Abstract
The present study was performed to develop regression-based prediction equations for skeletal muscle (SM) mass by ultrasound and to investigate the validity of these equations in Japanese adults. Seventy-two Japanese men (n=38) and women (n=34) aged 18–61 years participated in this study and were randomly separated into two groups: the model development group (n=48) and the validation group (n=24). The total and regional SM mass were measured using magnetic resonance imaging (MRI) 1.5 T-scanners with spin-echo sequence. Contiguous transverse images (about 150 slices) with a slice thickness of 1 cm were obtained from the first cervical vertebra to the ankle joints. The volume of SM was calculated from the summation of digitized cross-sectional area. The SM volume was converted into mass units (kg) by an assumed SM density of 1.04 kg l−1. The muscle thickness (MTH) was measured by B-mode ultrasound (5 MHz scanning head) at nine sites on the anatomical SM belly. Strong correlations were observed between the site-matched SM mass (total, arm, trunk body, thigh, and lower leg) by MRI measurement and the MTH × height (in m) in the model development group (r=0.83–0.96 in men, r=0.53–0.91 in women, P<0.05). When the SM mass prediction equations were applied to the validation group, significant correlations were also observed between the MRI-measured and predicted SM mass (P<0.05). The predicted total SM mass for the validation group was 19.6 (6.5) kg and was not significantly different from the MRI-measured SM mass of 20.2 (6.5) kg. Bland–Altman analysis did not indicate a bias in prediction of the total SM mass for the validation group (r=0.00, NS). These results suggested that ultrasound-derived prediction equations are a valid method to predict SM mass and an alternative to MRI measurement in healthy Japanese adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The accurate estimation of skeletal muscle (SM) mass in vivo is an important tool for studying the age-related loss of SM mass. A number of techniques are available for measuring/estimating SM mass (Lee et al. 2001), including magnetic resonance imaging (MRI), computed tomography (CT), anthropometry (Lee et al. 2000), bioelectrical impedance analysis (BIA, Janssen et al. 2000), urinary creatinine (Wang et al. 1996), whole-body 40 K counting (Wang et al. 2003), and dual-energy X-ray absorptiometry (DXA, Kim et al. 2002). Of these techniques, CT and MRI are considered the gold standards.
Three key points should be considered when studying age-related loss of muscle mass. First, age-related loss of muscle mass occurs in all populations, and therefore any available technique should be safe for all populations. Second, any useful technique should be portable and capable of taking measurements in the field. Although the DXA method can be used to accurately estimate SM mass, it is not portable. DXA involves the generation of small muscle doses of radiation during measurement, potentially precluding its use in certain populations. In general, field methods for estimating SM mass are derived from either anthropometric measurements or bioelectrical impedance analysis (Janssen et al. 2000; Lee et al. 2000; Martin et al. 1990). However, these methods are not as accurate as DXA (Kim et al. 2002). Greater rates of age-related loss of SM occur in the thigh, lower leg, and lower trunk regions, while only moderate losses occur in the upper-trunk and arm regions (Kanehisa et al. 2004; Miyatani et al. 2003; Reimers et al. 1998). Therefore, it is of critical importance to be able to measure the regional body segments. Neither BIA nor DXA are capable of accurate separation of SM mass from the trunk region.
Ultrasound has been widely employed for accurate measurement of the SM size in vivo (Abe et al. 1994, 1997; Ichinose et al. 1998; Kearns et al. 2001; Kubo et al. 2003; Lindstrom et al. 2000; Starkey et al. 1996). A compact-type ultrasound machine weighs approximately 10 kg, making it easily portable. In addition, B-mode ultrasound has been shown to be highly reliable and valid in previous studies involving measurement of muscle thickness (MTH, Baumgartner et al. 1998; Fukunaga et al. 1989; Reeves et al. 2004; Reimers et al. 1998). These characteristics make ultrasound a useful alternative to the more expensive imaging methods for assessing changes in SM mass. Therefore, it would seem reasonable that ultrasound-derived MTH should be used to accurately predict total and/or regional SM mass in vivo. However, there are no ultrasound-derived prediction equations for the estimation of total and/or regional SM mass. Thus, the present study was performed to develop regression-based prediction equations for estimating SM mass using ultrasound and to investigate the validity of these equations in Japanese adults.
Methods
Subjects
Seventy-two healthy Japanese men and women aged 18–61 years randomly separated into two groups—48 in the development group and 24 in the validation group. All subjects were physically active but did not include athletes. None of the subjects reported any known pathology or current medication use. The purpose, procedures, and risks of the study were explained to each subject prior to inclusion and all subjects gave their written informed consent before participating in the study. The Ethical Commission of Tokyo Metropolitan University approved this study. Subjects were instructed to fast (12 h) prior to each laboratory visit. Body mass was measured to the nearest 0.1 kg using an electronically calibrated scale with each subject wearing only minimal clothing. Standing height was measured to the nearest 0.1 cm using a standard height scale.
Measurement of the SM mass
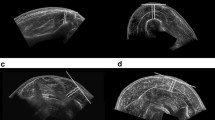
The total body SM volume was measured using MRI (Signa 1.5 T, GE, Milwaukee, WI, USA). Scanning was performed in a sequence with a repetition time of 1,300 ms, echo time of 9.9 ms, slice thickness of 1.0 cm, and inter-slice gap of 0 cm. The subjects lay in the magnet in the prone position with their hands placed on their abdomen. The MRI scanned from the first cervical vertebra to the malleolus lateralis, resulting in a total of approximately 150 images for each subject (Fig. 1). The total time for the MRI measurement was 15 min for each subject. All images traced the segment of the SM area without connective tissue, blood vessels, fat tissue, or abdominal organs by highly trained analyst (Abe et al. 2003). These traced images were scanned into a personal computer and the anatomical cross-sectional area was determined using image analysis software (NIH Image v5.0). The total body SM volume was defined as the sum of the anatomical cross-sectional area and converted to mass units (kilogram) by multiplying the total SM volume (l) by the assumed constant density for adipose tissue-free SM (1.04 kg l−1). The SM mass was separated into discrete regions using anatomical landmarks visible in the scanned images: lower leg (the articular surface of medial condyle to the malleolus lateralis), thigh (the femoral neck to the articular surface of medial condyle), trunk (the first cervicales vertebra to the femoral neck), and arm (the axillary fossa to the styloid process of the radius). The estimated the coefficient of validation (CV) for SM mass measurements from test–retest was 2%.
Ultrasound MTH and measurements
B-mode ultrasonographic MTHs were taken at nine sites from the anterior and posterior surfaces of the body as described previously by Abe et al. (1994). The sites were: lateral forearm, anterior and posterior upper arm, abdomen, subscapula, anterior and posterior thigh, anterior and posterior lower leg. Nine anatomical landmarks for the sites are noted below.
Lateral forearm
On the anterior surface, 30% proximal between the styloid process and the head of the radius.
Anterior and posterior upper arm
On the anterior and posterior surface, 60% distal between the lateral epicondyle of the humerus and the acromial process of the scapula.
Abdomen
At a distance 2–3 cm to the right of the umbilicus.
Subscapula
At a distance of 5 cm, directly below the inferior angle of the scapula.
Anterior and posterior thigh
On the anterior and posterior surface, midway between the lateral condyle of the femur and the greater trochanter.
Anterior and posterior lower leg
On the anterior and posterior surface, 30% proximal between the lateral malleolus of the fibula and the lateral condyle of the tibia.
The MTHs were scanned using a real-time linear electronic scanner with a 5 MHz scanning head (SSD-500, Aloka). The scanning head was prepared with water-soluble transmission gel that provided acoustic contact without depression of the skin surface. The scanner was placed perpendicular to the tissue interface at the marked sites. The MTH was measured directly from the screen using electronic calipers and determined to be the distance from the adipose tissue–muscle interface to the muscle–bone interface (Fig. 2). The reliability of image reconstruction and distance measurements was confirmed by comparing the ultrasonic and manual measurements of tissue thickness in human cadavers, and the CV of this MTH measurement was 1% (Fukunaga et al. 1989). Body height was used to express the length factor of the muscle, because MTH is only a predictor of muscle area. Although body segments were not analyzed, that is, length of forearm, upper arm, thigh and lower leg, in the prediction model, the predicted accuracy (r 2 value) was similar to using body height. As the equation to calculate the volume of a cylinder is π × r 2 × h, it may be suitable for MTH2 × height for the predicting variable. However, using MTH2 × height, these correlation coefficients were lower than the MTH × height dependent on the body site: for example, the sum of nine sites or thigh MTH multiplied height versus total body SM mass in men and women. Therefore, the predicted variables were simply determined as MTH [in centimeters (cm)] × height [in meters (m)].
Statistical analysis
All measurements and calculated values were expressed as the mean (SD). Student’s t test was used for comparison of variables between the development and validation groups. The differences between the MRI-measured and ultrasound-predicted SM mass were tested for significance by paired t test. Pearson’s product correlations were calculated between the MRI-measured SM mass and the MTH × height at each body site. Simple-regression analyses were performed separately in men and women and combined. Stepwise multiple-regression analyses were performed for the MTH × height or the other variables (e.g., MTH, height, body mass, age) and MRI-measured SM mass. Using ANCOVA, regression standards were compared to determine whether there were gender differences in the relationship between the MRI-measured SM mass and the ultrasound MTH × height in total and regional segments. The prediction equations obtained from the model-development group were then validated in the cross-validation group using the approach suggested by Bland and Altman (1986). Thus, the differences between MRI-measured total SM mass and predicted SM mass are plotted against the mean of MRI-measured and predicted SM mass. The alpha level for testing significance was set at P<0.05. All statistical analyses were completed using Stat View v5.0 for windows (SYS Institute).
Results
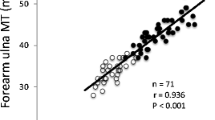
The subject characteristics are presented in Table 1. Significant correlations were observed between the site-matched SM mass by MRI and the ultrasound MTH in both men and women (Table 2). Significant simple correlations were observed between the site-matched SM mass (arm, trunk, thigh, lower leg, and total body) by MRI measurement and the ultrasound MTH × height in the model development group for men (n=26, r=0.83–0.96, P<0.05, SEE=0.2–2.2 kg) and women (n=22, r=0.53–0.91, P<0.05, SEE=0.5–2.9 kg, Table 2). Regression analysis using ANCOVA showed that there were no gender differences in the relationship between the MRI-measured SM mass and the ultrasound MTH × height in total and regional segments. In the model-development group (n=48), the sum of nine sites MTH × height was closely correlated with MRI-measured total body SM mass (total SM mass = 0.687 × UT9 × height − 15.122, n=48, r=0.98, P<0.001, SEE=1.1 kg, Fig. 3) where the units of MTH and height are cm and m, respectively. Stepwise multiple-regression analyses were performed for MRI-measured SM mass and MTH × height or the other variables (e.g., MTH, height, body mass, body mass index, age). These variables were not independently associated with MRI-measured SM mass. Therefore, stepwise regression analysis indicated that the MTH × height was independently associated with MRI-measured SM mass. The SM mass prediction equations were applied to the validation group, and significant correlations were also observed between the MRI-measured and predicted SM mass for total and all regional segments of the SM mass (n=24, r=0.89–0.97, P<0.05, SEE=0.05–0.10 kg). There was a significant correlation between the MRI-measured total SM mass and the ultrasound MTH × height in the validation group (Fig. 4). The predicted total SM mass in the validation group was 19.6 (6.5) kg, and was not different from the MRI-measured SM mass of 20.2 (6.5) kg (Table 3). Bland-Altman analysis did not indicate a bias in prediction of the total SM mass for the validation group (Fig. 5).
Discussion
Ultrasound is widely used for evaluating in vivo human body composition because it is a safe, noninvasive, and portable method (Brodie 1988). Traditionally, it has been used as an alternative to skinfold caliper to measure subcutaneous adipose tissue. Jones et al. (1986) established the validity of using ultrasound to measure adipose thickness by comparing soft-tissue radiograph-derived adipose thickness in 24 adult subjects with depth gauge measured adipose on a cadaver. The statistics of the regression equation showed a very close agreement between the ultrasound and the measurements on a cadaver (the equations for the four sites involved anterior and posterior thigh, lateral and medial calf, r=0.87–0.99). Strong precision for ultrasound-measured MTH has been reported for trunk and appendicular SM thickness (Abe et al. 1994; Ishida et al. 1992). Although there is a study of the prediction equations for body composition by B-mode ultrasound (Abe et al. 1994), to our knowledge, there are no published studies that have developed prediction equations for SM mass (kilogram units), which have been validated by MRI. The primary purpose of the present study was to develop accurately regression-based prediction equations of the total and regional SM mass using ultrasound-measured MTH in healthy Japanese adults. The sum of the ultrasound-measured MTH × height was closely predicted by MRI-measured total and regional SM mass in both men and women. The predicted total and regional SM mass corresponded to the site-matched MRI-measured SM mass in the validation group.
Although MRI is the gold standard for measuring SM mass, its use is limited because it is expensive and not portable. MRI is used primarily for basic or clinical research. Other methods of predicting SM mass suffer from poor accuracy and large error. Recently, Lee et al. (2000) developed a model for prediction of SM mass that used skinfold-corrected upper arm, thigh, and calf girths. Data from the present study were applied to Lee et al.’s equations, and the predicted total SM mass was significantly lower and higher than the MRI-measured SM mass in men (1.0 kg difference, P<0.05) and in women (2.5 kg difference, P<0.05), respectively. Moreover, the SEE of this anthropometrical model was higher than that of the DXA prediction model (Kim et al. 2002). These results suggested that although estimations using anthropometric measurements are easy to use for field studies, this method provides a low degree of accuracy in individual subjects (Table 4). BIA-derived prediction models have also been reported to show large SEE and small r 2. The ultrasound prediction model used in the present study gave a high r 2 value comparable to that of the DXA prediction model and produced a lower SEE as compared to the other methods for estimating total SM mass (Table 4). Ultrasound is portable due to the small weight of the equipment and has the advantage of rapid measurement time. Scanning time for the nine sites used in the present study was approximately 3 min, which was about fivefold faster than MRI and sevenfold faster than DXA. These are ideal characteristics for field studies.
Shih et al. (2000) reported that there were no gender differences in estimated lower-limb SM mass using the DXA-derived prediction model in healthy subjects (207 men and women). It is well known that body fat distribution and fat mass is different between men and women, however, SM mass estimated by ultrasound was not different between men and women in the present study (Fig. 3). These data are in agreement with the data of Abe et al. (2003) who reported that SM distribution was similar in men and women. Although Shih et al.’s model produced a high r 2 (0.89) and low SEE (1.06 kg), it is limited to the prediction of lower-limb SM. Greater gender differences in upper-body SM, rather than lower-body SM mass, have been observed previously. This is most likely because women have a larger proportion of SM mass in their lower body (Gallagher et al. 1997). The lower correlation coefficients in women for trunk SM mass as compared to men is therefore likely to be associated with the gender-specific differences in muscle distribution.
In many previous studies, B-mode ultrasound MTH has been used as an index of SM variation with aging (Kubo et al. 2003; Reimers et al. 1998), space flight or bed rest (Lindstrom et al. 2000; Abe et al. 1997), resistance training (Starkey et al. 1996), and in sports athletes (Ichinose et al. 1998; Kearns et al. 2001). Ultrasound measurement has been shown to be a reliable and accurate method for estimating the MTH. However, there are no quantitative data indicating that ultrasound MTH reflects the site-specific muscle volume or mass. Fukunaga et al. (2000) reported that muscle volume is a major determinant of joint torque in humans. Thus, muscle volume or mass can be used to evaluate variations in muscle function in humans without strength tests. This is especially important for safety in assessment of muscle function or age-related loss of muscle mass in elderly subjects.
Baumgartner et al. (1998) arbitrarily defined clinically significant sarcopenia, that is, age-related loss of muscle mass, as an appendicular muscle mass more than two standard deviations (<−2SD) below the mean of a young reference group (Gallagther et al. 1997). Consequently, the percentage of muscle loss was associated with a threefold to fourfold increase in the likelihood of disability in older individuals. However, this method uses appendicular muscle mass (i.e., involved the muscles of the upper and lower extremities). Using ultrasound, Reimers et al. (1998) reported that the thigh muscle mass decreased significantly with age, whereas upper arm muscles remained nearly unchanged. In addition, it has been reported using ultrasound methods that the abdominal MTH in the elderly (70–79 years) was 31% lower than that in young men and women (20–29 years), and this loss was greater than that in thigh MTH in these groups (26%, Miyatani et al. 2003). Thus, it should be possible to evaluate sarcopenia by estimating the regional SM mass. The prediction equations derived in the present study can be used to evaluate segmental SM mass, such as the trunk body. Therefore, this method can be used to determine relevant indexes of age-related loss of muscle mass in healthy adults.
There are several limitations to the present study that warrant discussion. First, the loss of muscle mass in sarcopenia is a relatively slow process (0.37 kg year−1 for the whole body; Song et al. 2004). This limited one to the use of ultrasound-derived equations with a SEE of 1.1–2.8 kg in the present study, evaluated every year. Evaluation of the loss of muscle mass will require data for over 5 years. Second, the age-related lost muscle mass can be replaced by intermuscular adipose tissue. MTH measurement is unable to differentiate between muscles of the same MTH, but with different amounts of intermuscular adipose tissue. Thus, the MTH measurement may overestimate SM mass in some aged subjects. Kent-Braun et al. (2000) reported that noncontractile cross-sectional area (cm2) in the ankle dorsiflexor muscles is different by approximately 9% between young and old subjects. This value becomes 3% when converted to the length dimension (cm). In the present study, even this difference could not be evaluated with the ultrasound MTH.
In summary, the results of ultrasound MTH and MRI-measured total or regional SM mass at nine sites were examined in two independent groups of Japanese adult men and women to develop prediction equations for estimating total and regional SM mass. The results indicated that ultrasound MTH×height can be used to accurately and reliably estimate total or regional SM mass in healthy Japanese adults.
References
Abe T, Kondo M, Kawakami Y, Fukunaga T (1994) Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol 6:161–170
Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T (1997) Effects of 20 days bed rest on muscle morphology. J Gravit Physiol 4:S10–S14
Abe T, Kearns CF, Fukunaga T (2003) Gender differences in MRI-measured whole-body skeletal muscle mass and distribution in young Japanese adults. Br J Sports Med 37:436–440
Brand JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 8:307–310
Brodie DA (1988) Techniques of measurement of body composition. Part II. Sports Med 5:74–98
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755-63
Fukunaga T, Mastuo A, Ishida Y, Tsunoda N, Uchino S, Ohkubo M (1989) Study for measurement of muscle and subcutaneous fat thickness by ultrasonic B-mode method. Jpn J Med Ultrason 16:170–177
Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. (2000) Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand 172:249–255
Gallagher D, Visser M, Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB (1997) Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol 83:229–239
Ichinose Y, Kanehisa H, Ito M, Kawakami Y, Fukunaga T (1998) Morphological and functional differences in the elbow extensor muscle between highly trained male and female athletes. Eur J Appl Physiol Occup Physiol 78:109–114
Ishida Y, Carroll ML, Pollock JE (1992) Reliability of B-mode ultrasound for the measurement of body fat thickness. Am J Hum Biol 4:511–520
Janssen I, Heymsfield SB, Baumgartner RN, Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471
Jones PRM, Davies PSW, Norgan NG. (1986) Ultrasonic measurements of subcutaneous adipose tissue thickness in man. Am J Phys Anthrop 71:359–363
Kanehisa H, Miyatani M, Azuma K, Kuno S, Fukunaga T (2004) Influences of age and sex on abdominal muscle and subcutaneous fat thickness. Eur J Appl Physiol 91:534–537
Kearns CF, Isokawa M, Abe T (2001) Architectural characteristics of dominant leg muscles in junior soccer players. Eur J Appl Physiol 85:240–243
Kent-Braun JA, Ng AV, Young K. (2000) Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol 88:662–668
Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D (2002) Total body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 76:378–383
Kubo K, Kanehisa H, Azuma K, Ishizu M, Kuno SY, Okada M, Fukunaga T (2003) Muscle architectural characteristics in women aged 20–79 years. Med Sci Sports Exerc 35:39–44
Lee RC, Wang ZM, Heo M, Ross R, Janssen I, Heymsfield SB (2000) Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr 72:796–803
Lee RC, Wang ZM, Heymsfield SB. (2001) Skeletal muscle mass and aging: regional and whole-body measurement methods. Can J Appl Physiol 26:102–122
Lindstrom A, Kvist A, Piersma T, Dekinga A, Dietz MW (2000) Avian pectoral muscle size rapidly tracks body mass changes during flight, fasting and fuelling. J Exp Biol 203(Pt 5):913–919
Martin AD, Spenst LF, Drinkwater DT, Clarys JP (1990) Anthropometric estimation of muscle mass in men. Med Sci Sports Exerc 22:729–733
Miyatani M, Kanehisa H, Azuma K, Kuno S, Fukunaga T (2003) Site-related differences in muscle loss with aging. A cross-sectional survey on the muscle thickness in Japanese men aged 20 to 79 years. Int J Sport Health Sci 1:34–40
Reeves ND, Maganaris CN, Narici MV (2004) Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 91: 116–118
Reimers CD, Harder T, Saxe H (1998) Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. J Neurol Sci 159:60–66
Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D (2004) Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 79:874–880
Starkey DB, Pollock ML, Ishida Y, Welsch MA, Brechue WF, Graves JE, Feigenbaum MS (1996) Effect of resistance training volume on strength and muscle thickness. Med Sci Sports Exerc 28:1311–1320
Shih R, Wang Z, Heo M, Wang W, Heymsfield SB (2000) Lower limb skeletal muscle mass: development of dual-energy X-ray absorptiometry prediction model. J Appl Physiol 89:1380–1386
Wang ZM, Gallagher D, Nelson M, Matthews DE, Heymsfield SB (1996) Total body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr 63:863–869
Wang ZM, Zhu S, Wang J, Pierson RN Jr, Heymsfield SB (2003) Whole-body skeletal muscle mass: development and validation of total-body potassium prediction models. Am J Clin Nutr 77:76–82
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanada, K., Kearns, C.F., Midorikawa, T. et al. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol 96, 24–31 (2006). https://doi.org/10.1007/s00421-005-0061-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-0061-0