Abstract
Orthostatic intolerance is common after space flight and head-down tilt (HDT) bed rest. We hypothesized that HDT-induced impairments of arterial blood pressure (AP) control would be more marked during exercise and that recovery of baroreflex function after very long-term HDT would be delayed. Six subjects were studied before (BDC) during (day 60, D60; D113) and after (recovery day 0, R0; R3; R15) 120 days of HDT. Supine resting subjects were exposed to repeated 1 min passive tilts to upright at 3-min interval. During 50 W steady-state exercise corresponding tilt had a 2-min duration at 4-min interval. The amplitudes of the tilt-induced transient beat-by-beat deviations in AP and rate (HR) were determined during the gravity transients. At rest these deviations did not change over time, but during exercise the total peak-to-nadir range of deviations in systolic AP (SAP) at up-tilt and down-tilt increased to 168±16% (mean±SEM) of BDC at D113 with no clear recovery upto and including R15. Counter-regulatory HR responses were not increased proportionally and especially not tachycardic responses to up-tilt, resulting in a reduction of baroreflex sensitivity (ΔRR-interval/ΔSAP) by 55±9% of BDC at D113 with no recovery upto and including R15. We conclude that prolonged bed rest cause long-lasting impairments in AP control and baroreflex function in exercising humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthostatic intolerance and decreased exercise performance are frequently observed after head-down tilt (HDT) bed rest (Ferretti et al. 1997; Traon et al. 1998) and after space flight (Buckey et al. 1996; Levine et al. 1996). Levine et al.(1997) studying resting subjects, and Sundblad et al. (2000b), studying exercising subjects, found impairments of stroke volume (SV) after HDT. They suggested that the SV impairments at least in part could account for the orthostatic intolerance and the decreased exercise capacity. In the earlier studies both orthostatic and exercise responses after bed rest and space flight were characterized by a marked tachycardia. This tachycardia is not unequivocally congruent with the result from several other studies that have provided evidence for an impaired carotid-cardiac chronotropic baroreflex function after bed rest (Convertino et al. 1990; Convertino and Fritsch 1992; Hughson et al. 1994) and after spaceflight (Fritsch et al. 1992; Fritsch-Yelle et al. 1994). However, these investigators utilized different methods to assess baroreflex function, and they studied baroreflex function during supine rest only. On the other hand, Sundblad et al. (2000c) studying exercising subjects after 42 days of HDT, found no alterations of the arterial-cardiac chronotropic baroreflex sensitivity (BRS) during tilt-table-induced deviations in arterial blood pressure (AP). It may very well be that a more prolonged absence of the normal gravity-induced baroreceptor loading/unloading cycles is required for obtaining impairments of reflex cardiovascular control also during exercise. In support of such an effect of actual and simulated weightlessness on cardiovascular control during physical activity, Spaak et al. (2001), studying the muscular chemoreflex, found attenuated cardiovascular responses to sustained handgrip both after 6–12 months space flight and (in the same group of subject as in the present study) after 120 days of HDT.
The present study was undertaken in an attempt to address the above-mentioned disparities. Therefore, for the first time, baroreflex and hemodynamic responses to sudden shifts of posture both at rest and during exercise were studied in the same group of subjects, both during and after a HDT of 120 days duration. It was hypothesized that an impaired arterial-cardiac baroreflex function would manifest itself as a diminished chronotropic response to tilt-induced deviations in AP. It was also hypothesized that any potential impairment of effector organ functions would become evident during tilt-induced orthostatic challenges, and manifest themselves as larger than normal deviations of AP. Finally, it was also hypothesized that these blood pressure deviations would be more apparent when cardiovascular demands increase during exercise, as compared to rest.
Methods
The experiments were at the Institute for Biomedical Problems, Moscow, during the winter and spring of 1997, in collaboration with the European Space Agency. The experimental protocol was approved by the Russian National Committee of Bioethics of the Russian Academy of Sciences and by the Ethics Committee of Karolinska Institute, Stockholm, Sweden, and informed consent had been obtained from the subjects.
Subjects and study design
Six male subjects entered and completed the study. At the onset of the study, their age, height, and weight were 31 (23–42) (mean [range]) years, 1.81 (1.71–1.90) m and 80 (63–114) kg. They were healthy and had passed an extensive medical examination. They spent 120 days at rest in −6° HDT posture. There were occasional deviations from HDT during this period; subjects were transported in supine posture to different laboratories, and some of the laboratory tests called for other postures than HDT or supine. Including the present measurements, which partly took place during the HDT period, each subject spent a total of 935–965 min (≈0.5% of the total time) in postures other than HDT, out of which 440 min were at +6° and the remainder between +30 and +90°. Experiments were performed 1–2 weeks before HDT (baseline) on day 60 of HDT (D60) and on day 113 (D113). During the recovery period, experiments were performed on the first day after HDT (R0) and after 3, 10 and 14–17 days of recovery (R3, R10, R15).
The present study on baroreflex and haemodynamic responses to sudden changes of posture was performed in conjunction with studies of cardiac output and diffusing capacity during steady-state rest (Montmerle et al. 2002) and exercise (Spaak et al. 2005). All measurements and interventions were performed by staff from the author’s laboratory.
Procedures
On D60 and D113, subjects came to the laboratory supine on a gurney, on R0 sitting in a wheelchair, and otherwise walking. Subjects were instrumented in supine position and then placed on a tilt frame equipped with a cycle ergometer for dynamic leg exercise. Subjects were studied at rest with their feet on a support below the pedals, and during 50-W exercise, where the crank axis of the ergometer was at the level of the heart in supine posture. The tilt frame could be moved rapidly (≈2 s) between supine (0°) and upright (80°) postures. The position of the subject was secured by separate supports for the lower back, the upper back and the head, by a bicycle saddle, and by shoulder supports. The arms of the subject rested on a support structure in front of the chest.
After a minimum of 6 min of rest at 0°, subjects were tilted rapidly to 80° for 60 s and then back to 0°. This procedure was repeated five times with 120 s of 0° in between. Thereafter a similar tilt sequence was performed during 50-W steady-state dynamic leg exercise: after a 6 min warm-up period of exercise in supine posture, the exercising subjects were tilted for 120 s to 80°, thereafter back to 0° for 120 s. This sequence was repeated five times. No signs of orthostatic intolerance were observed during these procedures besides the marked HR increases in upright posture during exercise.
Equipment and measurements were essentially the same as in a previous, shorter bed rest study (Sundblad et al. 2000b, c). Briefly, continuous beat-by-beat AP and heart rate (HR) recordings were obtained from a photoplethysmographic finger-cuff device (Finapres 2300, Ohmeda, Englewood, CO, USA) and an ECG, respectively. The hydrostatic pressure differences between the finger cuff and two reference sites were determined with open-ended fluid-filled catheters (Linnarsson and Rosenhamer 1968) connected to pressure transducers (Sensortechnics GmbH, Pucheim, Germany). One reference site was the heart level defined as the intersection of a transversal line through the fourth intercostal space at the sternum and the mid-axillary line, and the other was the carotid sinus.
Data acquisition and analysis
Data were stored at 100 Hz per channel with a PC-based data collection system (Biopac MP 100, with software AcqKnowledge 3.1, Biopac Systems Inc., Goleta, CA, USA). Off-line analyses were performed with LabView 5.1 software (National Instruments, Austin, TX, USA), and included determinations of AP at a level halfway between the heart and the carotid (Sundblad et al. 2000a,b,c). HR, R–R interval (RRI) and mean blood pressure (MAP) can, by definition, not be known until the cardiac cycle is completed. In the present off-line analysis, these data were shifted back in time to the RRI when they actually occurred.
Beat-by-beat systolic AP (SAP), MAP, HR, and RRI were analysed for tilt-induced peaks and nadirs. Tilt-induced blood pressure and chronotropic responses (ΔSAP, ΔMAP, ΔHR, ΔRRI) were determined as the difference between a pre-tilt average between 30 and 5 s before a tilt and a subsequent peak or nadir. For each subject, variable, rest/exercise condition, tilt direction and experiment day, five sets of data were obtained, one for each tilt repetition. The highest and the lowest response values were discarded and an average was computed from the three remaining values. This procedure was chosen to minimize the impact of spurious peak and nadir readings.
Arterial-cardiac chronotropic BRS was computed as the ratio of a tilt-induced chronotropic response over the preceding blood pressure response (Linnarsson et al. 1996; Sundblad et al. 2000c). For ease of comparison with other studies, BRS estimates were obtained both as ΔHR/ΔMAP and as ΔRRI/ΔSAP.
As an index of the overall efficiency of the blood pressure control to meet short-term orthostatic challenges, the ranges of SAP and MAP fluctuations were determined over whole up-tilt/down-tilt sequences. These ranges were computed as the differences between the highest and the lowest AP and HR values that were obtained in the beat-by-beat recording for each up-/down-tilt sequence.
Statistics
Statistical analyses was performed using an analysis of variance (ANOVA) with Dunnett’s Post-hoc test when applicable. Significance was accepted at P values below 0.05. Results are expressed as mean ± standard error of the mean (SE).
Results
Figure 1 shows typical time courses of beat-by-beat mean- and systolic APs and HR during a single up-tilt/down-tilt sequence in one exercising subject. The figure also indicates how parameters such as peak/nadir tilt-induced responses were extracted from individual recordings. Both at rest and during exercise, the sudden transitions between 0 and 80° and the reverse were associated with large but short-lasting changes in blood pressure and HR. In the resting experiments, the amplitudes of these blood pressure fluctuations did not change between experimental occasions, but during exercise, tilt-induced fluctuations of SAP and MAP were markedly increased during and after HDT (Fig. 2). Thus, the total peak-to-nadir range of down-tilt-induced transient increases and up-tilt-induced transient decreases of SAP was widened to 163±13 and 168±16% of the BDC range at D60 and D113, respectively. Corresponding values during recovery were 157±15, 144±20 and 154±26%, at R0, R3 and R15, respectively, that is, with no significant recovery during the post-HDT observation period.
Time courses of heart rate (HR), mean arterial pressure (MAP) and systolic arterial pressure (SAP) at a site half-way between the heart and the carotid sinus in one subject performing 50-W steady-state exercise and being passively tilted from supine (0°) to upright (80°) and the reverse. The extractions of pre-tilt levels and of peak or nadir response amplitudes and peak-to-nadir response ranges are exemplified. The instant of 30° tilt angle is indicated by a vertical line. The beat-by-beat recording shows relatively large variations with time during single-tilt transients. For each subject the data for pre-tilt levels and peak/nadir values were based on five such transients
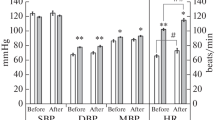
Group mean pre-tilt and peak/nadir values of SAP and MAP as defined in Fig. 1 during rest and 50-W exercise. Vertical lines connect pairs of pre-tilt values with tilt-induced peak/nadir values. BDC base-line data collection; D60, D113, after 60 and 113 days of −6° head down-tilt (HDT) bed rest; R0, R3, R15, after 0, 3 and 15 days of recovery. Asterisk significantly different level compared to BDC (P<0.05, N=6); Dagger significantly different response versus BDC; Double dagger significantly different range of responses versus BDC
The amplitudes of tilt-induced HR fluctuations (Fig. 3) did not change significantly with time when determined at rest, but during exercise HR fluctuations at down-tilt were significantly increased (Fig. 3) to 142±14 and 133±7% of baseline on D60 and D113, respectively. Amplitudes of tilt-induced HR fluctuations did not differ from baseline after HDT, but showed a trend of a more gradual return to BDC levels with 126±20%, 118±10% and 94±15% of BDC at R0, R3 and R15, respectively.
Heart rate (HR) values during the same conditions as in Fig. 2
Pre-tilt supine HR values at rest were elevated on R0 and R3, whereas the upright values (after the initial transient) did not differ from BDC during and after HDT. During exercise, all steady-state HR values in both postures were elevated from D60 and onwards compared to BDC. Pre-tilt supine SAP was elevated from D113 and onwards during rest, and from D60 and onwards during exercise. In contrast, upright SAP (after the initial transient) did not differ from BDC in any of the conditions (Fig. 3).
Arterial-cardiac chronotropic BRS is presented separately for rest (Fig. 4) and exercise (Fig. 5) due to different scales. At rest, the only significant alterations of BRS compared to baseline were for ΔRRI/ΔSAP on R0. During exercise, however, BRS computed as ΔRRI/ΔSAP was significantly depressed during the HDT and recovery periods, and both for up- and down-tilts (Fig. 5). For up-tilt, ΔRRI/ΔSAP was significantly reduced to 55±8 and 55±9% of baseline at D60 and D113 with no further change after HDT, when ΔRRI/ΔSAP averaged 49±6, 54±8 and 45±9% at R0, R3 and R15, respectively. Exercise BRS computed as ΔHR/ΔMAP during down-tilt was not changed during HDT but tended to be depressed during up-tilt from D60 and onwards (P=0.055–0.096) and was significantly depressed compared to control on R15.
Same variables and conditions as in Fig. 4, but during 50-W leg exercise
Table 1 lists the timing of tilt-induced changes in HR and MAP relative to the instant of 30° tilt angle, and response times until peak/nadirs of the same variables. Values for time delays and response times did not differ between experimental days. MAP started to change as soon as the tilt started and approximately 1 s before the 30° tilt angle was reached. HR started to change later than MAP; at rest, the delay in HR response from the onset of MAP change was about 1 s during up-tilt and about 4 s during down-tilt. Both at rest and during exercise, the peak/nadir responses of HR occurred later during up-tilt than during down-tilt.
Discussion
The ultimate purpose of the systemic circulation is to provide adequate perfusion to all the various tissues of the body under conditions of varying metabolic needs and environmental conditions, such as during rest versus exercise or supine versus upright posture. However, it appears that it is the AP rather than the tissue perfusion that is the most tightly controlled variable (Blomqvist and Stone 1983; Rowell et al. 1996). Tissue perfusion deficit appears to have a direct impact on cardiovascular control only during ischemic dynamic (Eiken et al. 1992; Sundberg and Kaijser 1992; Papelier et al. 1997) and isometric (Rowell and O’Leary 1990; Spaak et al. 1998) muscle activity. Its role during conditions of free-flow muscle perfusion has not been established (Papelier et al. 1997). It can therefore be argued that the most relevant test of the short-term cardiovascular control would be the ability to maintain AP rather than tissue perfusion. It is reasoned that the less efficient the cardiovascular control or function, the more the fluctuations would appear in AP during orthostatic cardiovascular challenges. Indeed, it is found that deviations of both mean- and systolic APs induced by up- and down-tilting were significantly increased during exercise in the group of subjects from D60 of the HDT and onwards, with no clear recovery upto and including R15. This trend was not found in the tilt tests performed during rest. These two observations show that, from a functional point of view, cardiovascular control during short-lasting gravitational challenges during the additional stress of dynamic exercise is impaired after long-term HDT, but the ability to control AP during the same challenges at rest appears to be satisfactory even after such an extended HDT period as 120 days.
Determinations of tilt-induced AP deviations will not in itself define the nature of the failure to defend the AP in the face of sudden orthostatic challenges during dynamic exercise; there might be impairments of cardiac chronotropic, cardiac inotropic and vasomotor control functions. These impairments may in turn be caused by alterations at the levels of the baroreceptors, the central processing of afferent signals, or in the effector organs. In the intact human, however, a separation of these categories is possible only to a limited extent. Several studies have demonstrated that sustained reductions of the normal baroreceptor loading/unloading cycles lead to a decreased carotid-cardiac chronotropic BRS, such as after bed rest (Convertino et al. 1990; Eckberg and Fritsch 1992), in quadriplegics (Convertino et al. 1991) and after spaceflight (Fritsch et al. 1992; Fritsch-Yelle et al. 1994). These decreases in carotid–cardiac BRS have all been tested by applying suction or positive pressure in a chamber around the neck, and they have all been tested during conditions of supine rest. Only two previous studies of HDT-induced changes in arterial–cardiac–chronotropic BRS during exercise have been performed, by Haruna et al. (1997) and Sundblad et al. (2000c) In contrast to the previous studies on resting subjects (Convertino et al. 1990; Convertino and Fritsch 1992; Eckberg and Fritsch 1992; Fritsch-Yelle et al. 1994), the BRS was found to be unchanged after 20 and 42 days of HDT, respectively. Haruna and Suzuki (1997) also tested the BRS during sitting and supine rest and found that post-HDT BRS was reduced when tested in supine position during rest but not during upright-seated rest and not, as mentioned, during exercise.
An important difference between the testing algorithms used during rest (Convertino et al. 1990, Convertino et al. 1992; Convertino and Fritsch 1992; Fritsch et al. 1992; Fritsch-Yelle et al. 1994) and later tests performed during exercise (Haruna and Suzuki 1997; Sundblad et al. 2000c) was that the BRS in the latter case was derived from changes of HR as a function of mean AP, rather than RRI as a function of systolic pressure. Traditionally, arterial-cardiac-chronotropic BRS has been defined in terms of RRI and systolic AP, since RRI has been shown to have a linear relationship to the vagal outflow to the heart (Parker et al. 1984), and since the arterial baroreceptors are sensitive to both static and pulsatile components of the AP (Eckberg and Sleight 1992). However, the non-linear inverse relationship between RRI and HR causes a given HR response to appear smaller when starting from a higher initial HR, if the chronotropic response is expressed in terms of RRI instead of HR. Therefore, several research groups (Melcher and Donald 1981; Potts et al. 1993; Papelier et al. 1994; Sundblad et al. 2000a), comparing BRS at different work intensities, and consequently at different HR, have chosen to quantify chronotropic responses to baroreflex stimuli in terms of HR in order to use a parameter that has both a linear relationship to arterial pressure and is independent of the initial HR. Also, when defining the input stimulus in studies of baroreflex control during exercise in humans, a majority of investigators have selected mean AP at the baroreceptor site rather than the systolic pressure (Potts et al. 1993; Toska and Eriksen 1993; Papelier et al. 1994; Linnarsson et al. 1996). Although it can be argued that both the static and the pulsatile components of AP contribute to the baroreceptor signal output (Eckberg and Sleight 1992), experiments with intact humans have indicated that when systolic AP on one hand, and diastolic and mean AP on the other hand are changed in opposite directions by a combination of fluid infusion and vasoactive pharmaceuticals (Sanders et al. 1988), HR and forearm vascular conductance appear to be more influenced by diastolic and mean AP than by systolic.
In an attempt to avoid problems of interpretation and to allow comparison with previous work with differing BRS testing paradigms, BRS was determined both as ΔHR/ΔMAP and as ΔRRI/ΔSAP. Responses to up-tilt and down-tilt were studied separately and it was found BRS is marginally reduced when studied at rest and then only during up-tilt (Fig. 4). When estimated during exercise, however, BRS was markedly reduced at D60 and D113, and showed no signs of recovery during the 15 days of the post-HDT observation period. It could be argued that since this decline in BRS was most obvious when tested as ΔRRI/ΔSAP, the decline could have been caused by an elevation of the pre-stimulus HR. As shown in Fig. 5, steady-state supine HR during exercise was increased during and after HDT, so the decline in BRS obtained during the up-tilts could potentially have been an artefact caused by changes in pre-stimulus (pre-tilt) HR. However, despite the fact that the exaggerated upright pre-tilt tachycardia at D113 and R0 recovered gradually at R3 and R15 towards the baseline level, BRS as obtained from down-tilts did not increase (Fig. 5). In summary, therefore, the present findings suggest a decline of BRS when determined within the range of arterial receptor pressure changes encountered during a sudden shift in posture. This decline was most obvious during exercise in combination with hypotensive stimuli but was not evident at rest in combination with hypertensive stimuli.
As BRS is defined as the ratio between tilt-induced chronotropic and baroreceptor blood pressure responses, a fall in BRS could be caused by either a reduced chronotropic response, an increased blood pressure response, or both. As already mentioned, it was found that the absolute deviations in mean and systolic APs induced by up- and down-tilting was significantly increased during exercise in the group of subjects from D60 of the HDT and onwards, with no clear recovery upto and including R15. This trend was not found in the tilt tests performed during rest. Thus, when comparing the exercise data in Figs. 2 and 3, it appears that the principal difference between baseline and HDT is a 60–70% increase of the absolute amplitude of the tilt-induced blood pressure responses to up- and down-tilts in combination with only a 30–40% corresponding increase of the combined absolute chronotropic responses to up- and down-tilts. For up-tilt responses corresponding figures were a 64–70% increase of the SAP response combined with a mere 8–14% increase of the HR response amplitude. Also, the approximately 50% reduction of BRS to up-tilt during exercise was accounted for by a relative decrease of the chronotropic responses.
In other words, the absolute HR responses to the more or less constant tilt-induced deviations in MAP and SAP during rest remained essentially unchanged, indicating a competent baroreflex regulation of both HR and AP. At exercise, the tilt-induced deviations in MAP and SAP were significantly increased at both up- and down-tilts, indicating an impaired baroreflex regulation of AP. The corresponding HR increases were essentially unchanged at up-tilt despite the more marked drops in AP, indicating an impaired tachycardic baroreflex response (Sundblad et al. 2000a). Generally, the corresponding HR decreases were markedly larger at down-tilt, also during and after HDT, indicating that the bradycardic, vagal HR control was less affected by HDT, than HR responses to up-tilt.
Interestingly, parallel studies of BRS on the same subjects were performed by Kamiya et al. (2000) using a different approach, studying baseline muscle sympathetic neve activity (MSNA) at supine rest, and MSNA responses to 30 and 60° head up-tilt for 5 min. Estimates of BRS were made during supine rest after 60 and 120 days of HDT from spontaneous systolic blood pressure and RRI sequences. Kamiya et al. (2000) confirm the results of an elevated supine resting HR after HDT, and they found attenuated BRS after both 60 and 120 days, which are supporting the present finding of reduced BRS to hypotensive stimuli at rest on R0, that is 1 day later. Their finding of attenuated arterial-cardiac BRS on D60, however, is at variance with the present findings; there are, however, important methodological differences between the two ways to assess BRS, which may account for the difference. Thus, the blood pressure stimulus employed by Kamiya et al. (2000) namely the variation of blood pressure during spontaneous breathing, is one order of magnitude smaller than the blood pressure deviations occurring during the present tilt experiments.
Kamiya et al. (2000) also found elevated baseline MSNA and enhanced MSNA responses to 5-min head up-tilt on D60 and D120. We speculate that the decreased arterial-cardiac chronotropic BRS was caused by sympato-vagal interaction: HR responses to short-lasting AP stimuli are mainly mediated through modulation of vagal outflow to the heart (Eckberg and Sleight 1992), and there is evidence that antecedent stimulation of cardiac sympathetic nerves slows the chronotropic response to vagal stimuli so that it becomes gradual rather than instantaneous (Yang et al. 1994) as is the case without antecedent sympathetic stimulation (Warner and Cox 1962). Thus vagally mediated HR responses to short-lasting stimuli, such as breath-synchronous arterial pressure fluctuations or tilt-induced changes in AP may not have time to develop fully in the presence of an increased sympathetic outflow to the heart. The origin of this increased sympathetic outflow after HDT is not known; Kamiya et al. (2000) speculate that a diminished cardiac size may result in a smaller inhibitory effect of cardiopulmonary baroreceptors on sympathetic outflow. Indeed, the present findings are compatible with such an explanation.
Sundblad et al. (2000a) studied exercising subjects, who were rapidly tilted between supine and upright and the reverse during pedalling at different work intensities. They modelled the HR responses to tilt using varying fractional contribution of rapid vagal and slower sympathetic modulations of HR (Warner and Cox 1962). They found that tachycardic responses to hypotensive stimuli during up tilt were markedly slower at 100 and 150 W than at 50 W, probably because of the increasingly dominating role of sympathetic inputs for tachycardic responses at the higher work loads. It is proposed that the greater relative effort required to perform 50-W exercise during and after compared to before HDT also contributed during exercise to a slowing, and thereby to an attenuation of tachycardic responses to hypotensive stimuli.
The extreme duration of the present study and the unique feature of having test sessions during the HDT period enabled to address the question whether impairments of cardiovascular functions become gradually more severe with longer HDT. The results of Kamiya et al. (2000) and the present exercise results support the notion that most if not all of the attenuation of arterial-cardiac-chronotropic BRS had occurred during the first 60 days of HDT, with no additional attenuation thereafter. Although gravity-dependent loading cycles are practically eliminated during strict HDT, the beat-by-beat stimulation from cardiac activity persists, and such stimulation may be sufficient for maintaining a certain level of arterial baroreflex function. Had, on the other hand, gravity-dependent baroreceptor loading cycles been solely responsible for maintaining baroreflex function, a gradual decline of baroreflex function over time, and much more severe impairments would have been expected.
Having established that long-term HDT results in some attenuation of arterial-cardiac-chronotropic BRS (Kamiya et al. 2000; present study), it is appropriate to consider the functional importance of this finding. Although calculation of BRS from the ratio ΔHR/ΔMAP appears to be less sensitive way to detect HDT-induced alterations of BRS than ΔRRI/ΔSAP (Figs. 4, 5) the former BRS-index probably provides a more fair estimate of the potential impact on the control of blood pressure, since mean AP changes are more directly proportional to concomitant changes in heart rate. For the hypertensive stimuli during down-tilt, there were no significant alterations of ΔHR/ΔMAP during either rest or exercise, and for hypotensive baroreflex stimuli during up-tilt there was no significant attenuation at rest, but a trend towards BRS attenuation in the exercise tests during HDT. These results are generally in agreement with those of Sundblad et al. (2000c), who found no changes of ΔHR/ΔMAP in exercising men after 42 days of HDT. However, the decreased BRS appears to be of modest functional significance both at rest and during light exercise, since both MAP and SAP steady-state levels (after the initial tilt-induced transients) did not change significantly in the upright position during and after HDT as compared to pre-HDT levels.
Finally a note on a specific consequence of the present tilting protocol: resting upright HR values did not differ between pre- and post-HDT. This observation is in apparent contrast to most previous studies, where orthostatic stress after HDT and space flight have been accompanied by an augmented tachycardiac response compared to pre-HDT/preflight controls (Beck et al. 1992; Buckey et al. 1996; Traon et al. 1998; Levine et al. 2002). Also Kamiya et al. (2000) found enhanced tachycardiac responses to head up-tilt after HDT in the very same subjects who participated in the present study. In the present study, resting subjects were exposed to 1 min at 80°, whereas in the cases of Kamiya et al.’s (2000) subjects were challenged with first 30° during 5 min and subsequently 60° during another 5 min. Also the other studies listed previously used challenges of 5–10 min duration and in some cases successive levels of increasing severity. With this additional longer duration, more slowly acting chronotropic mechanisms are given time to develop; Sander-Jensen et al. (1986) exposed normal subjects to 60°C passive head up-tilt and there was an increasing tachycardia between 10 and 20 min of 60° as well as continued reduction of AP. Taken together, the our data from 1 min at 80° and data from others from more extended orthostatic challenges suggest that it is only chronotropic mechanisms acting after the first minute are augmented after HDT and space flight.
In summary, the control of AP during sudden orthostatic and anti-orthostatic challenges became impaired during and after 120 days of head down-tilt bed rest. These impairments were not found at rest, but were evident as markedly increased deviations in AP upon orthostatic stimuli during exercise, and showed only a marginal normalization during a 15-day post-HDT observation period. The impairment of arterial-cardiac chronotropic BRS control was only evident during exercise, and then mainly for hypotensive stimuli, while hypertensive stimuli elicited a better maintained baroreflex response. This pattern of impairment suggests that the vagal control of HR remains essentially intact, whereas the regulation of HR during a state with increased sympathetic activity, becomes markedly impaired after HDT. Although the mechanism behind this alteration is not obvious, the protracted time course of recovery suggests that structural changes in the cardiovascular system are involved. This notion is supported by data from a parallel study performed with the same subjects; recovery of SV during supine exercise showed an equally protracted recovery (Spaak et al. 2005).
References
Beck L, Baisch F, Gaffney FA, Buckey JC, Arbeille P, Patat F, ten Harkel AD, Hillebrecht A, Schulz H, Karemaker JM, et al (1992) Cardiovascular response to lower body negative pressure before, during, and after ten days head-down tilt bedrest. Acta Physiol Scand Suppl 604:43–52
Blomqvist CG, Stone HL (1983). Cardiovascular adjustments to gravitational stress. American Physiological Society, Bethesda
Buckey JC Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG (1996) Orthostatic intolerance after spaceflight. J Appl Physiol 81:7–18
Convertino VA, Doerr DF, Eckberg DL, Fritsch JM, Vernikos-Danellis J (1990) Head-down bed rest impairs vagal baroreflex responses and provokes orthostatic hypotension. J Appl Physiol 68:1458–1464
Convertino VA, Adams WC, Shea JD, Thompson CA, Hoffler GW (1991) Impairment of carotid-cardiac vagal baroreflex in wheelchair-dependent quadriplegics. Am J Physiol 260:R576–R580
Convertino VA, Doerr DF, Guell A, Marini JF (1992) Effects of acute exercise on attenuated vagal baroreflex function during bed rest. Aviat Space Environ Med 63:999–1003
Convertino VA, Fritsch JM (1992) Attenuation of human carotid-cardiac vagal baroreflex responses after physical detraining. Aviat Space Environ Med 63:785–788
Eckberg DL, Fritsch JM (1992) Influence of ten-day head-down bedrest on human carotid baroreceptor-cardiac reflex function. Acta Physiol Scand Suppl 604:69–76
Eckberg DL, Sleight P (eds) (1992) Human baroreflexes in health and disease. Oxford University Press, New York, pp 199–200
Eiken O, Convertino VA, Doerr DF, Dudley GA, Morariu G, Mekjavic IB (1992) Characteristics of the carotid baroreflex in man during normal and flow-restricted exercise. Acta Physiol Scand 144:325–331
Ferretti G, Antonutto G, Denis C, Hoppeler H, Minetti AE, Narici MV, Desplanches D (1997) The interplay of central and peripheral factors in limiting maximal O2 consumption in man after prolonged bed rest. J Physiol 501(Pt 3):677–686
Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL (1994) Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol 77:1776–1783
Fritsch JM, Charles JB, Bennett BS, Jones MM, Eckberg DL (1992) Short-duration spaceflight impairs human carotid baroreceptor-cardiac reflex responses. J Appl Physiol 73:664–671
Haruna Y, Suzuki Y (1997) Blood pressure and heart rate responses to sudden change of posture during 20 days of simulated microgravity (−6 degrees head-down tilt). J Gravit Physiol 4:P37–38
Haruna Y, Suzuki Y, Kawakubo K, Gunji A (1997) Baroreflex during exercise in different postures before and after 20-days bed rest. J Gravit Physiol 4:S53–S57
Hughson RL, Maillet A, Gharib C, Fortrat JO, Yamamoto Y, Pavy-Letraon A, Riviere D, Guell A (1994) Reduced spontaneous baroreflex response slope during lower body negative pressure after 28 days of head-down bed rest. J Appl Physiol 77:69–77
Kamiya A, Iwase S, Kitazawa H, Mano T, Vinogradova OL, Kharchenko IB (2000) Baroreflex control of muscle sympathetic nerve activity after 120 days of 6 degrees head-down bed rest. Am J Physiol Regul Integr Comp Physiol 278:R445–R452
Levine BD, Lane LD, Watenpaugh DE, Gaffney FA, Buckey JC, Blomqvist CG (1996) Maximal exercise performance after adaptation to microgravity. J Appl Physiol 81:686–694
Levine BD, Zuckerman JH, Pawelczyk JA (1997) Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96:517–525
Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG (2002) Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 538:331–340
Linnarsson D, Rosenhamer G (1968) Exercise and arterial pressure during simulated increase of gravity. Acta Physiol Scand 74:50–57
Linnarsson D, Sundberg CJ, Tedner B, Haruna Y, Karemaker JM, Antonutto G, Di Prampero PE (1996) Blood pressure and heart rate responses to sudden changes of gravity during exercise. Am J Physiol 270:H2132–H2142
Melcher A, Donald DE (1981) Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol 241:H838–H849
Montmerle S, Spaak J, Linnarsson D (2002) Lung function during and after prolonged head-down bed rest. J Appl Physiol 92:75–83
Papelier Y, Escourrou P, Gauthier JP, Rowell LB (1994) Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J Appl Physiol 77:502–506
Papelier Y, Escourrou P, Helloco F, Rowell LB (1997) Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol 82:577–583
Parker P, Celler BG, Potter EK, McCloskey DI (1984) Vagal stimulation and cardiac slowing. J Auton Nerv Syst 11:226–231
Potts JT, Shi XR, Raven PB (1993) Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol 265:H1928–1938
Rowell LB, O’Leary DS (1990) Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69:407–418
Rowell LB, O’Leary DS, Kellogg JDL (1996) Integration of cardiovascular control systems in dynamic exercise. American Physiological Society, Bethesda
Sander-Jensen K, Secher NH, Astrup A, Christensen NJ, Giese J, Schwartz TW, Warberg J, Bie P (1986) Hypotension induced by passive head-up tilt: endocrine and circulatory mechanisms. Am J Physiol 251:R742–R748
Sanders JS, Ferguson DW, Mark AL (1988) Arterial baroreflex control of sympathetic nerve activity during elevation of blood pressure in normal man: dominance of aortic baroreflexes. Circulation 77:279–288
Spaak J, Sundblad P, Linnarsson D (1998) Human carotid baroreflex during isometric lower arm contraction and ischemia. Am J Physiol 275:H940–H945
Spaak J, Sundblad P, Linnarsson D (2001) Impaired pressor response after spaceflight and bed rest: evidence for cardiovascular dysfunction. Eur J Appl Physiol 85:49–55
Spaak J, Montmerle S, Sundblad P, Linnarsson D (2005) Long-term bed rest-induced reductions in stroke volume during rest and exercise: cardiac dysfunction vs. volume depletion. J Appl Physiol 98:648–654
Sundberg CJ, Kaijser L (1992) Effects of graded restriction of perfusion on circulation and metabolism in the working leg; quantification of a human ischaemia-model. Acta Physiol Scand 146:1–9
Sundblad P, Haruna Y, Tedner B, Linnarsson D (2000a) Short-term cardiovascular responses to rapid whole-body tilting during exercise. Eur J Appl Physiol 81:259–270
Sundblad P, Spaak J, Linnarsson D (2000b) Cardiovascular responses to upright and supine exercise in humans after 6 weeks of head-down tilt (−6 degrees). Eur J Appl Physiol 83:303–309
Sundblad P, Spaak J, Linnarsson D (2000c) Haemodynamic and baroreflex responses to whole-body tilting in exercising men before and after 6 weeks of bedrest. Eur J Appl Physiol 82:397–406
Toska K, Eriksen M (1993) Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. J Physiol 472:501–512
Traon AP, Sigaudo D, Vasseur P, Maillet A, Fortrat JO, Hughson RL, Gauquelin-Koch G, Gharib C (1998) Cardiovascular responses to orthostatic tests after a 42-day head-down bed-rest. Eur J Appl Physiol Occup Physiol 77:50–59
Warner HR, Cox A (1962) A mathematical model of heart rate control by sympathetic and vagus efferent information. J Appl Physiol 17:349–355
Yang T, Senturia JB, Levy MN (1994) Antecedent sympathetic stimulation alters time course of chronotropic response to vagal stimulation in dogs. Am J Physiol 266:H1339–H1347
Acknowledgements
The collaboration of the subjects and the staff of the Institute of Bio-Medical Problems, Moscow is gratefully acknowledged. This study was supported by the European Space Agency, the National Swedish Space Board, the Swedish Medical Research Council (Grant no. 5020) and Fraenckel’s Foundation for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linnarsson, D., Spaak, J. & Sundblad, P. Baroreflex impairment during rapid posture changes at rest and exercise after 120 days of bed rest. Eur J Appl Physiol 96, 37–45 (2006). https://doi.org/10.1007/s00421-005-0062-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-0062-z