Abstract
After decompression from dives, bubbles are frequently observed in the right ventricular outflow tract and may lead to vascular damage, pulmonary arterial hypertension and right ventricular overload. No data exist on the effect of open sea diving on the pulmonary artery pressure (PAP). Eight professional divers performed an open sea air dive to 30 msw. Before and postdive a Doppler echocardiographic study was undertaken. Systolic pulmonary artery pressure (SPAP) was estimated from measurement of peak flow velocity of the tricuspid regurgitant jet; the ratio between pulmonary artery acceleration times (AccT) and right ventricular ejection time (RVET) was used as an estimate of the mean PAP. No evidence of either patent foramen ovale or intra-pulmonary shunt was found in any subject postdive after performing a Valsalva maneuver. SPAP increased from 25 ± 3 to 33 ± 2 mmHg and AccT/RVET ratio decreased from 0.44 ± 0.04 to 0.3 ± 0.02 20 min after the dive, respectively. Pulmonary vascular resistance increased from 1.2 ± 0.1 to 1.4 ± 0.1 Woods Units. Postdive right ventricle end-diastolic and end-systolic volumes were increased for about 19% (P = 0.001) and 33% (P = 0.001) and right ejection fraction decreased about for 6% (P = 0.001). Cardiac output decreased from 4.8 ± 0.9 (l min−1) to 4.0 ± 0.6 at 40 min postdive due to decreases in heart rate and stroke volume. This study shows that a single open sea dive may be associated with right heart overload due to increased pressure in the pulmonary artery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurological injury is currently the most serious decompression-related problem in recreational divers. Decompression sickness (DCS) occurs when rapid exposure to reduced ambient pressure results in gas phase separation. Venous gas bubbles, when occurring without any acute clinical signs, have been termed “silent bubbles” (Behnke 1951). Although “silent” bubbles are asymptomatic, the occurrence of many bubbles is clearly linked to a high risk of DCS (Nishi 1990). We have previously reported that vascular bubbles lead to a reduction in endothelial function in the pig pulmonary artery (Nossum and Brubakk 1999) and that even a low bubble load was associated with delayed endothelial dysfunction in the rabbit 1–6 h after exposure (Nossum et al. 2002). Since venous bubbles were eventually trapped in the pulmonary circulation, they were assumed to have no further effects on the arterial circulation. It is further assumed that a right-to-left shunt (R-to-L), which is possible in the presence of a persistent foramen ovale (PFO), was necessary for the bubbles to affect the systemic arteries. Several authors (Wilmshurst et al. 1989; Moon et al. 1989) have shown that there is a relationship between the occurrence of a PFO and the incidence of neurological DCS. The other possibility that has recently been implicated as a possible pathway for crossing over of the venous bubbles is a large anatomical intra-pulmonary (I-P) shunt. Eldridge et al. (2004) have shown that even light exercise in man will allow ultrasonic contrast consisting of small gas bubbles to move through the pulmonary circulation and enter the arterial circulation in individuals without any PFO (Eldridge et al. 2004). However, we have recently shown that these I-P shunts are not recruited during postdive exercise (Dujić et al. 2005c). Furthermore, we have shown that simulated diving can lead to acute arterial dysfunction in man (Brubakk et al. 2005).
An increase in the pulmonary vascular resistance (PVR) after diving may lead to the increase in the pressure within the right heart, which can cause a reversal of the normal trans-septal pressure gradient. Other maneuvers that may potentiate R-to-L shunting of bubbles are any other situations resulting in increased venous return to the right heart, such as coughing and the Valsalva maneuver (Balestra et al. 1998). Recently, Diesel et al. (2002) showed no increase in systolic pulmonary artery pressure (SPAP) after simulated altitude decompressions inducing high grade venous gas bubbles; we have confirmed this finding in recreational air divers after a simulated chamber dive (Valic et al. 2005). The results of human studies seem to contradict the animal data, which show a pulmonary artery pressure (PAP) increase (Vik et al. 1993; Butler et al. 1989).
The physiological stress of open sea diving in comparison with simulated, dry chamber dives is greater due to additional factors such as immersion, exercise, and cold water. We have recently shown that a single open sea air dive is associated with acute, postdive depressions in lung respiratory function and cardiac output (Dujić et al. 2005a). Marabotti et al. (1999) have also found that a recreational scuba dive is associated with cardiac changes 2 h after a dive, suggesting a right ventricular overload and an impairment of both right and left ventricular diastolic performance.
Accordingly, the primary aim of this study was to investigate whether the PAP was increased after a single open sea air dive. The second aim was to investigate whether a Valsalva maneuver recruited I-P shunts after diving, causing paradoxical arterialization of the venous gas bubbles.
Methods
Study population
The study enrolled eight Croatian Navy divers aged 35.6 ± 3.6 years (mean ± SD, range 32–40), with the average body mass index of 25.9 ± 2.4 kg m−2 (range 21.4–29), height 1.81 ± 0.05 m (range 1.75–1.87), and body fat index 17.1 ± 3.0 (% body fat/kg). The subjects were all experienced divers with considerable diving experience (several thousand hours of air and oxygen diving). All participants were nonsmokers and had a valid medical certificate for diving, issued by official navy medical staff. At the time of the study, the divers showed no signs of acute or chronic illness. All experimental procedures were conducted in accordance with the Declaration of Helsinki, and were approved by Ethics Committee of the University of Split School of Medicine. Each method and potential risks were explained to the participants in detail and they gave their written informed consent before the experiment.
Maximal O2 uptake\( (\ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }}) \) and maximal heart rate (HRmax) were determined in all divers 1 week before the experiments using incremental protocol on the bicycle ergometer (Marquette Hellige Medical Systems 900 ERG, Milwaukee, USA). The subjects were initially warmed up during 10 min with cycling at 25 W. The starting load was 50 W, with further 25 W increases each following minute up to exhaustion. This occurred within 11–14 min in all subjects. Detailed information about \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) measurement is presented elsewhere (Dujić et al. 2005b). The \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) for the group was 42.2 ± 4.5 ml kg−1 min−1 and the HRmax at \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) was 178.8 ± 8.5 beats per min (bpm).
Timeline of measurements
Cardiovascular parameters were measured with ultrasound 30 min before, and 20 and 40 min after field dive. \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} \) was measured a week before dive.
Location and duration of the study
The study was performed at the military base of the Croatian Navy Forces over a 1 week period. The diving site was located in the vicinity of the base, where the divers were transported by a powerboat, during a 10 min drive. The site was chosen because it allowed us to perform dives of the suitable depth and duration. Sea temperature at bottom and at the decompression stop was 14°C for all dives, and outside temperature varied between 15 and 18°C.
Dive protocol
All dives were performed by divers equipped with wet suits in accordance with the Croatian Navy and US Navy diving manual (US Navy 1996). Depth of the dive was set to 30 msw with a descent rate of 10 m min−1, and each pair of divers was supplied with a diving computer (Mosquito, Suunto, Finland). The divers were told to swim on the bottom over the distance of 500 m, mimicking 30% of their \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }}; \) the distance covered was controlled by the personnel on the powerboat and the work load by subjects themselves by monitoring their preselected HR during the dive (Polar S810i HR monitor, Polar, Finland). The divers swam at the bottom for 500 m in 30 min with a speed of 17 m min−1. After 30 min at bottom, the ascent rate to decompression depth was 9 m min−1, with a decompression stop at 3 m for 3 min. During the decompression period, the divers were told not to perform any exercise as we have shown that this reduces the bubble formation (Dujić et al. 2005b). HR data were downloaded to a personal computer and later analyzed.
Echocardiography study
After the completion of the diving protocol, the divers were transported to the facility where they took a brief shower before further monitoring. After the shower, the subjects were placed in the supine position and an echocardiograph investigation with a phase array probe (1.5–3.3 MHz) using a Vivid 3 Expert ultrasonic scanner (GE, Milwaukee, USA) was conducted. All echocardiography investigations were performed by the same, experienced cardiologist (AO). The occurrence of gas bubbles in the right heart and the pulmonary artery was determined. Ultrasound images were graded according to a previously reported method (Eftedal and Brubakk 1997) as follows: (0) no bubbles, (1) occasional bubbles, (2) at least one bubble/4th heart cycle, (3) at least one bubble/cycle, (4) continuous bubbling, at least one bubble per cm2 in all frames and (5) “white-out”, individual bubbles cannot be seen (this grade has been observed only in animals). The grading system is non-linear when compared to the actual number of bubbles in the pulmonary artery. Therefore, bubble grades were converted into bubbles per cm2 as described by Nishi et al. (2003).
Doppler ultrasound was used to estimate PAP before and after the dive. This was done with two methods, pulsed and continuous Doppler echocardiography, in each diver.
After determination of the bubble grade, the transducer was pointed to the outflow tract of the right heart, with pulse wave Doppler sample volume positioned at a level of pulmonary valve annulus. The PA flow velocity was recorded during cessation of breathing on an S-VHS videotape. Doppler measurements were averaged over three consecutive cardiac cycles. AccT (the time interval between the onset and peak of pulmonary flow velocity, in ms), RVET (the time interval from the onset to termination of the systolic pulmonary flow velocity, in ms) and R–R interval (ms) (the time interval between two R waves of electrocardiogram) were measured. The AccT/RVET ratio was calculated for each cardiac cycle. The baseline predive AccT/RVET ratio was a mean of two measurements. We used AccT/RVET as an index of the mean PAP because others have shown a good relationship between AccT/RVET and invasively measured PAP in healthy subjects (r = −0.91) (Kitabatake et al. 1983). Systolic PAP (SPAP) was measured with spectral Doppler ultrasound by measuring peak flow velocity of the regurgitant jet through the tricuspid valve (TR) during systole (Yock and Popp 1984). The peak flow velocity, or \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{{\max }} \), of the TR jet was used to calculate right ventricular systolic pressure (RVSP). Typically, five measurements were averaged to provide an assessment of \( \ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{{\max }} \) at each point in the exposure. For this study right atrial pressure (P RA) was assumed to be 5 mmHg. SPAP was calculated from the following equation: \( {\text {RVSP\,=\,4}}{\left( {{\text{TR}}\,\ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{{\max }} } \right)}^{2} \) + P RA. In the absence of pulmonary stenosis, RVSP and SPAP are the same. We have preselected eight subjects with present TR jet from the group of ten tested subjects. This prevalence of TR jet positive subjects is similar to other studies in athletes (Douglas et al. 1989) and healthy subjects (Singh et al. 1999). Our subjects were highly endurance trained special military divers with regular aerobic training sessions.
Pulmonary vascular resistance (PVR)
PVR was estimated from the ratio of peak tricuspid regurgitant velocity (TRV, m s−1) to the right ventricular outflow tract time–velocity integral (TVIRVOT, cm) obtained by Doppler echocardiography (TRV/TVIRVOT × 10 + 0.16, in Woods Units, WU). The normal range of PVR is from 1 to 4 (Abbas et al. 2003).
Cardiac dimensions
The apical four-chamber view was identified initially by palpation of the cardiac apex with the patient in the left lateral decubitus position. Transducer position was then adjusted as needed to obtain optimal images. The four-chamber view displays all four cardiac chambers, as well as the ventricular and atrial septa. This view was used for right ventricular end-systolic and end-diastolic volume (ESV and EDV) calculations according to the Simpson’s method. Because both end-diastolic and end-systolic measurements are needed for volume calculations (Campana et al. 2004), the electrocardiogram was continuously recorded. Ejection fraction of the right ventricle was calculated from the following equation: RVEF = (RVEDV–RVESV)/RVEDV. Images were acquired using ECG gating during acquisition, using the onset of R wave to identify end diastole.
Cardiac output was measured as a product of stroke volume (SV) and HR. SV was calculated from pulmonary artery cross sectional area (CSA) and velocity time integral (VTI). CSA was calculated from measured pulmonary artery diameter as CSA = π (D/2)2, where D is pulmonary artery diameter.
Before performing Valsalva maneuvers, the inter-atrial septum was located, and the ultrasonic probe was positioned to allow a clear view of both the right and left atrium. The subjects were asked to perform a high-strain Valsalva maneuver, which was held for ∼10 s before release. This was repeated twice with a resting interval of 2 min.
Ultrasound was used to measure flow mediated dilatation (FMD) of the brachial artery; the results of this part of the study are reported elsewhere.
Statistical analysis
Data are expressed as mean ± SD. The effect of diving on studied variables in different postdive times was evaluated by Friedman analysis of variance, whereas post-hoc comparisons were done by Wilcoxon sign rank test. Non-parametric tests were used because of the small sample size (N = 8). P < 0.05 was considered statistically significant. All analyses were done with Statistica 7.0 software (Statsoft, Inc., Tulsa, OK, USA).
Results
All eight divers successfully completed the designated protocol, and none developed any symptoms of DCS. In one participant no bubbles were found during the first 40 min at rest in the supine position, whereas in the other seven the dive profile produced venous bubbles (Table 1). No evidence of either PFO or I-P shunt was found in seven divers with postdive bubbles during the postdive Valsalva maneuvers (Fig. 1). Figure 1 shows a four-chamber view of the heart immediately after Valsalva maneuver of diver No. 6 with bubble grade 4, average SPAP of 37 mmHg and AccT/RVET ratio 0.27 (at 40 min after the dive).
Right ventricle (RV) and right atrium (RA) gas bubbles (grade 4) without signs of right-to-left shunting after Valsalva maneuver at 20 min (a) and 40 min (b) after reaching surface. The figure shows a four-chamber view of the heart immediately after Valsalva maneuver of diver No 6 with bubble grade 4, average SPAP of 37.1 mmHg and AccT/RVET ratio 0.27 (at 40 min after the dive)
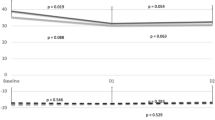
SPAP increased from 25 ± 3 to 33 ± 2 mmHg (P = 0.001), and AccT/RVET ratio decreased from 0.44 ± 0.04 predive to 0.3 ± 0.02 20 min after the dive (P = 0.001); Fig. 2a, b), an increase in PAP of about 32%. These differences are significant (P = 0.001 and P = 0.001, respectively). PVR increased significantly from 1.2 ± 0.1 to 1.4 ± 0.1 WU (P = 0.001) (Fig. 2c). Figure 3 shows the right ventricular outflow tract in diver No. 8 showed that AccT was reduced from 137.8 ms at baseline to 96 ms at 20 min after the dive, while RVET was prolonged from 328 to 380 ms (Fig. 3).
At postdive evaluation, right ventricle EDV and ESV were increased by 19% (P = 0.001) and 33% (P = 0.001), respectively, and right ejection fraction decreased by 6% (P = 0.001) (Fig. 4). Reduction in right ejection fraction was greater in divers with greater SPAP increase (P = 0.01). Cardiac output decreased significantly from 4.8 ± 0.9 (l min−1) at baseline to 4.0 ± 0.6 at 40 min (P = 0.001) after dive due to decreases in the heart rate and stroke volume (Fig. 5). Heart rate decreased from 61.3 bpm at baseline to 53.3 bpm at 20 min after dive.
The median bubble grade was 1.5 at 20 min and 2.0 at 40 min after dive, giving an average bubble load of 0.6 bubbles per cm2 over that time period. One of the divers had no bubbles during the observation time.
Discussion
We found that the PAP and PVR increased after a single open sea air dive to 30 m in professional divers. This was accompanied by an increase in the right ventricular end-diastolic and end-systolic volume and a decrease in the ejection fraction and cardiac output. This is contrary to what was observed after a simulated dry dive, where no increase in PAP was noted (Valic et al. 2005). Both dry and wet dives are associated with hyperoxia, increased density of breathing gas, and decompression stress with possible formation of venous bubbles and subsequent pulmonary microembolisation. Additional factors that have to be considered in open sea bounce dives are immersion, the mechanical load of the breathing apparatus, greater level of physical activity, and exposure to cold. Cold seawater may, by inducing peripheral cutaneous vasoconstriction, in conjunction with the immersion effect, potentiate greater central pooling of blood than in dry dives. The uptake and elimination of inert gas is dependent on blood flow during the dive. Exercise during the bottom phase of a dive increases the amount of gas taken up due to exercise and immersion induced increase in cardiac output (Flook 1997). In this study, divers performed light exercise \( {\left( {30\% \,\ifmmode\expandafter\dot\else\expandafter\.\fi{V}{\text{O}}_{{2\max }} } \right)} \) during the bottom phase of the dive to simulate working conditions, which could lead to an increase in gas uptake.
Mechanisms of increased PVR and PAP
In the present study, PAP was non-invasively estimated with two non-invasive methods, using continuous or pulsed Doppler echocardiography (Yock and Popp 1984; Kitabatake et al. 1983). We found increases in both systolic and mean PAP by about 30% after a single dive, as well as an increase in PVR by about 20%. This result is different to what was seen in previous studies using a simulated hyperbaric (Valic et al. 2005) or hypobaric protocol (Diesel et al. 2002), where no pressure increase could be seen. The increased PAP and PVR were not expected to cause any clinical symptoms, bearing in mind the large functional reserve in the pulmonary circulation and the good aerobic cardiovascular fitness of our subjects. At present, the mechanism causing increases in PAP and PVR after only a single field dive is unknown.
Although open sea diving may cause increased PAP after the dive, our findings showed that gas bubble formation seemed to play a minor role.
The exact mechanism(s) of increased PVR after the dive still remains elusive. Venous bubbles lodging in the pulmonary circulation may cause mechanical, humoral, and biochemical effects. Humoral factors, like tromboxane A2, histamine, endothelin and serotonin have been implicated in pulmonary microembolization by bubbles (Malik 1983). We and others (Nossum et al. 2002; Marabotti et al. 1999) have shown that venous bubbles may damage pulmonary endothelium. The increased PAP and PVR in the present study complements experimental studies reporting similar findings (Vik et al. 1993; Butler et al. 1989) in anaesthetized animals performing simulated dry dives. Venous gas emboli lead to an initial increase in PAP, followed by a decrease in arterial oxygen tension. The results of this study support previous studies reporting cardiac changes after open sea dives to 39 m (Dujić et al. 2005a) and 30 m (Marabotti et al. 1999). We have previously shown that postdive reductions in cardiac output were the result of decreased stroke volume, in presence of increased systemic vascular resistance and unchanged arterial blood pressure. In the present study, we found a similar reduction in cardiac output of about 20%, using a different method of measurement (echocardiography vs CO2 rebreathing). In animal models, the decrease in cardiac output after dives was related to reduced venous return and increased afterload of the right and/or left ventricle (due to increased PAP and systemic vascular resistance, respectively) (Bove et al. 1974; Vik et al. 1990; Butler et al. 1996). Marabotti et al. (1999) reported significant changes in cardiac function 2 h after a dive in recreational divers who produced bubbles. Right ventricular overload was present together with impairment of the left and right ventricular diastolic function; a reduction in left ventricular function was also seen in divers without detectable bubbles.
While simulated dry dives had no effect on ventricular function, an open water dive did, as shown in this study. We observed that arterial endothelial function was significantly reduced after a single simulated air dive (Brubakk et al. 2005) and showed that this effect was in part an effect of hyperoxia. As reported elsewhere (A. Obad et al., personal communication), endothelial function was also determined in the divers participating in this study, endothelial function after the dive was reduced by 63 vs 45% in the previous study. Thus it seems that an open water dive has a more depressive effect on the endothelium than a dry dive.
Immersion in thermoneutral water leads to an increase in thoracic blood volume of approximately 700 ml (Arborelius et al. 1972) and 180–240 ml of this was allotted to the heart volume with enlargement of all four chambers (Risch et al. 1978). Stroke volume is increased for about 50% after upright immersion up to the neck in healthy individuals (Meyer and Bucking 2004). This leads to an increase in both right ventricular dimensions and PAP and to PVR that in some cases can even lead to pulmonary edema (Pons et al. 1995). To our knowledge PAP values during prolonged immersion or swimming have not yet been measured. The reduction in endothelial function observed after the dive is probably also present in the heart, a reduction in endothelial function is an important factor in heart failure (Landmesser and Drexler 2005). Thus, immersion and hyperoxia, in addition to gas bubble formation caused by decompression, can reduce systemic as well as pulmonary artery and cardiac endothelial function and account for the observations.
Recently Eldridge et al. (2004) have shown that increasing exercise intensity causes R-to-L pulmonary shunting of agitated saline contrast in healthy humans, suggesting a recruitment of dormant I-P arteriovenous shunts. I-P shunting was fostered by an increased PAP during exertion and it was suggested that these shunts may act as a protective parallel vascular network helping to maintain high cardiac output during exercise (Eldridge et al. 2004). However, we have recently shown that I-P shunts are not recruited during exercise after open sea diving (Dujić et al. 2005c), thereby reducing the risk of arterial gas embolism with exercise after dives. R-to-L shunting is usually confirmed by the use of transthoracic, transesophageal echocardiography or transcranial Doppler after saline contrast infusion at rest, or after a Valsalva maneuver or a cough (Kerut et al. 2001). The Valsalva maneuver is frequently used to increase the sensitivity of contrast echocardiography. After the end of the Valsalva maneuver, increased venous return causes a significant rise in right atrial pressure with a leftward bulging of the interatrial septum (Balestra et al. 1998). We have found in this study that in no subject, the Valsalva maneuvers performed at the time of increased PAP after the dive, caused recruitment of dormant I-P arteriovenous shunts evidenced by the lack of R-to-L shunting of gas bubbles. This study supports previous findings (Dujić et al. 2005c) that I-P shunts are not recruited either during exercise or during other states of increased PAP, such as after diving.
Study limitations
Our study was performed on a small number of subjects and the results therefore have to be viewed with caution. The indirect measurements of PAP that we used have been used successfully in clinical setting, and recently these or similar methods have been used in environmental physiology investigations (e.g. Marabotti et al. 1999; Diesel et al. 2002; Valic et al. 2005). Still these non-invasive measurements might have produced inaccurate absolute estimates of some of the cardiovascular variables. Therefore, we utilized two methods for estimating PAP (AcT/RVET and TG jet) and both showed similar trend in postdive period. Furthermore, since every diver was his own control (predive vs postdive), we believe that the techniques employed are acceptable substitutes for invasive measurements (e.g. right heart catheterization).
In conclusion, there has been for many years a considerable controversy about the long-term effect of diving on the organism. We have recently shown that a single dry dive, even with a minimum bubble load, may impair arterial endothelial function (Brubakk et al. 2005). The present study shows that a single open sea dive may impair acutely the function of the pulmonary artery and the right heart, probably by the same mechanism. Further studies are needed to evaluate the importance of these findings as a possible mechanism for long-term changes of the cardiovascular system in recreational and professional divers.
References
Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ (2003) A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41:1021–1027
Arborelius M Jr, Ballidin UI, Lilja B, Lundgren CE (1972) Hemodynamic changes in man during immersion with the head above water. Aerosp Med 43:592–598
Balestra C, Germonpre P, Marroni A (1998) Intrathoracic pressure changes after Valsalva strain and other maneuvers: implications for divers with patent foramen ovale. Undersea Hyperb Med 25:171–174
Behnke AR (1951) Decompression sickness following exposure to high pressures. In: Fulton JF (ed) Decompression sickness. Saunders, Philadelphia, pp 53–89
Bove AA, Hallenbeck JM, Elliott DH (1974) Circulatory responses to venous air embolism and decompression sickness in dogs. Undersea Biomed Res 1:207–220
Brubakk AO, Duplančić D, Valic Z, Palada I, Obad A, Baković D, Wisloff U, Dujić Ž (2005) A single air dive reduces arterial endothelial function in man. J Physiol 566:901–906
Butler BD, Conkin J, Luehr S (1989) Pulmonary hemodynamics, extravascular lung water and residual gas bubbles following low dose venous gas emboli. Aviat Space Environ Med 60:1178–1182
Butler BD, Robinson R, Little T, Chelly JE, Doursout MF (1996) Cardiopulmonary changes with moderate decompression in rats. Undersea Hyperb Med 23:83–89
Campana C, Pasotti M, Monti L, Revera M, Serio A, Nespoli L, Magrini G, Scelsi L, Ghio S, Tavazzi L (2004) The evaluation of right ventricular performance in different clinical models of heart failure. Eur Heart J 6: F61–F67
Diesel DD, Ryles MT, Pilmanis AA, Balldin UI (2002) Non-invasive measurement of pulmonary artery pressure in humans with simulated altitude-induced venous gas emboli. Aviat Space Environ Med 73:128–133
Douglas PS, Berman GO, O’Toole ML, Hiller WD, Reicher N (1989) Prevalence of multivalvular regurgitation in atheletes. Am J Cardiol 64:209–212
Dujić Ž, Baković D, Marinović-Terzić I, Eterović D (2005a) Acute effects of single open-sea air dive and post-dive posture on cardiac output and pulmonary gas exchange in recreational divers. Br J Sports Med 39: e24
Dujić Ž, Palada I, Obad A, Duplančić D, Baković D, Valic Z (2005b) Exercise during three minute decompression stop reduces postdive venous gas bubbles in the field diving. Med Sci Sports Exerc 37:1319–1323
Dujić Ž, Palada I, Obad A, Duplančić D, Brubakk AO, Valic Z (2005c) Exercise-induced intrapulmonary shunting of venous gas emboli does not occur after open sea diving. J Appl Physiol 99:944–949
Eftedal O, Brubakk AO (1997) Agreement between trained and untrained observers in grading intravascular bubble signals in ultrasonic images. Undersea Hyperb Med 24:293–299
Eldridge MW, Dempsey JA, Havenkamp HC, Lovering AT, Hokanson JS (2004) Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol 97:797–805
Flook V (1997) The effect of exercise on decompression bubbles. A theoretical study. In: Mekjavic IB, Tipton MJ, Eiken O (eds) Proceedings of the XXIII annual scientific meeting of the European Underwater and Baromedical Societ. Bled, Slovenia, pp 55–61
Kitabatake A, Inous A, Asao M, Masuyama T, Tanoucji J, Morita T, Mishima M, Uematsu M, Shizaku T, Hori M, Abe H (1983) Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 68:302–309
Kerut EK, Norfleet WT, Plotnick GD, Giles TD (2001) Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 38:613–623
Landmesser U, Drexler H (2005) The clinical significance of endothelial dysfunction. Curr Opin Cardiol 20:547–551
Malik AB (1983) Pulmonary microembolization. Physiol Rev 63:1114–1207
Marabotti C, Chiesa F, Scalzini A, Antonelli F, Lari R, Franchini C, Data PG (1999) Cardiac and humoral changes induced by recreational scuba diving. Undersea Hyperb Med 26:151–158
Meyer K, Bucking J (2004) Exercise in heart failure: should aqua therapy and swimming be allowed? Med Sci Sports Exerc 36:2017–2023
Moon RE, Camporesi EM, Kisslo JA (1989) Patent foramen ovale and decompression sickness in divers. Lancet 1:513–514
Nishi RY (1990) Doppler evaluation of decompression tables. In: Lin YC, Shida KK (eds) Man in the sea. University of Hawaii Press, Honolulu, pp 297–316
Nishi R, Brubakk AO, Eftedal O (2003) Bubble detection. In: Brubakk AO, Neumann TS (eds) Bennet and Elliot’s physiology and medicine of diving, 5th, ed W.B. Saunders, London, pp 501–529
Nossum V, Brubakk AO (1999) Endothelial damage by bubbles in the pulmonary artery of the pig. Undersea Hyperb Med 26:1–8
Nossum V, Hjelde A, Brubakk AO (2002) Small amounts of venous gas embolism cause delayed impairment of endothelial function and increase polymorphonuclear neutrophil infiltration. Eur J Appl Physiol 86:209–214
Pons M, Blickenstorfer D, Oechslin E, Hold G, Greminger P, Franzeck UK, Russi EW (1995) Pulmonary oedema in healthy persons during scuba-diving and swimming. Eur Respir J 8:762–767
Risch WD, Koubenec HJ, Beckmann U, Lange S, Gauer OH (1978) The effect of graded immersion on heart volume, central venous pressure, pulmonary blood distribution, and heart rate in man. Pflugers Arch 374:115–118
Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ (1999) Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 83:897–902
US Navy diving manual (1996) Direction of Commander, Naval Sea System Command vol I (air diving)
Valic Z, Duplančić D, Baković D, Ivančev V, Eterović D, Wisløff U, Brubakk AO, Dujić Ž (2005) Diving-induced venous gas emboli do not change pulmonary artery pressure. Int J Sports Med 26:626–631
Vik A, Brubakk AO, Hennessy TR, Jenssen BM, Ekker M, Slordahl SA (1990) Venous air embolism in swine: transport of gas bubbles through the pulmonary circulation. J Appl Physiol 69:237–244
Vik A, Jensen BM, Eftedal O, Brubakk AO (1993) Relationship between venous bubbles and hemodynamic responses after decompression in pigs. Undersea Hyperb Med 20:233–248
Yock PG, Popp RL (1984) Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70:657–662
Wilmshurst PT, Byrne JC, Webb-Peploe MM (1989) Relation between interatrial shunts and decompression sickness in divers. Lancet 2:1302–1306
Acknowledgments
This study was supported by the Croatian Ministry of Science, Education and Sports, Grant No. 216006 and by the Norwegian Petroleum Dierctorate, Norsk Hydro, Esso Norge and Statoil under the ‘Dive contingency contract no 4600002328’ with Norwegian Underwater Intervention. Authors would like to thank Aleksandra Mišak for the editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dujić, Ž., Obad, A., Palada, I. et al. A single open sea air dive increases pulmonary artery pressure and reduces right ventricular function in professional divers. Eur J Appl Physiol 97, 478–485 (2006). https://doi.org/10.1007/s00421-006-0203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-006-0203-z