Abstract
The present study investigated the activity of the autonomic nervous system (ANS), a major influence in normal physiological function, and its association with unfavorable postmenopausal states in body composition, lipid and/or glucose metabolism, or cardiovascular profiles. Body composition, blood pressure, and blood profiles of lipid and glucose of 175 postmenopausal women were measured. Resting ANS activity was assessed by heart rate variability (HRV) power spectral analysis. To scrutinize the influence of ANS activity levels on postmenopausal obesity-related factors, we divided the subjects into a low group ( < 220 ms2) and a high group ( > 220 ms2), based on the total power of HRV. Low-frequency (P < 0.01) and high-frequency power (P < 0.01) were both significantly lower in the low group. No significant difference was found in age, age at menopause, or years after menopause between the two groups. In contrast, body mass index (P < 0.05), percentages of body fat (P < 0.01), and systolic (P < 0.01) and diastolic (P < 0.01) blood pressure were significantly greater in the low group. As to blood lipid profiles, triglycerides (P < 0.05), total cholesterol (P < 0.05), and low-density lipoprotein cholesterol (P < 0.05) were significantly higher in the low group. Our findings indicate that reduced sympatho-vagal activity is associated with higher postmenopausal body fat content, blood pressure, and blood lipid concentrations. This study further implies that such autonomic depression could be a crucial risk factor in undermining the health and, ultimately, the quality of life, of postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2002 National Nutrition Survey in Japan classified more than 30% of postmenopausal women over the age of 60 years as obese and revealed a significantly higher rate of obesity than that of the 1982 survey (Ministry of Health, Labor and Welfare, Japan 2004). The gynecological literature defines menopause as the permanent end of menstrual cyclicity secondary to ovarian failure, but it encompasses much more than just the cessation of menstruation, with the consequences being multifaceted (Wise et al. 1996). According to longitudinal and cross-sectional studies, postmenopausal women have greater total and abdominal adiposity and lower energy expenditure during rest and physical activity compared to that of premenopausal women (Lynch et al. 2002; Poehlman et al. 1995, 1997). In addition, recent research has shown deleterious effects of menopause on cardiovascular and metabolic processes that predispose women to a greater incidence of metabolic risk factors for cardiovascular disease such as dyslipidemia, hypercholesterolemia, hypertension, and insulin resistance (Carr 2003; Poehlman et al. 1997). Although a deficiency of estrogen production accompanied by aging is a major cause, it remains unclear whether other physiological mechanisms are also involved in undermining the health of postmenopausal women.
The autonomic nervous system (ANS) contributes to nearly every important homeostatic process in the body. This implies that the suppression of autonomic functioning could induce far-reaching adverse effects on health. Although autonomic abnormality in established adult obesity remains an unresolved issue (Jones et al. 1996; Scherrer et al. 1994), several human studies (Laederach-Hofmann et al. 2000; Peterson et al. 1988; Petretta et al. 1995; Tataranni et al. 1997) have strongly supported the MONA LISA (Most obesity known are low in sympathetic activity) hypothesis (Bray 1991). Clinical research has further demonstrated reduced autonomic function in patients suffering from diabetes mellitus with and without autonomic neuropathy (Hayashi et al. 1994; Singh et al. 2000), dyslipidemia (Danev et al. 1997; Doncheva et al. 2003), multiple metabolic syndrome disorders (Liao et al. 1998), or coronary heart disease (Hayano et al. 1990), one of the most prevalent forms of cardiovascular disease. Even individuals in the early stage of hypertension present autonomic dysfunction (Singh et al. 1998). Despite the recent findings that sympatho-vagal activity is altered after menopause (Brockbank et al. 2000; Davy et al. 1998; Liu et al. 2003), there is a paucity of information regarding the potential association of the autonomic dysregulation with obesity and/or other pathophysiological complications in postmenopausal women.
The electrocardiogram (ECG) R–R interval, or inter-beat interval of heart rate is determined by the net effect of sympathetic and parasympathetic input. Because it is accessible and noninvasive, the spectral analysis of heart rate variability (HRV) has gained popularity with broad applications as a functional indicator of the ANS (Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996). In addition, HRV power spectral analysis lightens the burden imposed on subjects during an experiment, as opposed to an invasive measurement, i.e., plasma catecholamine concentration and muscle sympathetic nerve activity, and offers a practical and valuable approach to evaluating the sympatho-vagal activity in climacteric clinical research (Brockbank et al. 2000; Davy et al. 1998; Liu et al. 2003).
Accordingly, the present study evaluated the resting ANS activity of postmenopausal women by means of the HRV power spectral analysis and investigated the association of reduced sympathetic and/or parasympathetic activity and unfavorable clinical states of body composition, lipid and/or glucose metabolism, or cardiovascular profiles after menopause.
Methods
Subjects and design
The present investigation was performed on 175 postmenopausal women (55.4 ± 0.4 years) who visited our climacteric clinic. The study protocol was approved by the Institutional Review Board of Tokyo Medical and Dental University and was performed in accordance with the Declaration of Helsinki. All subjects received an explanation of the nature and purpose of the study, and all gave their written informed consent to participate in the study. The subjects completed a standardized health questionnaire including medical history, medication, age at menopause, lifestyle, diet, smoking habits, alcohol consumption, and physical activity. In all cases, more than 6 months had passed since the last menstrual bleeding, the serum estradiol was lower than 20 pg ml−1 (73.42 pmol l−1), and the serum follicle-stimulating hormone level was more than 50 IU dl−1. None of the subjects exercised regularly, smoked, or drank heavily (consuming > 80 g alcohol/day). The subjects were not clinically diagnosed with diabetes mellitus, hypertension, cardiovascular disease, or other endocrine diseases, nor were they taking medications that could alter autonomic function and lipid metabolism. All subjects were asked not to consume any food or beverages containing alcohol or caffeine after 9:00 p.m. of the day preceding the study. The subjects were also instructed to abstain from alcohol and excessive physical activity for 24 to 48 h before testing.
Experimental procedure
The subjects came to the laboratory at approximately 9:00 a.m., and all experiments were performed in the morning. The room temperature was kept controlled at 25°C, quiet, and comfortable, with minimization of arousal stimuli. After the measuring of height and body weight, the percentage of body fat was determined by means of a bioelectrical impedance analyzer (SS-103, Sekisui, Tokyo, Japan). Body mass index (BMI) was calculated as body weight divided by height squared.
Blood was drawn from the antecubital vein with the subject seated comfortably. After accurate skin preparation, the subjects were fitted with ECG electrodes and rested for at least 20 min prior to the start of the experiment. After the resting period, the CM5 lead ECG was continuously monitored and recorded for 5 min during supine rest. All subjects breathed in synchrony to a metronome at 15 beats min−1 (0.25 Hz) to ensure that the respiratory-linked variations in heart rate did not overlap the lower-frequency heart rate fluctuations (below 0.15 Hz) from other sources.
R–R spectral analysis procedure
The ANS activity was non-invasively measured by HRV power spectral analysis. The analysis technique used in the present study has been applied in basic physiological and clinical research fields, and its validity and reliability has been previously confirmed (Amano et al. 2001; Hayashi et al. 1994; Moritani et al. 1995; Nagai and Moritani 2004). The procedure of R–R interval power spectral analysis has been described in great detail elsewhere (Hayashi et al. 1994; Moritani et al. 1995). Briefly, the ECG signal was amplified (MEG-6100, Nihon Kohden, Tokyo, Japan) and digitized via a 16-bit analog-to-digital converter (Model PS-2032GP, TEAC, Tokyo, Japan) at a sampling rate of 1,000 Hz. The digitized ECG signal was differentiated, and the resultant QRS spikes and the intervals of the impulses (R–R intervals) were stored sequentially on a hard disk for later analyses.
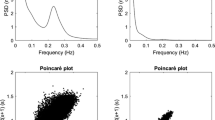
Before the R–R spectral analysis was performed, the stored R–R interval data were displayed and aligned sequentially to obtain evenly-spaced samples with an effective sampling frequency of 2 Hz (Rompelman et al. 1977) and displayed on a computer screen for visual inspection. Then, the direct current component and linear trend were completely eliminated by digital filtering for the band-pass between 0.03 and 0.5 Hz. The root mean square value of the R–R interval was calculated as representing the average amplitude. After passing through the Hamming window, power spectral analysis by means of a fast Fourier transform was performed on a consecutive 256-s time series of R–R interval data obtained during the test. The spectral powers were calculated for the following respective frequency band: the low frequency (LF) power (0.03 and 0.15 Hz), an indicator of both sympathetic and parasympathetic nervous system (PNS) activity; the high frequency (HF) power (0.15 and 0.5 Hz), which solely reflects the PNS activity; and the total power (0.03 and 0.5 Hz) representing the overall ANS activity (Fig. 1) (Amano et al. 2001; Hayashi et al. 1994; Moritani et al. 1995; Nagai and Moritani 2004).
Examples of ECG R–R interval changes and the corresponding power spectra for a postmenopausal woman in the resting condition. The technique of heart rate variability power spectral analysis used in the present study identifies two separate frequency components, low (0.03–0.15 Hz) and high (0.15–0.5 Hz), which are represented by the black and the white areas, respectively
Blood analyses
Blood samples were immediately transferred to siliconized tubes containing Na2 EDTA (1 mgml−1) and centrifuged at 4°C. Each serum was frozen and stored at –20°C until assay. Concentrations of glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides were measured by the Hitachi automated analyzer 7350 (Hitachi Ltd., Tokyo, Japan). Low density lipoprotein (LDL) cholesterol levels were determined by the Hitachi automated analyzer 7170 (Hitachi Ltd., Tokyo, Japan).
Statistical analyses
All statistical analyses were performed using a commercial software package (SPSS 12.0J for Windows, SPSS Inc., Tokyo, Japan). Statistical differences between groups were assessed by using Student’s unpaired t-test. Correlation coefficients and significance values between two variables were calculated by linear regression analysis. P values < 0.05 were considered statistically significant. Data are expressed as mean ± SE.
Results
The total power of HRV in all subjects showed skewed distribution, and 50 percentile was 220 ms2. To scrutinize potential influence of ANS activity levels on obesity-related factors, including body composition and metabolic and cardiovascular profiles after menopause, we divided the subjects into two groups based on the total power, i.e., low group (total power < 220 ms2) and high group (total power > 220 ms2). The statistical analysis revealed that both the LF and HF power were significantly lower in the low group than in the high group (LF: 51.6 ± 3.1 vs. 261.1 ± 36.5 ms2, P < 0.01; HF: 59.8 ± 3.7 vs. 318.2 ± 27.1 ms2, P < 0.01).
Table 1 shows the physical characteristics of the low and high groups. No significant difference between the two groups was found either in age, age at menopause, or in years after menopause. BMI (P < 0.05) and percentages of body fat (P < 0.01) were, however, significantly greater in the low group than those in the high group. It should be noted that the BMI of the subjects in the low and high groups is in the average range of Japanese postmenopausal women according to the very recent National Health and Nutrition Survey in Japan (Ministry of Health, Labor and Welfare, Japan 2005).
The ratio of waist to hip was also higher in the low group compared to that in the high group, but no significant difference was detected. Both systolic (P < 0.01) and diastolic (P < 0.01) blood pressure was greater in the low group than in the high group.
Regarding blood profiles, the glucose level did not differ between the two groups (94.7 ± 1.1 vs. 95.9 ± 1.7 ng ml−1). As Fig. 2 presents, in contrast, the values of triglycerides (124.5 ± 8.6 vs. 97.9 ± 5.9 mg dl−1, P < 0.05), total cholesterol (224.5 ± 4.3 vs. 210.7 ± 3.6 mg dl−1, P < 0.05), and LDL cholesterol (127.8 ± 4.6 vs. 115.0 ± 3.5 mg dl−1, P < 0.05) were significantly higher in the low group than in the high group. The HDL cholesterol level was lower in the low group than that of the high group (73.2 ± 1.9 vs. 75.0 ± 2.1 mg dl−1); however, the difference in value did not reach statistical significance. Linear correlation analysis revealed no significant relationship between age and those four blood lipid concentrations in each group.
Discussion
Measurement of the HRV integrates pre-synaptic and post-synaptic end-organ response and provides a comprehensive quantitative and qualitative evaluation of neuro-autonomic function under various physiological conditions and clinical settings (Davy et al. 1998; Hayano et al. 1990; Hayashi et al. 1994; Liao et al. 1998; Matsumoto et al. 1999). Although quantification and interpretation of HRV remain an intricate issue (Conny et al. 1993; Eckberg 1997), the efficacy and applicability of the technique utilized in the present study have been shown in the previous research: The findings from a pharmacological blockade experiment with atropine, a parasympathetic muscarinic antagonist and propranolol, a β-adrenoceptor antagonist, in our laboratory (Hayashi et al. 1994; Matsumoto et al. 1999; Oida et al. 1997) supported the classical studies (Akselrod et al. 1981 Pagani et al. 1986) and confirmed: (1) HF power is associated solely with the PNS activity, and the LF power is jointly mediated by the PNS and sympathetic nervous system (SNS) activity; and (2) R–R interval variability and the integrated values of all the components of power spectra could reflect overall ANS activity. Taking these findings into consideration, it is plausible to assume that both the SNS and PNS activity were more reduced in the low group of the present study because the total power, together with the LF and the HF power, were all significantly lower compared to those in the high group.
As to the association of the total power with metabolic profiles, our recent clinical study (Amano et al. 2001) of obese middle-aged women shows that twelve weeks of moderate aerobic exercise training significantly increased the total power together with LF and HF power. These changes were associated with the decreases in adiposity and an improvement of cardiovascular capacity. The results imply that individuals with lower value of total power as found in the low group of the present study could be more susceptible to obesity and/or obesity-related disorders.
The alteration of autonomic function has been widely believed to contribute to energy metabolism, weight regulation, and consequently, the pathophysiology of obesity. Tataranni et al. ( 1997 ) demonstrated that urinary norepinephrine and epinephrine excretion rate correlated negatively with body weight gain and the development of central adiposity, respectively. The time and/or frequency domain analysis of HRV has been widely used in human obesity research and has provided notable findings: a series of our recent studies has found an association with obesity and decreased sympathetic responsiveness to various thermogenic perturbations (Matsumoto et al. 1999, 2000, 2001). Similarly, Peterson et al. (1988) and others (Petretta et al. 1995; Laederach-Hofmann et al. 2000) reported a negative correlation of sympathetic and parasympathetic activity with increasing BMI or percentages of body fat. In a study on weight gain, Hirsch et al. (1991) described an inverse relationship of the amount of weight gain with lower parasympathetic drive. While supporting the MONA LISA hypothesis (Bray 1991) as the earlier research demonstrated, the present investigation further suggests that lower activity in both sympathetic and parasympathetic branches of ANS is associated with increases in adiposity in postmenopausal women.
The present study has shown that the influence of reduced ANS activity extends to cardiovascular and blood lipid profiles. As observed in body composition, none of the clinical variables regarding the cardiovascular system or glucose or lipid metabolism deviated far from the normal ranges. Significant differences were detected, however, in systolic and diastolic blood pressure, triglycerides, total cholesterol, and LDL cholesterol between the low and the high groups. Novak et al. (1994) demonstrated that spectral powers of HRV were all significantly smaller in hypertensive patients compared to those in normotensive individuals. This unfavorable phenomenon has been found even the women, who have become hypertensive of late, in a population-based study (Singh et al. 1998). As to lipid metabolism, total cholesterol and LDL cholesterol were negatively associated with the HRV measures (Danev et al. 1997; Doncheva et al. 2003). Liao et al. (1998) have shown that multiple metabolic syndrome disorders had an additional adverse effect on sympatho-vagal activity beyond that of hypertension, diabetes, or dyslipidemia alone. Since the orchestrations of the ANS plays a major role in normal physiological function, these findings indicate that the suppression of autonomic functioning could cause more than just increases in adiposity, including broadly ranged medical disorders. In addition, the present study implies that the HRV spectral analysis serves as a productive and applicable device to uncover potential autonomic dysfunction in a subclinical and/or early stage of various pathophysiological conditions.
According to the climacteric research, menopause is associated with reduced energy expenditure during rest and physical activity, an accelerated loss of fat-free mass, and alteration of adipose tissue metabolism and fat oxidation (Ferrara et al. 2002; Lynch et al. 2002; Poehlman et al. 1995, 1997). The dysregulation of energy metabolism could induce an increase in total adiposity and a redistribution of fat to the abdominal region (Lynch et al. 2002; Poehlman et al. 1995, 1997). In addition, postmenopausal women have a well-documented, increased risk of metabolic syndrome including dyslipidemia, insulin resistance, and hypertension, and consequently, cardiovascular disease. Despite intricate underlying mechanisms, the pathogenic condition may be a direct result of ovarian failure, or alternatively, an indirect result of central fat redistribution with estrogen deficiency (Carr 2003). The epidemiological data show that women below the age of 50 years rarely develop cardiovascular disease, but the incidence is equal in men and women, 70 years of age (Carr 2003). As the clinical evidence indicates, menopause heralds the onset of physiologic changes that trigger detrimental effects on the metabolic and cardiovascular systems. Taken together with the present findings, it is conceivable that lower sympatho-vagal activity in the postmenopausal period, as observed in the low group, could be a risk factor in accelerating the worsening health profile of women.
In conclusion, our findings indicate an association of postmenopausal reduced sympatho-vagal activity with higher body fat content, blood pressure and blood lipid concentrations. As life expectancy increases, women in general experience a longer life after menopause. The association of menopause and increased risk of health hazards is well known. Thus, this study further implies that the reduced sympatho-vagal activity has an additional adverse effect on the health profile and ultimately, on the quality of life, of postmenopausal women.
References
Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222
Amano M, Kanda T, Ue H, Moritani T (2001) Exercise training and autonomic nervous system activity in obese individuals. Med Sci Sports Exerc 33:1287–1291
Bray GA (1991) Obesity, a disorder of nutrient partitioning: The MONA LISA hypothesis. J Nutr 121:1146–1162
Brockbank CL, Chatterjee F, Bruce SA, Woledge RC (2000) Heart rate and its variability change after the menopause. Exp Physiol 85:327–330
Carr MC (2003) The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88:2404–2411
Conny MA, Louis AA, Jeroen CW, Gerard BA, Herman P (1993) Heart rate variability. Ann Intern Med 118:436–447
Danev S, Nikolova R, Kerekovska M, Svetoslavov S (1997) Relationship between heart rate variability and hypercholesterolaemia. Cent Eur J Public Health 5:143–146
Davy KP, DeSouza CA, Jones PP, Seals DR (1998) Elevated heart rate variability in physically active young and older adult women. Clin Sci (Lond) 94:579–584
Doncheva NI, Nikolova RI, Danev SG (2003) Overweight, dyslipoproteinemia, and heart rate variability measures. Folia Med (Plovdiv) 45:8–12
Eckberg DL (1997) Sympathovagal balance: A critical appraisal. Circulation 96:3224–3232
Ferrara CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM (2002) Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab 87:4166–4170
Hayano J, Sakakibara Y, Yamada M, Ohte N, Fujinami T, Yokoyama K, Watanabe Y, Takata K (1990) Decreased magnitude of heart rate spectral components in coronary artery disease. Its relation to angiographic severity. Circulation 81:1217–1224
Hayashi T, Masuda I, Shinohara M, Moritani T, Nakao K (1994) Autonomic nerve activity during physical exercise and postural change: investigations by power spectral analysis of heart rate variability. Jpn J Biochem Exerc 6:30–37
Hirsch J, Leibel RL, Mackintosh R, Aguirre A (1991) Heart rate variability as a measure of autonomic function during weight change in humans. Am J Physiol 261:R1418–R1423
Jones PP, Snitker S, Skinner JS, Ravussin E (1996) Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol 270 (Endocrinol Metabo 33) E363–E366
Laederach-Hofmann K, Mussgay L, Ruddel H (2000) Autonomic cardiovascular regulation in obesity. J Endocrinol 164:59–66
Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, Cai J, Sharrett AR (1998) Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care 21:2116–2122
Liu CC, Kuo TB, Yang CC (2003) Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol 285:H2188–H2193
Lynch NA, Ryan AS, Berman DM, Sorkin JD, Nicklas BJ (2002) Comparison of VO2max and disease risk factors between perimenopausal and postmenopausal women. Menopause 9:456–462
Matsumoto T, Miyawaki T, Ue H, Kanda T, Zenji C, Moritani T (1999) Autonomic responsiveness to acute cold exposure in obese and non-obese young women. Int J Obes Relat Metab Disord 23:793–800
Matsumoto T, Miyawaki C, Ue H, Yuasa T, Miyatsuji A, Moritani T (2000) Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. J Nutr Sci Vitaminol 46:309–315
Matsumoto T, Miyawaki C, Ue T, Kanda T, Yoshitake Y, Moritani T (2001) Comparison of thermogenic sympathetic response to food intake between obese and non-obese young women. Obes Res 9:78–85
Ministry of Health, Labor and Welfare, Japan (2004) The national nutrition survey in Japan, 2002. Dai-ichi shuppan publishing, Co Ltd. Tokyo, Japan, pp 52
Ministry of Health, Labor, and Welfare (2005) Part 3 Investigation of the physical characteristics. The National Health and Nutrition Survey in Japan, 2003. http://www.mhlw.go.jp/bunya/kenkou/eiyou-chosa2–01/pdf/05a.pdf, pp 15
Moritani T, Hayashi T, Shinohara M, Mimasa F, Masuda I, Nakao K (1995) Sympatho-vagal activities of NIDDM patients during exercise as determined by heart rate spectral analysis. In: Kawamori R, Vranic M, Horton ES, Kubota M (eds) Glucose fluxes, exercise and diabetes. Smith-Gordon: Great Britain pp 91–96
Nagai N, Moritani T (2004) Effect of physical activity on autonomic nervous system function in lean and obese children. Int J Obes Relat Metab Disord 28:27–33
Novak V, Novak P, de Champlain J, Nadeau R (1994) Altered cardiorespiratory transfer in hypertension. Hypertension 23:104–113
Oida E, Moritani T, Yamori Y (1997) Tone-entropy analysis on cardiac recovery after dynamic exercise. J Appl Physiol 82:1794–1801
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59:178–193
Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA (1988) Body fat and the activity of the autonomic nervous system. N Engl J Med 318:1077–1083
Petretta M, Bonaduce D, de Filippo E, Mureddu GF, Scalfi L, Marciano F, Bianchi V, Salemme L, de Simone G, Contaldo F (1995) Assessment of cardiac autonomic control by heart period variability in patients with early-onset familial obesity. Eur J Clin Invest 25:826–832
Poehlman ET, Toth MJ, Gardner AW (1995) Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med 123:673–675
Poehlman ET, Toth MJ, Ades PA, Rosen CJ (1997) Menopause-associated changes in plasma lipids, insulin-like growth factor I and blood pressure: a longitudinal study. Eur J Clin Invest 27:322–326
Rompelman O, Coenen AJR, Kitney RI (1977) Measurement of heart-rate variability: part 1 - comparative study of heart-rate variability analysis methods. Med Biol Eng Comput 15:233–239
Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P (1994) Body fat and sympathetic nerve activity in healthy subjects. Circulation 89:2634–2640
Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D (1998) Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension 32:293–297
Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D (2000) Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 86:309–312
Task force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standard of measurements, physiological interpretation and clinical use. Circulation 93:1043–1065
Tataranni PA, Young JB, Bogardus C, Ravussin E (1997) A low sympathoadrenal activity is associated with body weight gain and development of central adiposity in Pima Indian men. Obes Res 5:341–347
Wise PM, Krajnak KM, Kashon ML (1996) Menopause: the aging of multiple pacemakers. Science 273:67–70
Acknowledgements
We wish to express our appreciation to Dr. Tatsuya Hayashi, Graduate School of Human and Environmental Studies, Kyoto University, for his constructive suggestions and cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, T., Matsumoto, T., Akiyoshi, M. et al. Body fat and blood lipids in postmenopausal women are related to resting autonomic nervous system activity. Eur J Appl Physiol 97, 542–547 (2006). https://doi.org/10.1007/s00421-006-0207-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-006-0207-8