Abstract
Large elastic artery stiffness increases with age and menopause is a mitigating factor in women. High-intensity resistance training (RT) increases arterial stiffness in young men and women. However, the effects of moderate intensity RT on central aortic pressure wave reflection in healthy postmenopausal women are unknown. Healthy sedentary normotensive postmenopausal women were randomly assigned to either 18 weeks (2 days/week) of RT (RES n = 13) or aerobic training (AER n = 10). Central aortic pressure wave reflection and brachial artery reactivity were assessed before and after training. Central aortic pressure wave reflection was evaluated by measuring aortic augmentation index (AIa) and round trip travel time (Δt p) of the reflected arterial pressure wave using applanation tonometry. Brachial artery reactivity was assessed using brachial artery flow mediated dilation (FMD). Eighteen weeks of RT did not change AIa (28.9 ± 1.9 vs. 28.5 ± 1.9%; mean ± S.E.M.) or Δt p (140.6 ± 2.4 vs. 141.2 ± 2.1 ms). In contrast, the AER group showed a decrease in AIa from 28.8 ± 2.1 to 25.1 ± 1.4% (P < 0.05) and an increase in Δt p from 137.3 ± 2.5 to 144.6 ± 2.9 ms (P < 0.05). Brachial FMD did not significantly change in either group following training. This prospective, randomized study demonstrated that moderate intensity whole body RT in previously sedentary, normotensive postmenopausal women does not alter central aortic pressure wave reflection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk of cardiovascular disease in women increases substantially after menopause (van der Schouw et al. 1996) and remains the major cause of mortality in this population (AHA 2003). The mechanism by which menopause exerts its effect on the cardiovascular system may be related to changes in estrogen deficiency on endothelial function, vascular smooth muscle phenotype, and elastin/collagen-related stiffening of large elastic arteries. Large elastic artery stiffness increases with age in both men and women (McEniery et al. 2005) and menopause appears to augment these age-related changes (Waddell et al. 2001; Zaydun et al. 2006). Moreover, increased arterial stiffness is an independent predictor of adverse cardiovascular events and mortality (Laurent et al. 2001; Weber et al. 2004).
Endurance exercise is associated with reduced arterial stiffness and central aortic arterial wave reflections in both young (Cameron and Dart 1994) and older healthy individuals (Tanaka et al. 2000), competitive endurance athletes (Edwards and Lang 2005), and patients with coronary artery disease (Edwards et al. 2004). However, less is known about the effects of resistance training (RT) on arterial stiffness. Two cross-sectional studies have suggested that young and middle aged resistance trained men demonstrate lower arterial compliance than their age matched sedentary counterparts (Bertovic et al. 1999; Miyachi et al. 2003). Three interventional studies have examined the effect of RT on arterial function. Miyachi et al. (2004) reported that 4 months of RT decreased central arterial compliance in young healthy men. Cortez-Cooper et al. (2005) reported that 11 weeks of high-intensity RT resulted in increases in arterial stiffness and wave reflection in young healthy women. In contrast, Rakobowchuk et al. (2005a) found that central arterial compliance was unaltered after 3 months of RT in young men. The aforementioned studies have all used young healthy individuals with no evidence of baseline arterial stiffness prior to testing. American Heart Association (AHA), American College of Sports Medicine (ACSM), and the American Diabetes Association (ADA), recommend RT 2–3 days/week in postmenopausal women to prevent osteoporosis, sarcopenia, obesity, and the clustering of cardiovascular risk factors associated with the metabolic syndrome. However, no study has investigated the effects of RT on arterial function in normotensive postmenopausal women who demonstrate baseline elevated arterial stiffness when compared to younger counterparts (McEniery et al. 2005).
The purpose of this study was to determine whether whole body RT alters central aortic pressure wave reflection in previously sedentary normotensive postmenopausal women. We hypothesized that 18 weeks of resistance training, when performed in accordance with the ACSM and AHA RT guidelines (Pollock et al. 2000; Pescatello et al. 2004), would not result in changes in central aortic pressure wave reflection in postmenopausal women.
Methods
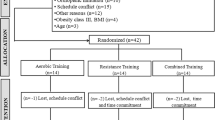
Twenty-six healthy (n = 26; age range 52–71 years), sedentary post-menopausal women were randomized at study entry on a 50/50 basis to either a group that performed RT or endurance training. The study was approved by the University of Florida Health Science Center Institutional Review Board and all subjects signed written informed consent to participate in the study. The women were approximately 8.6 years postmenopausal, nonsmokers, and were not receiving hormone replacement therapy. Additionally, subjects did not take any vasoactive medications known to affect arterial function, thyroid medication, or antihypertensive medication and had not participated in an exercise program at least 6 months before study entry. Subjects were randomly assigned to 18 weeks of RT (RES n = 13) or to 18 weeks of aerobic training (AER n = 13). Data were collected and analyzed at the beginning and end of exercise training at least 24 h after the last RT or walking exercise session. All measurements were performed by the same investigator while the subjects were in a supine position in a quiet, temperature controlled room (21–23°C). Subjects were asked to abstain from caffeine and alcohol for at least 12 h prior to vascular measurements.
Pulse waveform analysis
Following a 10 min rest period in a supine position resting heart rate (HR) and brachial systolic, diastolic, and pulse blood pressure measurements were performed in triplicate in the left arm using an automated non-invasive blood pressure (BP) cuff (Omron, Inc). An average of the three HR and BP measurements were used for resting values. Assessment of central aortic wave reflection characteristics was performed non-invasively using the SphygmoCor system (Version 7.0, AtCor Medical, Sydney, Australia). High-fidelity radial artery pressure waveforms were recorded by applanation tonometry of the radial pulse using a “pencil type” micromanometer (Millar Instruments, Houston, TX, USA). After obtaining 10 s of stable baseline waveform recordings, a generalized mathematical transfer function was used to synthesize a central aortic pressure waveform (Chen et al. 1997; Nichols and O’Rourke 2005). This technique is reproducible (Wilkinson et al. 1998; Siebenhofer et al. 1999) and the aortic pressure waveform was established by triplicate measurement in our laboratory on nonconsecutive days in young, healthy men with a mean coefficient of variation of 6.5% (Casey et al. 2006).
The central aortic pressure wave is composed of a forward traveling wave, generated by left ventricular ejection and a reflected wave that is returning to the ascending aorta from the periphery (Nichols and Singh 2002). The reflected wave is a summation of individual reflected waves generated at arterial and arterial–arteriolar junctions. High-resistance arterioles are considered to be the major sites of wave reflection in the circulatory system. The aortic augmentation index (AIa) is defined as reflected wave amplitude divided by pulse pressure and is expressed as a percentage (Murgo et al. 1980). The forward and reflected waves travel in opposite directions along the artery at the same velocity. The round trip travel time (Δt p) of the forward traveling wave from the ascending aorta to the major reflection site and back is measured from the foot of the forward traveling pressure wave to the foot of the reflected wave. Δt p is inversely related to arterial pulse wave velocity and arterial stiffness, and directly related to the distance to the reflecting site (Nichols and Singh 2002). A stiffer arterial system results in faster wave travel and quicker return of the reflected wave and thus a lower Δt p. AIa is an index of wave reflection, which is a manifestation of systemic arterial stiffness. Because an inverse linear relationship exists between AIa and HR (Wilkinson et al. 2002), the SphygmoCor software normalizes AIa values to 75 bpm. Assessment of central arterial pressure waves are described in detail by Nichols and Singh (2002).
Brachial artery reactivity
Brachial artery flow mediated dilation (FMD) was assessed non-invasively in the right arm using a high-resolution ultrasound machine (ATL Ultramark 9) equipped with a 10.5 MHz transducer (ATL) as described by Celermajer et al. (1992). Briefly, resting baseline end diastolic brachial diameters were obtained with the transducer placed 3–5 cm above the antecubital crease. A blood pressure cuff placed on the upper arm was then inflated to 200 mmHg for 5 min followed by a rapid deflation. The transducer was held in the same position for the duration of cuff inflation to ensure the same section of the brachial artery was measured before and after cuff inflation. The brachial artery was imaged and recorded for 3 min following cuff deflation. Ultrasound images were recorded on a super VHS videocassette for later off-line manual analysis using specialized image analysis software (Image Pro, Data Translation, Inc.) Brachial artery diameters were determined during end-diastole by measuring the distance between the anterior and posterior wall of the intima (Corretti et al. 2002). Brachial FMD was expressed as absolute (Δmm) and as a percent increase from baseline (FMD%).
Muscular strength
Muscle strength was assessed by a 1-RM test using variable resistance MEDX training equipment. Six exercises were used for strength testing: chest press, leg extension, leg press, lat pulldown, seated row, and overhead press. Prior to testing, subjects warmed up each muscle group by doing 5–10 repetitions using a lightweight. After a 2-min rest, each subject began the process of reaching her 1-RM. The determination of each subject’s 1-RM was achieved within five attempts. Each attempt was followed by 2 min of rest. Strength testing was conducted at the start of the study and following 18 weeks for the RES group.
Exercise training
Exercise training was performed at the “Living Well Center” at the University of Florida, Gainesville, FL. Exercise training was administered as per ACSM guidelines for an older population (ACSM 2000). The RES group exercised on two nonconsecutive days per week. Each session consisted of a 5-min warm-up on a treadmill followed by approximately 30 min of resistance training. Subjects performed one set of 12 repetitions of the following ten exercises using variable resistance MEDX training equipment: leg extension, leg curl, leg press, lat pulldown, seated row, chest press, overhead press, bicep curl, triceps extension, and abdominal crunch. Initial resistance for subjects was set at 50% of their 1-RM. The weight was increased by 5% after the subject could successfully complete two consecutive exercise sessions of 12 repetitions with proper form and control. The AER group also exercised on two nonconsecutive days per week. Each session consisted of 30–40 min of treadmill walking between 65 and 80% of their predicted HR reserve (Karvonen Formula). HR was determined using a monitor (Polar Electro Inc.). Exercise intensity (speed and/or grade) was adjusted throughout the intervention to keep the subjects within their training zone. An exercise specialist monitored both the RES and AER group during exercise and made adjustments to workloads and equipment when needed.
Statistical analysis
Outcomes data are reported as mean ± S.E.M. Continuous variables were analyzed by a two-way analysis of variance (ANOVA) with repeated measures of AIa, Δt p, HR, brachial and aortic blood pressures, and brachial FMD before and after 18 weeks of resistance or aerobic training. When a significant group-by-time interaction was observed, within-group comparisons between time points were performed using Tukey’s post hoc analysis. Measurements of strength were compared using a paired Student’s t test. All statistical analyses were performed using SPSS 12.0 for Windows (SPSS, Inc., Chicago, IL, USA). An alpha level of P < 0.05 was required for statistical significance.
Results
Of the 26 subjects who underwent initial testing, 3 subjects in the AER group dropped out. Two dropped due to injuries unrelated to the exercise intervention and one due to non-compliance with the training regimen. Therefore, data presented are the means for 13 RES and 10 AER subjects. There were no significant differences in height, weight, body mass index (BMI), age, years postmenopausal, HR, or peripheral blood pressure between the two groups at study entry (Table 1). Weight and BMI did not change following training in either the RES or AER group.
Hemodynamic and pulse wave analysis
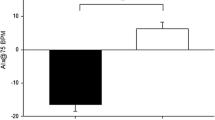
At study entry values for AIa and Δt p did not differ between groups. Resting HR, and brachial and aortic blood pressures did not change after 18 weeks of training in either group (Table 2). AIa decreased (28.8 ± 2.1 to 25.1 ± 1.4%; P < 0.05) following 18 weeks of aerobic training but AIa did not change in the RT group (28.9 ± 1.9 vs. 28.5 ± 1.9%). Additionally, there was an increase (137.3 ± 2.5 to 144.6 ± 2.9 ms; P < 0.05) in Δt p in the AER group but Δt p did not change in the RT group (140.6 ± 2.4 vs. 141.2 ± 2.1 ms). Relative changes in AIa and Δt p are presented in Fig. 1.
Aortic wave reflection characteristics before and after 18 weeks of resistance training (RES) or aerobic training (AER). a Aortic augmentation index (AIa); b Round trip travel time of reflected pressure wave from ascending aorta to peripheral reflecting sites and back (Δt p). Data represent mean ± SEM. * P ≤ 0.05 baseline versus 18 weeks within group
Brachial FMD
Resting brachial artery diameter did not change in either the RES or AER group following training. There were no significant changes in relative or absolute FMD of the brachial artery following training in either group (Table 2).
Muscle strength
1-RM muscle strength in the RT group increased on all six machines used for criterion measures of muscle strength (P < 0.01). Mean changes for the chest press, lat pulldown, seated row, overhead press, leg extension, and leg press, are presented in Fig. 2.
Discussion
This randomized study examined the impact of RT on arterial stiffness in postmenopausal women. The principal finding is that 18 weeks of moderate intensity whole body RT does not alter central aortic pressure wave reflection as assessed by AIa. Second, whole body RT had no effect on brachial artery reactivity. These results suggest that RT, when performed in accordance with ACSM and AHA guidelines (Pollock et al. 2000; Pescatello et al. 2004), does not have a deleterious effect on arterial function in otherwise healthy, but sedentary normotensive postmenopausal women.
In contrast to our findings, RT increases central artery stiffness in young women. Cortez-Cooper et al. (2005) reported that 11 weeks of high-intensity RT in young healthy women results in an increase of arterial stiffness. Although the authors found an increase in augmentation index of the carotid artery, both the RT and control groups demonstrated an increase in the carotid–femoral pulse wave velocity (PWV) with the greatest increase in PWV occurring in the untrained control group. There are several possible explanations for the disparate results. The current investigation studied arterial function in older postmenopausal women, whereas the previous work studied young healthy females. Ageing results in a progressive increase in arterial stiffness in both women and men as assessed by AIa (McEniery et al. 2005). Average AIa for older women (50–59 years) is 33% whereas in younger females (20–29 years) the average AIa is 9% (McEniery et al. 2005). Consequently, subjects in the present investigation would have had higher levels of arterial stiffness at study entry when compared to younger counterparts, which could attenuate the magnitude of change in arterial stiffness following RT.
Discrepancy between results of the two studies may also be explained by differences in the RT protocol. Cortez-Cooper et al. (2005) used a RT protocol consisting of high intensity ‘super sets’ and an extremely high volume (up to six sets per exercise), both of which are not commonly recommended for the majority of the population and are usually performed only by competitive athletes (Pollock et al. 2000; Pescatello et al. 2004). An interventional RT study that utilized high intensity training protocols in novice male weight trainers, also reported increases of central arterial stiffness (Miyachi et al. 2004). In contrast, Rakobowchuk et al. (2005a) employing similar vascular measurement techniques, demonstrated that central arterial compliance was unaltered after 3 months of RT in young men when they used a progressive training protocol which increased intensity but not the volume of exercise over 12 weeks.
Studies that have reported increases in arterial stiffness following RT have speculated on the possible physiological mechanisms involved (Miyachi et al. 2004; Cortez-Cooper et al. 2005). Stiffness of a vessel is controlled by distending pressure, structural elements within the vessel wall, mainly elastin and collagen, and functional components of the smooth muscle cells. It is unlikely that structural changes in the arterial wall would occur over short durations (e.g. 2–4 months). Increased levels of sympathetic nervous system activity can enhance the basal vasoconstrictor tone of vascular smooth muscle and ultimately play a role on the compliance of peripheral arteries. Heavy RT increases resting norepinephrine levels in older men (Pratley et al. 1994). However, 16 weeks of RT did not change resting levels of catecholamines in postmenopausal women (Ryan et al. 1995). Sympathetic nervous system activity was not assessed in the current study or the studies that have reported increases in arterial stiffness following RT. In large elastic and muscular arteries, stiffness can also be influenced by endothelial function (Wilkinson et al. 2004). Endothelial function, as assessed by brachial FMD, has an inverse relationship with central arterial stiffness (Nigam et al. 2003). Brachial artery reactivity in postmenopausal women was not altered by 18 weeks of RT, which is consistent with findings in younger subjects (Rakobowchuk et al. 2005b) and may explain why there was not an increase in central aortic pressure wave reflection following RT.
Walking for only 30–40 min 2 days per week resulted in improved wave reflection characteristics (i.e. AIa and Δt p). Although there was a trend towards improved brachial artery FMD in the aerobic group, it did not reach statistical significance. Habitual aerobic training may modify the elastin content of the large elastic arteries (Matsuda et al. 1993). However, with the short duration and small volume of training used in the current study, we speculate that such structural changes are unlikely. One other possible mechanism for vascular adaptations in the aerobic group is that walking decreased AIa by modifying risk factors for atherosclerosis, such as total and LDL cholesterol concentrations, fasting glucose, or mean arterial pressure (Seals 2003). However, there were no changes in mean arterial BP in the aerobic group.
This study examined the relationship between RT and arterial function in healthy, previously sedentary, postmenopausal women. The results of this study suggest that 18 weeks of moderate intensity whole-body RT does not alter central aortic pressure wave reflection in this population. This finding is in contrast to previous studies that have reported that RT has adverse effects on arterial function in younger men and women (Miyachi et al. 2004; Cortez-Cooper et al. 2005).
References
ACSM. (2000) Guidelines for exercise testing and prescription, 6th ed. Lippincott Williams and Wilkins, Philadelphia
AHA (2003) Heart disease and stroke statistics: 2003 Update. Dallas
Bertovic DA, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA (1999) Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension 33:1385–1391
Cameron JD, Dart AM (1994) Exercise training increases total systemic arterial compliance in humans. Am J Physiol 266:H693–H701
Casey DP, Pierce GL, Nichols WW, Braith RW (2006) Measurement of pulse wave velocity and augmentation index is reproducible in young, healthy men. Med Sci Sport Exer 38(5):S185
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE (1992) Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340:1111–1115
Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA (1997) Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 95:1827–1836
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265
Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, Tanaka H (2005) Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens 18:930–934
Edwards DG, Lang JT (2005) Augmentation index and systolic load are lower in competitive endurance athletes. Am J Hypertens 18:679–683
Edwards DG, Schofield RS, Magyari PM, Nichols WW, Braith RW (2004) Effect of exercise training on central aortic pressure wave reflection in coronary artery disease. Am J Hypertens 17:540–543
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A (2001) Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37:1236–1241
Matsuda M, Nosaka T, Sato M, Ohshima N (1993) Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol 66:122–126
McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR (2005) Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46:1753–1760
Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H (2003) Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 41:130–135
Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H (2004) Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 110:2858–2863
Murgo JP, Westerhof N, Giolma JP, Altobelli SA (1980) Aortic input impedance in normal man: relationship to pressure wave forms. Circulation 62:105–116
Nichols WW, O’Rourke MF (2005) McDonald’s blood flow in arteries: theoretical, experimental and clinical principles, 5th edn. Arnold, London
Nichols WW, Singh BM (2002) Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 17:543–551
Nigam A, Mitchell GF, Lambert J, Tardif JC (2003) Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol 92:395–399
Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA (2004) American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 36:533–553
Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, Bazzarre T (2000) AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the committee on exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 101:828–833
Pratley R, Nicklas B, Rubin M, Miller J, Smith A, Smith M, Hurley B, Goldberg A (1994) Strength training increases resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J Appl Physiol 76:133–137
Rakobowchuk M, McGowan CL, de Groot PC, Bruinsma D, Hartman JW, Phillips SM, MacDonald MJ (2005a) Effect of whole body resistance training on arterial compliance in young men. Exp Physiol 90:645–651
Rakobowchuk M, McGowan CL, de Groot PC, Hartman JW, Phillips SM, MacDonald MJ (2005b) Endothelial function of young healthy males following whole body resistance training. J Appl Physiol 98:2185–2190
Ryan AS, Pratley RE, Elahi D, Goldberg AP (1995) Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol 79:818–823
van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD (1996) Age at menopause as a risk factor for cardiovascular mortality. Lancet 347:714–718
Seals DR (2003) Habitual exercise and the age-associated decline in large artery compliance. Exerc Sport Sci Rev 31:68–72
Siebenhofer A, Kemp C, Sutton A, Williams B (1999) The reproducibility of central aortic blood pressure measurements in healthy subjects using applanation tonometry and sphygmocardiography. J Hum Hypertens 13:625–629
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR (2000) Aging, habitual exercise, and dynamic arterial compliance. Circulation 102:1270–1275
Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA (2001) Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens 19:2205–2212
Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B (2004) Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 109:184–189
Wilkinson IB, Franklin SS, Cockcroft JR (2004) Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 44:112–116
Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ (1998) Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 16:2079–2084
Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR (2002) Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens 15:24–30
Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, Motobe K, Hori S, Yamashina A (2006) Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis 184:137–142
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casey, D.P., Pierce, G.L., Howe, K.S. et al. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100, 403–408 (2007). https://doi.org/10.1007/s00421-007-0447-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0447-2