Abstract
O2 uptake \((\dot{V}O_{2})\) kinetics were examined during the follicular (F) and luteal (L) phases of the menstrual cycle to determine if there was an effect of altered sex hormones on the \(\dot{V}O_{2}\) response to moderate-intensity exercise. Seven healthy women (age 21 ± 2 years; mean ± SD) performed six transitions from 20 W to moderate-intensity exercise (∼ 90 % \(\hat{\theta}_{{{\rm L}}}\)) during the F and L phase. \(\dot{V}O_{2}\) was measured breath-by-breath and deoxyhemoglobin/myoglobin (Δ HHb) was determined by near infrared spectroscopy. Progesterone and estrogen were significantly (P < 0.05) elevated during the L compared to F phase. \(\dot{V}O_{2}\) kinetics \((\tau \dot{V}O_{2})\) were not different in the two phases of the menstrual cycle (F, 22 ± 5 s; L, 22 ± 6 s; 95% confidence intervals ±4 s) nor was the time course of the Δ HHb response (F, TD 11 ± 2 s, τ 11 ± 3 s; L, TD 12 ± 2 s, τ 12 ± 11 s; τHHb 95% confidence intervals ±3 s). Respiratory exchange ratio (RER) was not different between phases for baseline or steady-state exercise and the blood lactate response to exercise was not different. In conclusion, \(\dot{V}O_{2}\) kinetics at the onset of moderate-intensity exercise are not affected by the phase of the menstrual cycle in young females suggesting either no change in, or no effect of metabolic activation on the on-transient kinetics of moderate-intensity exercise. Additionally, the similar adaptation of Δ HHb in combination with unchanged \(\dot{V}O_{2}\) suggests that there were no differences in the adaptation of local muscle O2 delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adaptation of pulmonary O2 uptake during the transition to exercise \((\dot{V}O_{2}\) kinetics) provides insight into control of aerobic metabolism, specifically mitochondrial oxidative phosphorylation. It has been suggested that \(\dot{V}O_{2}\) kinetics are limited either by the rate of muscle enzyme activation and provision of substrates (i.e., metabolic inertia) (Grassi 2001), by bulk muscle blood flow and O2 availability (Hughson et al. 2001), or by a combination of both (for reviews on the topics see Jones and Poole 2005a).
Generally, in those studies that examined \(\dot{V}O_{2}\) kinetics at the onset of exercise, only male subjects have been used, or if female subjects have been included, there has been no control for the phase of the menstrual cycle. The female ovarian sex hormones estrogen and progesterone undergo large changes in their plasma concentrations throughout the menstrual cycle. The absolute plasma concentrations of estrogen and progesterone are greater (and the estrogen-to-progesterone ratio lower) in the luteal compared to the follicular phase of the menstrual cycle. As estrogen and progesterone have been implicated in metabolic and cardiovascular responses to exercise (see below) it is important to determine whether the activation of muscle O2 consumption is influenced by the phase of the menstrual cycle.
Estrogen supplementation has been shown to increase FFA availability in rats (Kendrick and Ellis 1991; Rooney et al. 1993; Ellis et al. 1994) and FFA oxidation in human males (Devries et al. 2005; Hamadeh et al. 2005), as well as elevating the maximal activities of enzymes associated with increased fat metabolism in rats [carnitine palmitoyltransferase I (CPT-I) and β-hyroxyacyl-CoA dehydrogenase (β-HAD)] (Campbell and Febbraio 2001). Additionally, estrogen and progesterone together may facilitate carbohydrate sparing (Kalkhoff 1982; Campbell and Febbraio 2002) and contribute to a greater reliance on fat oxidation during prolonged exercise.
Importantly, estrogen and progesterone treatment in rats resulted in elevated expression of pyruvate dehydrogenase kinase (PDK) (Campbell et al. 2003), an enzyme associated with the mitochondrial pyruvate dehydrogenase (PDH) complex. PDK catalyzes the phosphorylation and subsequent inactivation of PDH. Delayed activation of PDH at the onset of exercise has been implicated as a possible site of metabolic inertia (and thus, delayed activation of muscle O2 consumption) during the transition to exercise (Timmons et al. 1998). If PDH activity was suppressed during the transition to moderate-intensity exercise during the luteal phase of the menstrual cycle (when estrogen and progesterone concentrations are high) the provision of carbohydrate-derived substrate for the tricarboxylic acid (TCA) cycle and subsequently reducing equivalents for the electron transport chain (ETC) may be expected to be decreased, possibly contributing to a slowed activation of muscle O2 consumption at the onset of exercise. Therefore, while we are unable to measure PDH directly in the present study, our ability to measure, with high levels of confidence, pulmonary \(\dot{V}O_{2}\) kinetics, which reflect the adaptation of muscle O2 consumption, will allow us to observe changes in the time course of adaptation of muscle O2 consumption that may occur as a result of any ovarian hormone-induced suppression of PDH activation during the transition to exercise.
Alternatively, muscle O2 delivery has been implicated in limiting the activation of pulmonary \(\dot{V}O_{2}\) (and muscle O2 consumption) kinetics during the transition to exercise. At present, the effect of menstrual cycle phase on muscle blood flow and O2 delivery during exercise is unclear. There is evidence that estrogen and progesterone supplementation and the menstrual cycle may alter blood flow control (Sudhir et al. 1997; Ettinger et al. 1998; Moreau et al. 2003; Kirwan et al. 2004). However, estrogen and progesterone or menstrual cycle phase has recently been reported to have no effect on resting blood flow (Cooper et al. 2006) or on the post-exercise return of blood flow to resting levels (Cooper et al. 2006). To date, we are not aware of any studies that have examined the adaptation of muscle blood flow during the transition to exercise in the different phases of the menstrual cycle.
Near-infrared spectroscopy (NIRS)-derived measures of muscle deoxygenation (Δ HHb) provides information on muscle O2 extraction and thus the balance between local muscle O2 utilization and local muscle O2 delivery (DeLorey et al. 2003) in the region of NIRS interrogation. When used in combination with measures of pulmonary \(\dot{V}O_{2}\) kinetics (providing information on the adaptation of muscle O2 utilization), the Δ HHb signal provides qualitative information on the adaptation of local muscle perfusion (relative to local O2 consumption) during the transition to exercise.
Therefore, the purpose of this study was to examine the effect of menstrual cycle phase on pulmonary \(\dot{V}O_{2}\) and NIRS-derived deoxygenation (Δ HHb) kinetics during the transition to moderate-intensity exercise. It was hypothesized that (1) \(\dot{V}O_{2}\) kinetics would be slower in the luteal compared to the follicular phase of the menstrual cycle, and (2) NIRS-derived deoxygenation kinetics also would be slower in the luteal phase, consistent with a slower rate of muscle O2 utilization and no, or only small changes in the rate of local O2 delivery.
Methods
Subjects
Seven females were recruited to participate in this study. All subjects were regularly active with five being members of the university rugby team. All subjects were healthy and not taking any medications. None of the subjects had used oral contraceptives for the 6 months prior to participating in this study and all reported having regular menstrual cycles during this period. All subjects were informed of potential risks and provided informed consent. The study was approved by The University of Western Ontario Ethics Committee for Research on Human Subjects.
Timing of menstrual cycle testing
The start of each menstrual cycle was marked by the onset of menstrual flow (menstruation). Testing in each of the follicular and luteal phases was performed in random order with testing in the follicular phase being performed 3–5 days after the onset of menses and testing in the luteal phase being performed 5–8 days before the onset of the next menstruation. Testing in each phase of the menstrual cycle was performed at approximately the same time of day and subjects were instructed to consume a small meal high in carbohydrate and low in fat ∼2 h before each test. Each phase of the menstrual cycle was confirmed by means of blood samples showing appropriate estrogen and progesterone concentrations within each phase.
Exercise protocol
Prior to the start of testing all subjects performed an incremental ramp exercise test (25 W/min) to the limit of tolerance on an electronically braked cycle ergometer (model H-300-R, Lode) for determination of the estimated lactate threshold \(\hat{\theta}_{{{\rm L}}}\) and peak \(\dot{V}O_{2} (\dot{V}O_{2}\) peak) [for detailed explanation see (Gurd et al. 2005)]. From the results of this ramp test, a moderate-intensity work rate (WR) was selected that would elicit a \(\dot{V}O_{2}\) corresponding to ∼90% of the \(\dot{V}O_{2}\) at \(\hat{\theta}_{{{\rm L}}}\) and this WR was used for all subsequent tests. This incremental ramp exercise test was used only for determination of an appropriate WR in the moderate-intensity domain. Confirmation of whether this WR represented moderate-intensity exercise subsequently was verified by examining the \(\dot{V}O_{2}\) profile and the plasma lactate− concentrations in exercise. We did not control for the phase of the menstrual cycle during this preliminary testing.
Following the initial visit subjects reported to the laboratory on two consecutive days during each of the follicular and luteal phases of the menstrual cycle. During each visit subjects performed three step-transitions to the WR corresponding to 90% \(\hat{\theta}_{{{\rm L}}}\) with each transition lasting 6 min. The first transition was preceded by 6 min baseline cycling (20 W) and each subsequent transition was separated by 8 min of baseline cycling. This resulted in six transitions for each subject in each phase of the menstrual cycle. This protocol was used to maximize the number of transitions that could be obtained within each specific phase of the menstrual cycle, thereby maximizing the confidence of the parameter estimations for the \(\dot{V}O_{2}\) kinetic response. It was demonstrated previously that a prior bout of moderate-intensity exercise does not affect the \(\dot{V}O_{2}\) kinetic response to a subsequent bout of moderate-intensity exercise (Burnley et al. 2000; Behnke et al. 2005) and this was confirmed in the current study by comparing the parameter estimates for each of the three individual exercise transitions.
On the first day of testing within each of the follicular and luteal phases of the menstrual cycle, and with the subject resting in a supine position, a percutaneous Teflon catheter (Angiocath, 21 gauge) was placed in a dorsal hand vein. A venous sample was drawn from the back of the hand for determination of estrogen and progesterone concentrations. The hand and forearm were then wrapped in a heating pad with additional heating provided by a heat lamp in order to “arterialize” the blood. Arterialized-venous blood was drawn during baseline cycling at 120 and 60 s prior to the step change in WR, and at 30, 60, 120, 240, and 360 s during the transition to the first of the three bouts of moderate-intensity exercise for determination of plasma lactate− concentration. On the second day of testing in each phase NIRS was used to measure changes in concentration of deoxyhemoglobin/myoglobin (Δ HHb) in the exercising leg (see below). Only data from the first of the three transitions were used for analysis of the Δ HHb signal as the NIRS-derived signals are affected by changes in the balance between O2 utilization and O2 availability if adequate recovery is not provided between subsequent moderate-intensity square-wave transitions.
Materials
Gas-exchange measurements were similar to those previously described (Babcock et al. 1994). Briefly, inspired and expired flow rates were measured with a low dead-space (90 ml) bi-directional turbine (Alpha technologies, VMM 110, Bellingham, WA, USA), which was calibrated before each test with a syringe of known volume (3 l). Inspired and expired gases were sampled continuously at the mouth and analyzed for concentrations of O2, CO2, and N2 by mass spectrometry (Innovision, AMIS 2000, Lindvedvej, Denmark) after calibration with precision-analyzed gas mixtures. Changes in gas concentration were aligned with gas volumes by measuring the time delay for a square-wave bolus of gas passing the turbine to the resulting changes in fractional gas concentrations as measured by the mass spectrometer. Data collected every 20 ms were transferred to a computer, which aligned concentrations with the volume data to build a profile of each breath. Breath-by-breath alveolar gas exchange was calculated by the algorithms of Beaver et al. (1981).
Arterialized-venous blood samples were analyzed for plasma lactate− concentration with selective electrodes (StatProfile 9 Plus blood gas-electrolyte analyzer, Nova Biomedical, Mississauga, ON, Canada). The electrodes were calibrated before each test and at regular intervals throughout the analysis. Blood concentrations of progesterone and estrogen were determined using standard analysis kits (Gamma-Dynacare Medical Laboratories, Oakville, ON, Canada).
Near-infrared spectroscopy (NIRS; Hamamatsu NIRO 300, Hamamatsu Photonics KK, Hamamatsu, Japan) was used to measure continuously changes in concentration of local muscle deoxy-hemoglobin/myoglobin (Δ HHb) of the vastus lateralis muscle of the right leg. Optodes were placed on the belly of the muscle mid-way between the lateral epicondyle and the greater trochanter of the femur. Details describing the placement of the NIRS probe and NIRS measurement was described previously (DeLorey et al. 2003). Briefly, the NIRS unit uses four different wavelength laser diodes (775, 810, 850, and 910 nm) pulsed in rapid succession, with the reflected light detected by the photomultiplier tube. The intensity of incident and transmitted light was recorded continuously at 1 Hz and, along with the relevant specific extinction coefficients and optical path length [assuming a differential path length factor = 3.83 (Kowalchuk et al. 2002)], used for online estimation and display of the relative concentration changes from the “zero” set during the resting baseline of Δ HHb. The raw attenuation signals (in optical density units) were transferred to computer and stored for further analysis. The NIRS-derived Δ HHb signal used in this study is assumed to reflect local muscle O2 extraction and thus provide an estimate of changes in the balance between local muscle O2 delivery and O2 utilization within the NIRS field of interrogation (De Blasi et al. 1993; Ferrari et al. 1997).
Data analysis and curve fitting
The breath-by-breath \(\dot{V}O_{2}\) data obtained during each step increase in WR were filtered and linearly interpolated at 1 s intervals. To ensure the appropriateness of this protocol, the \(\dot{V}O_{2}\) response parameters for the different transitions were compared, as no differences were found between the individual transitions, all six transitions were averaged together. Each transition was time-aligned, ensemble-averaged to yield a single profile and time-averaged into 10 s bins to give a single response for each subject. The on-transient response to moderate-intensity exercise was modeled as a mono-exponential of the form
where Y (t) represents the variable at any time (t); Y (BSL) is the baseline value of Y before the step increase in WR; Amp the amplitude (i.e., steady-state increase in Y above baseline); τ the time constant (i.e., the time taken to reach 63% of the steady-state response); and TD is the time delay. \(\dot{V}O_{2}\) data were fit from the phases 1–2 transition to the end of exercise, as previously described (Rossiter et al. 2001).
Respiratory exchange ratio (RER) was calculated as the ratio \({{\dot{V}\hbox{CO}}}_{{{2}}}{{/\dot{V}\hbox{O}}}_{{{2}}}\) using the averaged single response data for each subject. The RER was calculated during the last 30 s of baseline exercise (i.e., steady-state at 20 W), and during the last 30 s of constant-load steady-state exercise at 90% \(\hat{\theta}_{{{\rm L}}}.\)
The time delay before an increase in Δ HHb after exercise onset (Δ HHb-TD) was determined by second-by-second data and corresponded to the time to the first point demonstrating a consistent increase above the nadir of the signal. The NIRS-derived Δ HHb was subsequently time-averaged into 5 s bins for each subject. The Δ HHb data between the Δ HHb-TD and 90 s (corresponding to the duration of the phase 2 \(\dot{V}O_{2}\) response) approximated an exponential-like response and thus these data were modeled with an exponential function of the form given in equation Eq. 1 to determine the time course of muscle Δ HHb (τ Δ HHb). The effective time constant (τ′ = Δ HHb − TD + τ Δ HHb) was calculated to provide a description of the overall time course for muscle Δ HHb.
Statistical analysis
Parameter estimates for \(\dot{V}O_{2}\) and Δ HHb during moderate-intensity exercise in the follicular and luteal phases of the menstrual cycle and blood hormone concentrations were analyzed by a one-way repeated measures ANOVA. Changes in blood lactate− concentration were analyzed using a two-way ANOVA. Statistical significance was accepted at P < 0.05. All data are presented as mean (±SD).
Results
Subject characteristics are summarized in Table 1. Subjects cycled at an average WR of 74 (6) W, eliciting a \(\dot{V}O_{2}\) corresponding to 88 (4) % \(\hat{\theta}_{{{\rm L}}},\) and 45 (8) % \(\dot{V}O_{2}\) peak.
Menstrual cycle and hormonal concentrations
Females were tested in the follicular phase, 3.6 (1.1) days (day 1) and 4.8 (1.1) days (day 2) after the start of menstruation, and in the luteal phase, 7.2 (1.9) days (day 1) and 5.6 (2.2) days (day 2) before the start of the subsequent menstruation. The average length of menstrual cycle for these females was 28 (1) days. The mean venous blood estrogen and progesterone concentrations were 96 (24) pmol/l and 2 (1) nmol/l, respectively, in the follicular phase, and 276 (143) pmol/l and 23 (19) nmol/l, respectively, in the luteal phase. All subjects presented with higher (P < 0.05) venous blood estrogen and progesterone concentrations, and lower (P < 0.05) ratio of estrogen-to-progesterone, during the luteal compared to the follicular phase of the menstrual cycle.
\(\dot{V}O_{2}\) kinetics
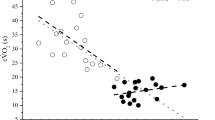
The pulmonary \(\dot{V}O_{2}\) response profiles for transitions to moderate-intensity exercise for a representative subject and the group mean responses for both the follicular and luteal phases of the menstrual cycle are presented in Fig. 1, and a summary of the parameter estimates for the \(\dot{V}O_{2}\) response are presented in Table 2. The time constant for the fundamental, phase 2 \(\dot{V}O_{2}\) response \((\tau \dot{V}O_{2})\) was not different between the follicular (22 ± 5) and the luteal (22 ± 6) phases of the menstrual cycle (Table 2; Fig. 2). Additionally, none of the subjects demonstrated an individual difference in \(\tau \dot{V}O_{2}\) as determined by their 95% confidence intervals (4 ± 1 s). There were no differences related to the menstrual cycle for baseline \(\dot{V}O_{2}, \dot{V}O_{2}\) amplitude, or end-exercise \(\dot{V}O_{2}\) during moderate-intensity exercise (Table 2).
\(\dot{V}O_{2}\) profile showing model best-fit line and residuals for a representative subject (a) and the group mean responses (b) during the transition to moderate-intensity cycle exercise during the follicular (closed circles and black line) and luteal (open circles and gray line) phases of the menstrual cycle. In b, each dot represents the average \(\dot{V}O_{2}\) within a 20 s time bin
Δ HHb
The Δ HHb response profiles for transitions to moderate-intensity exercise for a representative subject are shown in Fig. 3 and a summary of the parameter estimates for the NIRS-derived Δ HHb response is presented in Table 3. There were no difference related to the phase of the menstrual cycle for any of the NIRS-derived Δ HHb parameters.
Lactate
Plasma lactate− concentration increased (P < 0.05) ∼0.5 mmol/l above baseline levels by 120 s of exercise, with no differences between the follicular and luteal phases of the menstrual cycle (Fig. 4).
Blood lactate concentration during the transition to moderate-intensity exercise for females during the follicular (closed circles) and luteal (open circles) phases of the menstrual cycle. Asterisks represent significant difference (P < 0.05) from baseline for both follicular and luteal phases of the menstrual cycle
RER
Steady-state RER data are summarized in Table 4. RER was greater at end-exercise than at baseline but there were no differences between the follicular and luteal phases of the menstrual cycle.
Discussion
This is the first study to examine the adaptation of pulmonary \(\dot{V}O_{2}\) and muscle deoxygenation (Δ HHb) during the transition to moderate-intensity exercise in mid-follicular and mid-luteal phases of the menstrual cycle in healthy young female adults. The major findings of this study were that the adaptation of both pulmonary \(\dot{V}O_{2}\) and muscle Δ HHb were not affected by the phase of the menstrual cycle and thus the associated changes in plasma ovarian hormone (i.e., estrogen and progesterone) concentrations. Also, the steady-state baseline and end-exercise RER and the plasma lactate concentration throughout the exercise transition were not affected by the phase of the menstrual cycle. Together these results suggest that potential metabolic and/or cardiovascular changes related to ovarian hormonal differences that are associated with different phases of the menstrual cycle do not alter the time course of muscle O2 utilization (as reflected by pulmonary \(\dot{V}O_{2}\) kinetics).
The mid-follicular and mid-luteal phases of the menstrual cycle were identified based the subjects’ knowledge and history of their normal menstrual cycle. In the present study, the onset of menstruation represented the start of the menstrual cycle and the mid-luteal and mid-follicular phases of the menstrual cycle corresponded to, respectively, 3–5 days after the onset of menstruation and 5–8 days prior to the start of the next menstruation. This timing was confirmed with blood sampling showing that all subjects presented with elevated plasma estrogen and progesterone levels, and lower concentration ratio of estrogen-to-progesterone during the luteal compared to the follicular phases of the menstrual cycle. In general, the hormone concentrations measured in the present study are somewhat lower than the relatively large range of average concentrations reported for estrogen and progesterone in the follicular (estrogen, 99–300 pmol/l; progesterone, 1–2 nmol/l) and luteal phases (estrogen, 310–920 nmol/l; progesterone, 32–47 nmol/l) of the menstrual cycle (Broocks et al. 1990; Suh et al. 2002; Casazza et al. 2004; Lutoslawska et al. 2006; Lynn et al. 2007). All subjects reported a history of regular menstrual cycles, and thus the lower values found in this group of subjects may reflect the relative training status of these women, with 5 of 7 subjects being members of the university varsity rugby team (Broocks et al. 1990). However, based on accurate self-reporting by the subjects and confirmation by the measured blood ovarian hormones concentrations, we are confident that the testing was done within the appropriate menstrual cycle phase for each subject.
\(\dot{V}O_{2}\) kinetics
We hypothesized that females would demonstrate slower pulmonary \(\dot{V}O_{2}\) kinetics in the luteal compared to the follicular phase on the menstrual cycle based on findings, in rats (Campbell et al. 2003), showing greater PDK activity associated with elevated plasma estrogen and progesterone concentrations. Higher PDK activity (and ratio of PDK-to-pyruvate dehydrogenase phosphatase (PDP) activity) would inhibit PDH activity and thus limit the provision of carbohydrate-derived acetyl CoA into the TCA cycle and reducing equivalents (NADH and FADH2) to ETC during the transition to exercise. Activation of PDH has been implicated as contributing to metabolic inertia, and thus limiting the activation of mitochondrial O2 utilization during the transition to exercise. However, in the present study, the activation of pulmonary \(\dot{V}O_{2}\) kinetics and, presumably, muscle O2 utilization were not different in the two phases of the menstrual cycle, despite increases in blood estrogen and progesterone concentrations between the follicular and luteal phases. Also, steady-state RER values were not different in either phase of the menstrual cycle. Thus, combined, these results suggest that there was no measurable effect of cyclical changes in plasma estrogen and progesterone concentrations on substrate oxidation during the transition to short-duration (i.e., 6 min) constant-load moderate-intensity exercise.
The finding in the present study that the RER was not different in either phase of the menstrual cycle contrasts with studies of more prolonged exercise which report that females preferentially utilize fat as a fuel source during the luteal phase of the menstrual cycle (Bonen et al. 1983; Dombovy et al. 1987; Hackney et al. 1994; Hackney 1999; Zderic et al. 2001; Campbell et al. 2001), although others report no differences in RER between phases of the menstrual cycle (Braun et al. 2000; Horton et al. 2002, 2006). In the present study, RER was measured during the first 6 min of exercise, whereas in previous studies measurements were made later in exercise (>10 min) where an effect of menstrual cycle phase on substrate utilization has been observed (Braun et al. 2000; Zderic et al. 2001; Campbell et al. 2001). At the early stage of exercise studied in the present study, it is unlikely that fatty acid oxidation contributes significantly to ATP production regardless of the hormonal status, but rather carbohydrate stores will be preferentially utilized in these early stages of the transition to exercise (i.e., >10 min). Thus, the ability to detect a change in substrate utilization may depend on the timing of measurement and the discrepancies of this timing in the literature may potentially explain the inconsistent findings regarding substrate utilization and menstrual cycle phase.
A rise in plasma lactate− concentration reflects, in part, the imbalance between pyruvate production and oxidation (Spriet et al. 2000), and thus muscle lactate accumulation in (and its efflux from) muscle. The finding that the plasma lactate− concentration was similar within different phases of the menstrual cycle early in exercise is consistent with our finding of no differences in \(\dot{V}O_{2}\) kinetics and suggests that the contributions of both oxidative and substrate-level phosphorylation to ATP synthesis were similar in both the follicular and luteal phases. The finding of no differences in the plasma lactate− concentration within the two phases of the menstrual cycle is in agreement with some (Bonen et al. 1983; Nicklas et al. 1989; Dean et al. 2003) but not all (Jurkowski et al. 1981; Lavoie et al. 1987; McCracken et al. 1994; Galliven et al. 1997) studies that have measured plasma lactate− during exercise across menstrual cycle phase. In those studies that observed lower plasma lactate− concentration in the luteal phase, measurements were made during more prolonged exercise (i.e., >10 min) (Jurkowski et al. 1981; Lavoie et al. 1987; Galliven et al. 1997) or during recovery from exercise (McCracken et al. 1994), which suggests that a carbohydrate-sparing effect associated with the luteal phase is not manifest until later in exercise when fatty acids are more likely to be mobilized and utilized.
Muscle Δ HHb kinetics
No differences were observed for any of the kinetic parameters for the NIRS-derived Δ HHb response in the two phases of the menstrual cycle. Changes in the Δ HHb signal reflect the balance between local muscle O2 delivery and O2 utilization (and thus O2 extraction) in the region of tissue being interrogated by the NIRS signal. When NIRS-derived measures of muscle deoxygenation (i.e., Δ HHb) are used in combination with measures of pulmonary \(\dot{V}O_{2}\) kinetics (reflecting the kinetics of muscle O2 utilization), it is possible to speculate on the adaptation of local muscle perfusion and muscle O2 delivery based on the relationship: \(Q_{{{\left(t \right)}}} \propto {\hbox{phase 2}}\;\dot{V}O_{{2}{{(t)}}} /\Delta {\hbox{HHb}}_{{(t)}}\) (DeLorey et al. 2003). The presence of a period of no change in the Δ HHb signal (i.e., Δ HHb-TD) following the onset of exercise suggests that the increase in O2 delivery is matched to the increase in muscle O2 utilization during the immediate transition (i.e., initial seconds) to exercise (Behnke et al. 2001, 2002; Grassi et al. 2003; DeLorey et al. 2003). The similarity in the Δ HHb-TD in the two phases of the menstrual cycle suggests that the increase in local muscle O2 delivery also was matched to the increase in O2 utilization. The similar τ Δ HHb in combination with a similar \(\tau \dot{V}O_{2}\) in each of the menstrual cycle phases suggests that the O2 delivery response (and O2 extraction) were similar throughout the transition to moderate-intensity exercise. While no study to date has demonstrated altered blood flow in the different menstrual cycle phases, Ettinger et al. (1998) observed higher muscle sympathetic nerve activity during the early (days 1–4; low estrogen/low progesterone) compared to late (days 10–14; high estrogen/low progesterone) follicular phase accompanying similar heart rate and mean arterial pressure and suggested that this might result in attenuated blood flow. Additionally, Kolka and Stephenson (1997) measured forearm blood flow during leg exercise and demonstrated a higher steady-state blood flow in the luteal phase. Our finding of an unchanged Δ HHb response in the presence of an unchanged \(\dot{V}O_{2}\) response is not consistent with these findings but is in agreement with the recent results demonstrating no effect of menstrual cycle phase or estrogen and progesterone on resting calf blood flow (Cooper et al. 2006). Thus, in combination, the lack of change in the Δ HHb-TD and τ Δ HHb accompanying the similar \(\dot{V}O_{2}\) response in the follicular and luteal phases suggest that there is no effect of menstrual cycle phase on the adaptation of muscle blood flow or O2 delivery during the transition to moderate-intensity exercise.
Previous studies have suggested that O2 availability does not limit \(\dot{V}O_{2}\) kinetics during the transition to upright cycling (Jones and Poole 2005b). However, recently we observed a speeding of pulmonary \(\dot{V}O_{2}\) kinetics when moderate-intensity exercise was preceded by a bout of heavy-intensity exercise and this speeding was accompanied by a greater muscle perfusion (as determined by NIRS-derived Δ HbO2 and Δ HbTOT) immediately prior to and throughout the transition to moderate-intensity exercise, suggesting that muscle perfusion and O2 delivery, in part, were limiting the rate of adaptation of muscle O2 utilization (Gurd et al. 2005, 2006). In the present study, estimated muscle O2 availability was not different between phases of the menstrual cycle, and thus differences in \(\dot{V}O_{2}\) kinetics between the luteal and follicular phases of the menstrual cycle would not be expected even if O2 availability was limiting muscle O2 consumption.
Limitations
As with any study examining the effects of menstrual cycle certainty regarding timing of testing is always a potential limitation. Based on the subjects’ knowledge of their normal menstrual cycle and confirmation of appropriate blood hormonal levels for each phase of the menstrual cycle, we are confident that testing was done in the proper phase and thus represents the physiological response during the follicular and luteal phases of the menstrual cycle. While we are confident in our measures of pulmonary \(\dot{V}O_{2}\) and muscle deoxygenation kinetics, our findings in this relatively athletic subject population may not be generalized to a non-athletic, sedentary, female population.
The hypotheses for the current study were based on reports that estrogen and progesterone may inhibit PDH activity through activation of PDK activity. In the present study, because of the invasive nature of the muscle biopsy technique, we were unable to measure either PDH or PDK activity in muscle. However, we are confident that our measures of pulmonary \(\dot{V}O_{2}\) and muscle deoxygenation provide an accurate reflection of local muscle O2 utilization during the transition to exercise and that, in this study, there were no effects of menstrual cycle on activation of pulomonary \(\dot{V}O_{2}\) (or muscle O2 utilization). The question of whether the menstrual cycle, and its accompanying changes in ovarian hormone concentrations, has any effect on PDH activity in females during the transition to exercise remains to be answered.
Conclusion
In summary, these findings demonstrate that any hormonal differences between the follicular and luteal phases of the menstrual cycle had no measurable effect on the adaptation of pulmonary O2 uptake (and presumably, muscle O2 utilization) during the transition to moderate-intensity exercise in healthy, young female adults. The similar adaptation of NIRS-derived Δ HHb in the two phases of the menstrual cycles combined with no change in pulmonary \(\dot{V}O_{2}\) kinetics suggests that there were no differences in the adaptation of local muscle blood flow and O2 delivery. Also, similarities in the RER and plasma lactate− concentration in the two phases of the menstrual cycle suggest that the contribution of carbohydrate-derived substrate to ATP production was similar, at least during the immediate transition to exercise. Finally, a practical implication of these findings is that when using young, healthy, fit female subjects in studies examining pulmonary \(\dot{V}O_{2}\) kinetics, it is not be necessary to control for each phase of the menstrual cycle in the study design.
References
Babcock MA, Paterson DH, Cunningham DA, Dickinson JR (1994) Exercise on-transient gas exchange kinetics are slowed as a function of age. Med Sci Sports Exerc 26:440–446
Beaver WL, Lamarra N, Wasserman K (1981) Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51:1662–1675
Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC (2001) Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126:53–63
Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC (2002) Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol 133:229–239
Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC (2005) Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146:259–268
Bonen A, Haynes FJ, Watson-Wright W, Sopper MM, Pierce GN, Low MP, Graham TE (1983) Effects of menstrual cycle on metabolic responses to exercise. J Appl Physiol 55:1506–1513
Braun B, Mawson JT, Muza SR, Dominick SB, Brooks GA, Horning MA, Rock PB, Moore LG, Mazzeo RS, Ezeji-Okoye SC, Butterfield GE (2000) Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol 88:246–256
Broocks A, Pirke KM, Schweiger U, Tuschl RJ, Laessle RG, Strowitzki T, Horl E, Horl T, Haas W, Jeschke D (1990) Cyclic ovarian function in recreational athletes. J Appl Physiol 68:2083–2086
Burnley M, Jones AM, Carter H, Doust JH (2000) Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol 89:1387–1396
Campbell SE, Febbraio MA (2001) Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab 281:E803–E808
Campbell SE, Febbraio MA (2002) Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab 282:E1139–E1146
Campbell SE, Angus DJ, Febbraio MA (2001) Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am J Physiol Endocrinol Metab 281:E817–E825
Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D (2003) 17beta-estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. J Mol Endocrinol 31:37–45
Casazza GA, Jacobs KA, Suh SH, Miller BF, Horning MA, Brooks GA (2004) Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol 97:302–309
Cooper BC, Sites CK, Fairhurst PA, Toth MJ (2006) Evidence against a role for ovarian hormones in the regulation of blood flow. Fertil Steril 86:440–447
De Blasi RA, Cope M, Elwell C, Safoue F, Ferrari M (1993) Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur J Appl Physiol Occup Physiol 67:20–25
Dean TM, Perreault L, Mazzeo RS, Horton TJ (2003) No effect of menstrual cycle phase on lactate threshold. J Appl Physiol 95:2537–2543
DeLorey DS, Kowalchuk JM, Paterson DH (2003) Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95:113–120
Devries MC, Hamadeh MJ, Graham TE, Tarnopolsky MA (2005) 17-{beta}-estradiol supplementation decreases glucose Ra and Rd with no effect on glycogen utilization during moderate intensity exercise in men. J Clin Endocrinol Metab 90:6218–6225
Dombovy ML, Bonekat HW, Williams TJ, Staats BA (1987) Exercise performance and ventilatory response in the menstrual cycle. Med Sci Sports Exerc 19:111–117
Ellis GS, Lanza-Jacoby S, Gow A, Kendrick ZV (1994) Effects of estradiol on lipoprotein lipase activity and lipid availability in exercised male rats. J Appl Physiol 77:209–215
Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI (1998) Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 85:2075–2081
Ferrari M, Binzoni T, Quaresima V (1997) Oxidative metabolism in muscle. Philos Trans R Soc Lond B Biol Sci 352:677–683
Galliven EA, Singh A, Michelson D, Bina S, Gold PW, Deuster PA (1997) Hormonal and metabolic responses to exercise across time of day and menstrual cycle phase. J Appl Physiol 83:1822–1831
Grassi B (2001) Regulation of oxygen consumption at exercise onset: is it really controversial? Exerc Sport Sci Rev 29:134–138
Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P (2003) Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95:149–158
Gurd BJ, Scheuermann BW, Paterson DH, Kowalchuk JM (2005) Prior heavy-intensity exercise speeds VO2 kinetics during moderate-intensity exercise in young adults. J Appl Physiol 98:1371–1378
Gurd B, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM (2006) Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J Physiol 577:985–986
Hackney AC (1999) Influence of oestrogen on muscle glycogen utilization during exercise. Acta Physiol Scand 167:273–274
Hackney AC, McCracken-Compton MA, Ainsworth B (1994) Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Int J Sport Nutr 4:299–308
Hamadeh MJ, Devries MC, Tarnopolsky MA (2005) Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J Clin Endocrinol Metab 90:3592–3599
Horton TJ, Miller EK, Glueck D, Tench K (2002) No effect of menstrual cycle phase on glucose kinetics and fuel oxidation during moderate-intensity exercise. Am J Physiol Endocrinol Metab 282:E752–E762
Horton TJ, Miller EK, Bourret K (2006) No effect of menstrual cycle phase on glycerol or palmitate kinetics during 90 min of moderate exercise. J Appl Physiol 100:917–925
Hughson RL, Tschakovsky ME, Houston ME (2001) Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29:129–133
Jones AM, Poole DC (2005b) Oxygen uptake dynamics: from muscle to mouth—an introduction to the symposium. Med Sci Sports Exerc 37:1542–1550
Jones AM, Poole DC (2005a) Oxygen uptake kinetics in sport, exercise and medicine. Routledge, London
Jurkowski JE, Jones NL, Toews CJ, Sutton JR (1981) Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol 51:1493–1499
Kalkhoff RK (1982) Metabolic effects of progesterone. Am J Obstet Gynecol 142:735–738
Kendrick ZV, Ellis GS (1991) Effect of estradiol on tissue glycogen metabolism and lipid availability in exercised male rats. J Appl Physiol 71:1694–1699
Kirwan LD, MacLusky NJ, Shapiro HM, Abramson BL, Thomas SG, Goodman JM (2004) Acute and chronic effects of hormone replacement therapy on the cardiovascular system in healthy postmenopausal women. J Clin Endocrinol Metab 89:1618–1629
Kolka MA, Stephenson LA (1997) Effect of luteal phase elevation in core temperature on forearm blood flow during exercise. J Appl Physiol 82:1079–1083
Kowalchuk JM, Rossiter HB, Ward SA, Whipp BJ (2002) The effect of resistive breathing on leg muscle oxygenation using near-infrared spectroscopy during exercise in men. Exp Physiol 87:601–611
Lavoie JM, Dionne N, Helie R, Brisson GR (1987) Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. J Appl Physiol 62:1084–1089
Lutoslawska G, Niedbalska M, Skierska E, Keska A, Szpocinska-Byszewska E, Zolnowska M (2006) Plasma proinsulin, C-peptide and sex hormone concentrations in regularly menstruating premenopausal females with ovulatory and anovulatory menstrual cycles. J Sports Med Phys Fitness 46:138–142
Lynn BM, McCord JL, Halliwill JR (2007) Effects of the menstrual cycle and sex on postexercise hemodynamics. Am J Physiol Regul Integr Comp Physiol 292:R1260–R1270
McCracken M, Ainsworth B, Hackney AC (1994) Effects of the menstrual cycle phase on the blood lactate responses to exercise. Eur J Appl Physiol Occup Physiol 69:174–175
Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR (2003) Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol 547:309–316
Nicklas BJ, Hackney AC, Sharp RL (1989) The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int J Sports Med 10:264–269
Rooney TP, Kendrick ZV, Carlson J, Ellis GS, Matakevich B, Lorusso SM, McCall JA (1993) Effect of estradiol on the temporal pattern of exercise-induced tissue glycogen depletion in male rats. J Appl Physiol 75:1502–1506
Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ (2001) Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol 537:291–303
Spriet LL, Howlett RA, Heigenhauser GJ (2000) An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc 32:756–763
Sudhir K, Elser MD, Jennings GL, Komesaroff PA (1997) Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension 30:1538–1543
Suh SH, Casazza GA, Horning MA, Miller BF, Brooks GA (2002) Luteal and follicular glucose fluxes during rest and exercise in 3-h postabsorptive women. J Appl Physiol 93:42–50
Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL (1998) Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. Am J Physiol 274:E377–E380
Zderic TW, Coggan AR, Ruby BC (2001) Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol 90:447–453
Acknowledgments
This study was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grants. Additional support was provided by a UWO Academic Development Fund Grant and infrastructure support of the Canadian Foundation for Innovation (CFI) and Ontario Innovation Trust (OIT). Financial support to B. J. Gurd was provided by an Ontario Graduate Scholarship. We wish to acknowledge the technical support provided by Brad Hansen, and the subjects for their participation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gurd, B.J., Scheid, J., Paterson, D.H. et al. O2 uptake and muscle deoxygenation kinetics during the transition to moderate-intensity exercise in different phases of the menstrual cycle in young adult females. Eur J Appl Physiol 101, 321–330 (2007). https://doi.org/10.1007/s00421-007-0505-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0505-9