekin, B.M., Resmi, H. et al. The apoptotic response to strenuous exercise of the gastrocnemius and solues muscle fibers in rats. Eur J Appl Physiol 102, 515–524 (2008). https://doi.org/10.1007/s00421-007-0612-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0612-7