Abstract
The purpose of this study was to examine the effect of carbohydrate (CHO) feeding during the second of two 90-min cycling bouts (EX1 started at 09:00 and EX2 started at 13:30) at 60% \(\dot{V}\hbox{O}_{2\max} \) on leucocyte redistribution, neutrophil degranulation and oxidative burst and plasma IL-6 and stress hormone responses. This study consisted of two trials, which were completed in a counterbalanced order and separated by at least 4 days. Subjects (n=9) consumed a lemon flavoured 10% w/v CHO (glucose) or placebo (PLA) beverage during EX2: 500 ml just before exercise and 250 ml every 20 min during exercise. Venous blood samples were taken 5 min before exercise, immediately post-exercise, and 18-h post-EX2 for both trials. The main findings of this study were that ingestion of CHO compared with PLA during EX2 better maintained plasma glucose concentration, blunted the responses of plasma adrenaline, ACTH, cortisol, GH and IL-6, and attenuated the leukocytosis and monocytosis, but had no effect on neutrophil degranulation and oxidative burst activity. Furthermore, the immunoendocrine disturbances induced by two bouts of prolonged exercise returned to resting values within 18 h. These findings suggest that ingestion of CHO compared with PLA during the second of two bouts of 90-min cycling at 60% \(\dot{V}\hbox{O}_{2\max} \) better maintains plasma glucose, blunts hypothalamic–pituitary–adrenal activation, and attenuates leucocyte trafficking, but does not affect neutrophil function. Furthermore, the disturbances of immunoendocrine responses induced by two bouts of prolonged exercise on the same day recover within 18 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prolonged strenuous exercise affects the circulating numbers and functions of immune cells. These effects are thought to be largely mediated by the actions of elevated levels of circulating stress hormones (e.g. adrenaline, cortisol and GH) and altered regulatory cytokines (Mackinnon 1999). Although the effects of a single acute bout of exercise on immune system function are quite well established (Pedersen and Hoffman-Goetz 2000) and some studies have shown that a second bout of exercise performed on the same day induces greater plasma stress hormone responses and leucocyte mobilisation compared with a single identical exercise bout (Ronsen et al. 2001a, b), it is still not clear how repeated bouts of exercise on the same day influence immune function.
As part of their routine training regimen, elite athletes may train several times each day. Repeated bouts of strenuous exercise with short recovery intervals may depress immunity and thus it is of interest to know how nutritional interventions can minimise the impact of heavy training on immunity and performance. To date, the most effective and common strategy applied to maintain immunocompetence and improve endurance exercise performance is to ingest carbohydrate (CHO)-rich drinks during exercise (Costill and Hargreaves 1992; Coyle et al. 1983; Gleeson and Bishop 2000). Ingestion of CHO compared with placebo (PLA) during a single prolonged exercise bout promotes CHO availability, better maintains blood glucose concentration, lessens hypothalamic–pituitary–adrenal (HPA) activation and diminishes the perturbation of circulating leucocyte counts and function (Bishop et al. 1999; Mitchell et al. 1990; Nieman et al. 1997).
Neutrophils are derived from stem cells and mature in the bone marrow and constitute 50–60% of the total blood leucocytes (Smith 1997). After stimulation, activated neutrophils function through a series of actions, including adherence to endothelial cells (attachment), migration into the inflammatory site (chemotaxis), and phagocytosis and killing of microorganisms (Parslow et al. 2001). Neutrophils destroy invading pathogens via both oxygen-dependent (release of reactive oxygen species, ROS) and oxygen-independent (release of proteases) mechanisms (Fukatsu et al. 1996; Johnson et al. 1998). It has been recently suggested that neutrophils serve as a last line of defence to block the “open window” during the period of immunodepression after intensive prolonged exercise (Pedersen 1999). However, several studies reported that endurance exercise temporarily reduced neutrophil phagocytosis (Blannin et al. 1996), degranulation response to bacterial stimulation on a per cell basis (Blannin et al. 1997), and oxidative burst activity in vitro (Gabriel et al. 1994; Pyne et al. 1996).
Many factors may influence neutrophil mobilisation and function during exercise, such as adrenaline, cortisol, GH and IL-6 (Benschop et al. 1996; Mullen et al. 1995; Pyne 1994; Ruy et al. 1997; Suzuki et al. 1996; Tintinger et al. 2001). Interestingly, plasma concentrations of these parameters are increased during prolonged exercise together with a reduction in CHO availability. During a second exercise bout, liver and muscle glycogen content may be compromised by the previous exercise bout. This may induce an energy crisis in the working muscle, affecting sympathetic nervous system (SNS) and HPA activation. Therefore, we hypothesised that CHO supplementation during the second of two prolonged exercise bouts would be particularly effective in minimising immunoendocrine responses. Furthermore, previous studies demonstrated that the immunodepression induced by intensive strenuous exercise may last for several hours post-exercise (Mackinnon 1999). Hence, the aims of the present study were to determine the effect of carbohydrate supplementation during the second of two prolonged cycling bouts on changes in leucocyte redistribution, plasma stress hormones, IL-6, and bacterially stimulated neutrophil degranulation and PMA (phorbol-12- myristate-13-acetate)-induced oxidative burst activity in vitro. In addition, we also examined if the aforementioned parameters recover from the influence of the two bouts of 90-min cycling at 60% \(\dot{V}\hbox{O}_{2\max} \) within 18 h.
Methods
Subjects
Nine male volunteers (age 28.7±1.6 years, height 174±2 cm, body mass 74.4±3.2 kg, \(\dot{V}\hbox{O}_{2\max} \) 50.3±2.4 ml kg−1 min−1; means ± SEM), who were recreationally active and familiar with cycling, participated in the study. After receiving written information and passing a Health Questionnaire screen, subjects signed an informed consent. Subjects were requested to complete the dietary record sheet on the day prior to trial 1 and then repeated the same diets according to the dietary record sheet before trial 2. Subjects were also asked not to perform any strenuous exercise or consume alcohol or medication for 2 days before each trial. The protocol was approved by the Ethics Committee of Loughborough University before the study began.
Preliminary measurements
Maximal oxygen uptake was estimated by means of a continuous incremental exercise test on a cycle ergometer (Monark 874E, Monark Exercise AB, Sweden) to volitional exhaustion. Subjects began cycling at 70 W with increments of 35 W every 3 min. The cadence was maintained at 70 rpm and heart rate was monitored using radiotelemetry. During the third minute of each work rate increment, expired gas was collected in Douglas bags. An oxygen/carbon dioxide analyzer (Servomax 1400B, Crowborough, UK) was used along with a dry gas meter (Harvard Apparatus, Edenbridge, UK) for the determination of \(\dot{V}\hbox{O}_{2} \) and \(\dot{V}\hbox{CO}_{2}\) From the \(\dot{V}\hbox{O}_{2}{\text{-work\;rate\;relationship}},\) the work rate equivalent to 60% \(\dot{V}\hbox{O}_{2\max} \) was interpolated.
Experimental procedures
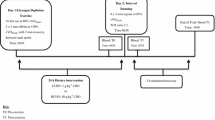
The subjects completed two trials in a counterbalanced order, each separated by at least 4 days. Subjects arrived at the laboratory at 08:30 after fasting from 23:00 the previous day and were asked to empty the bladder before body mass was recorded. Subjects then performed two bouts of 90-min cycling (EX1 started at 09:00 and EX2 started at 13:30) at 60% \(\dot{V}\hbox{O}_{2\max} \) at 70 rpm on the same ergometer used to determine \(\dot{V}\hbox{O}_{2\max}.\) Subjects were given a lemon flavoured 10% w/v CHO (glucose) beverage or artificially sweetened placebo during the second exercise bout: 500 ml just before exercise and 250 ml every 20 min during exercise. Subjects were asked to consume each drink within 3 min. Heart rate was recorded continuously during exercise by radiotelemetry. Ratings of perceived exertion (RPE) were obtained at 15-min intervals. Expired gas was collected at 30 and 60 min during both exercise bouts for 1 min to confirm exercise intensity. Venous blood samples were taken 5 min before exercise, immediately post-exercise, and 18-h post EX2. Subjects were asked not to consume any food throughout trials (including the 3-h recovery interval between EX1 and EX2) until 2 h after EX2. Thereafter, food intake was allowed ad libitum until 23:00 so that the 18-h post-EX2 sample was collected following another overnight fast. Water ingestion was allowed ad libitum during the first exercise bout and the recovery interval and also after 2 h post-EX2. The laboratory temperature and relative humidity were 24.5±0.2°C and 33±2%, respectively.
Blood collection and analysis
Venous blood samples were taken from an antecubital vein by venepuncture, and were collected into three vacutainer tubes (Becton Dickinson, Oxford, UK). Blood samples in two K3EDTA vacutainers (4 ml) were used for haematological analysis including haemoglobin, haematocrit, and total and differential leucocyte counts using an automated haematology analyser (AC •T 5diff analyser, Beckman Coulter, UK) and to determine changes in the plasma concentrations of stress hormones and interleukin-6. Plasma volume changes were calculated according to Dill and Costill (1974). From blood taken into a lithium heparin vacutainer (7 ml), 1 ml was immediately added to an eppendorf tube (1.5 ml capacity) containing 50 μl of bacterial stimulant solution (Stimulant, Sigma, Poole, UK). Blood and bacterial stimulant were mixed by gentle inversion and then incubated for 1 h at 37°C, with gentle mixing every 20 min. After incubation, the mixture was centrifuged for 2 min at 15,000 g. The supernatant was immediately stored at −80°C prior to analysis of elastase concentration. The amount of elastase released per neutrophil in response to bacterial stimulation was calculated according to Robson et al. (1999).
A microplate luminometer cell activation kit (Knight Scientific Limited, Plymouth, UK) was used to measure the neutrophil oxidative burst activity. Sample analysis was performed in duplicate as follows: 20 μl of K3EDTA whole blood sample was added into a dilution tube with 2 ml of blood dilution buffer (HBSS without calcium and magnesium but with 20 mM HEPES, pH 7.4). A 20 μl aliquot of each diluted sample was then added into an opaque white microplate well. Ninety microlitre reconstitution and assay buffer (HBSS with 20 mM HEPES, pH 7.4) was then added into each well followed by the addition of 20 μl reconstituted Adjuvant-K and 50 μl Pholasin (10 μg ml−1). The microplate was placed into a luminometer (Anthos Lucy 1 Microplate Luminometer, Anthos Labtec Instrument, Austria) after adding 20 μl PMA (phorbol-12- myristate-13-acetate, 5 μg ml−1) into each well. After 1 min shaking and incubation at 37°C, Pholasin-enhanced chemiluminescence (CL) was measured at 1 min intervals for 30 min, and the incremental area under the curve (IAUC) was calculated. The oxidative burst activity per cell was calculated by dividing the IAUC by the neutrophil count in each sample. The intra-assay coefficient of variation was 5.7% for the chemiluminescence assay.
The remaining K3EDTA and heparinized whole blood was spun at 1,500 g for 10 min in a refrigerated centrifuge at 4°C within 10 min of sampling. The plasma obtained was immediately stored at −80°C prior to analysis. Plasma aliquots were analysed to determine the concentration of glucose (GOD-PAP method, Randox, Ireland) using an automatic photometric analyser (Cobas-Mira plus, Roche). Human growth hormone (GH), cortisol (both DRG Instruments GmbH, Germany), adrenaline (IBL GmbH, Hamburg, Germany), interleukin-6 (IL-6) (Diaclone Research, France) and elastase (Merck, Lutterworth, UK) were determined using enzyme-linked immunosorbant assay (ELISA) kits. The intra-assay coefficient of variation was 1.3, 2.4, 6.9, 12.7, 1.6, and 3.9% for glucose, GH, cortisol, adrenaline, IL-6, and elastase, respectively.
Statistical analysis
All results are presented as mean values and standard errors of the mean (±SEM). Data were checked for normality, homogeneity of variance and sphericity before statistical analysis, and where appropriate the Huynh–Feldt method was applied for adjustment of degrees of freedom for the F tests. Data were analysed using a two-factor (trial × time) repeated measures ANOVA with post hoc Tukey and paired t tests, where appropriate. For the blood variables, the time points used in the ANOVA were pre-EX2 and the post-EX2 since the intervention (CHO or PLA) occurred during EX2. Comparison of immunoendocrine responses between pre-trial and 18 h post-EX2 (both at 09:00) were examined using paired t tests. P, t, and adjusted F values are presented and statistical significance was accepted at P<0.05.
Results
Physiological variables and RPE
There were significant main effects of time and interactions between trial and time for RPE (time: P= 0.001 and interaction: P=0.005) and body mass loss (time: P=0.006 and interaction: P=0.017). There was a significant main effect of trial (P=0.025) and an interaction between trial and time (P=0.004) for percentage change in plasma volume. A main effect of time was found for \(\%\dot{V}\hbox{O}_{2\max} \) (P=0.023) and HR (P=0.001) (Table 1).
Leucocyte counts
There was a significant main effect of trial for the blood counts of leucocytes (P=0.031, Fig. 1a) and monocytes (P=0.011, Fig. 1d), with values higher at post-EX2 in PLA compared with CHO. There was a significant main effect of time for the blood leucocyte (P<0.001), neutrophil P=0.006, Fig. 1b), lymphocyte (P<0.001, Fig. 1c) and monocyte (P<0.001) counts, with higher values at post-EX2 than pre-EX2 in both trials. Furthermore, there was a significant interaction between trial and time for blood leucocyte (P=0.004), lymphocyte (P=0.035) and monocyte (P=0.003) counts. No difference was found between pre-EX1 and 18 h-p-EX2.
Stress hormones
There was a significant main effect of trial for plasma concentrations of adrenaline (P=0.018, Fig. 2a), ACTH (P=0.011, Fig. 2b), cortisol (P=0.007, Fig. 2c) and GH (P=0.005, Fig. 2d), with higher levels at post-EX2 in PLA compared with CHO. A significant main effect of time was observed for ACTH (P=0.002) and cortisol (P<0.001), with higher levels at post-EX2 compared with pre-EX2 in both trials. There was also a significant main effect of time for plasma adrenaline (P=0.009), which was higher at post-EX2 than pre-EX2 only in PLA. Furthermore, there was a significant interaction between trial and time for plasma adrenaline (P=0.031), ACTH (P=0.012), cortisol (P=0.010) and GH (P=0.006). No difference was found between pre-EX1 and 18 h-p-EX2.
Glucose and IL-6
There was a significant main effect of trial for plasma glucose (P<0.001, Fig. 3a) and IL-6 (P=0.035, Fig. 3b), which were higher at post-EX2 in PLA compared with CHO. A significant main effect of time for plasma IL-6 was observed (P=0.001). Furthermore, there was an interaction between trial and time for plasma glucose (P<0.001) and IL-6 (P = 0.030). No difference was found between pre-EX1 and 18 h-p-EX2.
Neutrophil degranulation and oxidative burst
There was no main effect of trial, time, or interaction between trial and time for bacterially-stimulated neutrophil elastase release in total or on per cell basis (Fig. 4a, b) and total PMA-induced oxidative burst (Fig. 5a) from pre-EX2 to post-EX2. However, there was a significant main effect of time for PMA-induced oxidative burst per neutrophil (P=0.050), which showed a lower value at post-EX2 compared with pre-EX2 in PLA (Fig. 5b). No difference was found between pre-EX1 and 18 h-p-EX2.
Discussion
The main findings of this study were that ingestion of CHO compared with PLA during EX2 better maintained plasma glucose concentration, blunted the responses of plasma adrenaline, ACTH, cortisol, GH and IL-6, and attenuated the leucocytosis and monocytosis, but had no effect on bacterially-stimulated neutrophil degranulation and PMA-induced oxidative burst activity. Furthermore, the immunoendocrine disturbances induced by two bouts of prolonged exercise returned to resting values within 18 h.
The plasma glucose level at the end of EX2 was as low as 3.75±0.22 mM (Fig. 3a), reaching the threshold for inducing elevated plasma adrenaline (3.8±0.1 mM) and GH (3.7±0.1 mM) secretion (Schwartz et al. 1987). Since a low blood glucose level is also associated with HPA activation and stress hormone secretion (Mitchell et al. 1990), it was not surprising to find higher concentrations of plasma adrenaline, ACTH, cortisol and GH at post-EX2 in PLA than CHO in this study.
EX2 elicited significant increases in circulating counts of total leucocytes and subsets, whereas ingestion of CHO compared with PLA blunted the leucocytosis (Fig. 1a) and monocytosis (Fig. 1d), but not the neutrophilia (Fig. 1b) and lymphocytosis (Fig. 1c). It is well known that acute exercise leads to a significant but reversible redistribution of leucocyte subsets between the circulation, marginated pools and the bone marrow (Gleeson and Bishop 1999). This exercise-induced mobilisation of leucocytes is linked to elevated plasma concentrations of stress hormones (Benschop et al. 1996). Catecholamines exert an immediate effect, initiating a lymphocytosis within 10 min and subsequently evoke a neutrophilia and monocytosis with a relative lymphopenia (Benschop et al. 1996). The later rise of plasma cortisol during a prolonged strenuous exercise induces a further neutrophilia from the spleen and the bone marrow into the circulation (Toft et al. 1994) but mobilises other leucocyte subsets from the circulation into the bone marrow, lymphoid, skin and injured tissue (Toft et al. 1992; Wira et al. 1990). The leucocyte trafficking during EX2 is likely mediated by the elevated plasma adrenaline (Fig. 2a) rather than cortisol (Fig. 2c) or GH (Fig. 2d) since we did not find a lymphopenia at post-EX2. Moreover, Nieman (1997) has suggested that cortisol does not dominate leucocyte mobilisation until the duration of exercise is in excess of 90 min and the effect of GH on neutrophil trafficking may not occur until 2 h after infusion (Kappel et al. 1993).
No significant effect of CHO ingestion on neutrophil degranulation (Fig. 4) and oxidative burst activity (Fig. 5) was found in the present study. The results support the findings of previous studies, which reported that CHO ingestion during exercise did not affect neutrophil degranulation on per cell basis (Bishop et al. 2002; Lancaster et al. 2003). Although there were no differences in bacterially stimulated elastase release per neutrophil between pre-EX2 and post-EX2 in both trials, a significant decrease was observed at post-EX1 compared with pre-EX1 in both trials (Fig. 4b). The findings were similar to previous studies, which demonstrated that neutrophil degranulation on per cell basis fell after 2-h cycling at 60% \(\dot{V}\hbox{O}_{2\max} \) (Walsh et al. 2000) and following cycling to fatigue (98±7 min in the CHO trial) at 75% \(\dot{V}\hbox{O}_{2\max} \) (Bishop et al. 2001). Nakagawa et al. (1998) reported that the circulating neutrophilia after dexamethasone infusion was due to the release of neutrophils from the bone marrow (10%) and marginated pools (61%) and prolongation of neutrophil intravascular half-life (29%) in rabbits. The neutrophils released from the bone marrow appear to have a lower content of granular digestive enzymes compared with fully mature neutrophils (Pyne 1994). Therefore, an important factor determining the effect of exercise on neutrophil degranulation response to bacterial stimulation on a per cell basis may be the mobilisation of neutrophils, although many other factors seem to be associated with neutrophil degranulation, such as the level of intracellular cAMP (Ottonello et al. 1997), phagocytic activity (Morozov et al. 2003), platelet–neutrophil contacts (Losche et al. 1996), adrenaline (Tintinger et al. 2001), glucocorticoids (Liles et al. 1995) and IL-6 (Johnson et al. 1998). This suggestion is supported by observations in the present study: a neutrophilia during EX1 coincided with a decline in bacterially stimulated elastase release, whereas the similar neutrophilia in CHO and PLA during EX2 was associated with similar values of elastase release per neutrophil throughout the experimental protocol. Gleeson et al. (1998) reported that intensive exercise induces spontaneous neutrophil degranulation in vivo. Morozov et al. (2003) further indicated that elevated circulating glucocorticoid levels during exercise might stimulate neutrophil degranulation and result in a decrease of the granule protein content in neutrophils. These suggestions may partly explain the insignificant decline of neutrophil degranulation on per cell basis with a significant neutrophilia during EX2 in both trials.
In the present study, the PMA-induced neutrophil oxidative burst activity (determined by CL) on per cell basis did not decline during EX1 until pre-EX2. The CL was only further decreased during EX2 on the PLA trial. However, there was no significant difference between CHO and PLA throughout the experimental protocol. A few studies have examined the effect of exercise on PMA-induced neutrophil oxidative burst activity and the results were inconsistent. Pyne et al. (1996) reported that PMA-induced CL decreased 41% during the first bout of 40 min running at a heart rate of ∼140 beats min−1 and the CL values did not change further during the 1 h recovery interval or in a second bout of identical exercise. However, Suzuki et al. (1999) showed that the PMA-induced CL of isolated neutrophils was increased after 90 min cycling at ∼53% \(\dot{V}\hbox{O}_{2\max}. \) Boyum et al. (2002) also reported that the PMA-induced CL of isolated neutrophils was increased after the second (50% \(\dot{V}\hbox{O}_{2\max} \) for 10 min and then 75% \(\dot{V}\hbox{O}_{2\max} \) for 65 min) of two cycling bouts on the same day. Moreover, CHO ingestion did not appear to affect PMA-stimulated intracellular H2O2 production (Smith et al. 1996). The neutrophils in the bone marrow are less mature with lower NADPH-dependent oxidase activity and superoxide response to PMA stimulation (Berkow and Dodson 1986), whereas the nitro blue tetrazolium (NBT)-negative neutrophils in the marginated pools are likely to produce less superoxide in response to in vitro stimulation (Suzuki et al. 1996). Therefore, the decline of the PMA-induced CL on per neutrophil basis at pre-EX2 and onwards in the present study may be due to the influx of these two types of neutrophils into the circulation. Other factors also appear to affect neutrophil oxidative burst activity, including adrenaline (Tintinger et al. 2001), GH (Ruy et al. 1997), repeated stimulation (Prasad et al. 1991), and cell isolation procedures (Fukuda and Schmid-Schonbein 2002). In the present study, the plasma IL-6 concentration was elevated during exercise and CHO ingestion blunted the IL-6 response compared with PLA. It is accepted that prolonged exercise elicits IL-6 production and release from contracting skeletal muscle into the circulation (Steensberg et al. 2000). The plasma IL-6 level is not markedly elevated until the later stage of prolonged exercise (glycogen- depleted state) and CHO ingestion during exercise attenuates the plasma IL-6 response (Febbraio and Pedersen 2002). The relatively low level of plasma IL-6 in the present study may reflect a sufficient CHO availability during EX2 and may not exert marked metabolic effects on hepatic glucose production, muscle glucose uptake, and lipolysis during exercise (Gleeson 2000).
In conclusion, ingestion of CHO compared with PLA during the second of two bouts of 90 min cycling at 60% \(\dot{V}\hbox{O}_{2\max} \) maintained better CHO availability, blunted HPA activation, and attenuated the leukocytosis and monocytosis. However, CHO ingestion had no effect on the neutrophil degranulation response to bacterial stimulation and oxidative burst activity induced by PMA on per cell basis. Furthermore, the disturbances of immunoendocrine responses induced by two bouts of prolonged exercise on the same day recovered within 18 h.
References
Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M (1996) Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun 10:77–91
Berkow RL, Dodson RW (1986) Purification and functional evaluation of mature neutrophils from human bone marrow. Blood 68:853–860
Bishop NC, Blannin AK, Walsh NP, Robson PJ, Gleeson M (1999) Nutritional aspects of immunosuppression in athletes. Sports Med 28:151–176
Bishop NC, Blannin AK, Walsh NP, Gleeson M (2001) Carbohydrate beverage ingestion and neutrophil degranulation responses following cycling to fatigue at 75% VO2 max. Int J Sports Med 22:226–231
Bishop NC, Gleeson M, Nicholas CW, Ali A (2002) Influence of carbohydrate supplementation on plasma cytokine and neutrophil degranulation responses to high intensity intermittent exercise. Int J Sport Nutr Exerc Metab 12:145–156
Blannin AK, Chatwin LJ, Cave R, Gleeson M (1996) Effects of submaximal cycling and long term endurance training on neutrophil phagocytic activity in middle aged men. Br J Sports Med 30:125–129
Blannin AK, Gleeson M, Brooks S, Cave R (1997) The effects of endurance training in the bacterially stimulated degranulation of human neutrophils in vitro. J Sport Sci 15:28
Boyum A, Ronsen O, Tennfjord VA, Tollefsen S, Haugen AH, Opstad PK, Bahr R (2002) Chemiluminescence response of granulocytes from elite athletes during recovery from one or two intense bouts of exercise. Eur J Appl Physiol 88:20–28
Costill DL, Hargreaves M (1992) Carbohydrate nutrition and fatigue. Sports Med 13:86–92
Coyle EF, Hagberg JM, Hurley BF, Martin WH, Whsani AA, Holloszy JO (1983) Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol 55:230–235
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Febbraio M, Pedersen BK (2002) Muscle derived interleukin-6 mechanisms for activation and possible biological roles. FASEB J 16:1335–1347
Fukatsu K, Sato N, Shimizu H (1996) 50-mile walking race suppresses neutrophil bactericidal function by inducing increases in cortisol and ketone bodies. Life Sci 58:2337–2343
Fukuda S, Schmid-Schonbein GW (2002) Centrifugation attenuates the fluid shear response of circulating leukocytes. J Leukoc Biol 72:133–139
Gabriel H, Muller HJ, Urhausen A, Kindermann W (1994) Suppressed PMA-induced oxidative burst and unimpaired phagocytosis of circulating granulocytes one week after a long endurance exercise. Int J Sports Med 15:441–445
Gleeson M (2000) Interleukins and exercise. J Physiol 529:1
Gleeson M, Bishop NC (1999) Immunology. In: Maughan RJ (ed) Basic and applied sciences for sports medicine. Butterworth-Heinemann, Oxford, pp 199–236
Gleeson M, Bishop NC (2000) Modification of immune responses to exercise by carbohydrate, glutamine and anti-oxidant supplements. Immunol Cell Biol 78:554–561
Gleeson M, Walsh NP, Blannin AK, Robson PJ, Cook L, Donelly AE, Day SH (1998) The effect of severe eccentric exercise-induced muscle damage on plasma elastase, glutamine and zinc concentrations. Eur J Appl Physiol 77:543–546
Johnson JL, Moore EE, Tamura DY, Zallen G, Biffl WL, Silliman CC (1998) Interleukin-6 augments neutrophil cytotoxic potential via selective enhancement of elastase release. J Surg Res 76:91–94
Kappel M, Hansen MB, Diamant M, Jorgensen JOL, Gyhrs A, Pedersen BK (1993) Effects of an acute bolus growth hormone infusion on the human immune system. Horm Metabol Res 25:579–585
Lancaster GI, Jentjen RLPG, Moseley L, Jeukendrup AE, Gleeson M (2003) Effect of pre-exercise carbohydrate ingestion on plasma cytokine, stress hormone, and neutrophil degranulation responses to continuous, high intensity exercise. Int J Sport Nutr Exerc Metab 13:1–18
Losche W, Dressel M, Krause S, Redlich H, Spangenberg P, Heptinstall S (1996) Contact-induced modulation of neutrophil elastase secretion and phagocytic activity by platelets. Blood Coagul Fibrinolysis 7:210–213
Mackinnon LT (1999) Advances in exercise immunology. Human Kinetics, Champaign
Mitchell JB, Costill DL, Houmard JA, Flynn MG, Fink WJ, Beltz JD (1990) Influence of carbohydrate ingestion on counterregulatory hormones during prolonged exercise. Int J Sports Med 11:33–36
Morozov VI, Pryatkin SA, Kalinski MI, Rogozkin VA (2003) Effect of exercise to exhaustion on myeloperoxidase and lysozyme release from blood neutrophils. Eur J Appl Physiol 89:257–262
Mullen PG, Windsor ACJ, Walsh CJ, Fowler AA, Sugerman HJ (1995) Tumor necrosis factor-á and interleukin-6 selectively regulate neutrophil function in vitro. J Surg Res 58:124–130
Nakagawa M, Terashima T, D’yachkova Y, Bondy GP, Hogg JC, Van Eeden SF (1998) Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation 98:2307–2313
Nieman DC (1997) Immune response to heavy exertion. J Appl Physiol 82:1385–1394
Nieman DC, Fagoaga OR, Butterworth DE, Warren BJ, Utter A, Davis JM, Henson DA, Nehlsen-Cannarelia SL (1997) Carbohydrate supplementation affects blood granulocyte and monocyte trafficking but not function after 2.5 h of running. Am J Clin Nutr 66:153–159
Ottonello L, Barbera P, Dapino P, Sacchetti C, Dallegri F (1997) Chemoattractant-induced release of elastase by lipopolysaccharide (LPS)-primed neutrophils; inhibitory effect of the anti-inflammatory drug nimesulide. Clin Exp Immunol 110:139–143
Parslow TG, Stites DP, Terr AI, Imboden JB (2001) Medical immunology, 10th edn. McGraw-Hill, New York
Pedersen BK (1999) Exercise and immune function. In: Schedlowski M, Tewes U (eds) Psychoneuroimmunology: an interdisciolinary introduction. Kluwer/Plenum, New York, pp 341–358
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 80:1055–1081
Prasad K, Chaudhary AK, Kalra J (1991) Oxygen-derived free radicals producing activity and survival of activated polymorphonuclear leukocytes. Mol Cell Biochem 103:51–62
Pyne DB (1994) Regulation of neutrophil function during exercise. Sports Med 17:245–258
Pyne DB, Baker MS, Smith JA, Telford RD (1996) Exercise and the neutrophil oxidative burst: biological and experimental variability. Eur J Appl Physiol 74:564–571
Robson PJ, Blannin AK, Walsh NP, Castell LM, Gleeson M (1999) Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med 20:128–135
Ronsen O, Haug E, Pedersen BK, Bahr R (2001a) Increased neuroendocrine response to a repeated bout of endurance exercise. Med Sci Sports Exerc 33:568–575
Ronsen O, Pedersen BK, Oritsland TR, Bahr R, Kjeldsen-Kragh J (2001b) Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J Appl Physiol 91:425–434
Ruy H, Jeong S-M, Jun C-D, Lee J-H, Kim J-D, Lee B-S, Chung H-T (1997) Involvement of intracellular Ca2+ during growth hormone-induced priming of human neutrophils. Brain Behav Immun 11:39–46
Schwartz NS, Clutter WE, Shah SD, Cryer PE (1987) Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest 79:777–781
Smith JA (1997) Exercise immunology and neutrophils. Int J Sports Med 18:S46–S55
Smith JA, Gray AB, Pyne DB, Baker MS, Telford RD, Weidemann MJ (1996) Moderate exercise triggers both priming and activation of neutrophil subpopulations. Am J physiol 270:R838–R845
Steensberg A, Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK (2000) Production of interleukin-6 in contracting human skeletal muscle can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242
Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, Totsuka M, Sato K, Yamaya K (1996) Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. J Appl Physiol 81:1213–1222
Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, Sugawara K, Yamaya K, Sato K (1999) Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol 87:1360–1367
Tintinger GR, Theron AJ, Anderson R, Ker JA (2001) The anti-inflammatory interactions of epinephrine with human neutrophils in vitro are acheieved by cyclic AMP-mediated accelerated resequestration of cytosolic calcium. Biochem Pharmacol 61:1319–1328
Toft P, Tonnesen E, Svendsen P, Pasmussen JW (1992) Redistribution of lymphocytes after cortisol administration. APMIS 1000:154–158
Toft P, Helbo-Hansen HS, Lillevang ST, Rasmussen JW, Christensen NJ (1994) Redistribution of granulocytes during adrenaline infusion and following administration of cortisol in healthy volunteers. Acta Anaesthesiol Scand 38:254–258
Walsh NP, Blannin AK, Bishop N, Robson PJ, Gleeson M (2000) Effect of oral glutamine supplementation on human neutrophil lipopolysaccharide-stimulated degranulation following prolonged exercise. Int J Sport Nutr Exerc Metab 10:39–50
Wira CR, Sandoe CP, Steele MG (1990) Glucocorticoid regulation of the humoral immune system. I. In vivo effects of dexamethasone on IgA and IgG in serum and at mucosal surfaces. J Immunol 144:142–146
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, TL., Gleeson, M. The effects of carbohydrate supplementation during the second of two prolonged cycling bouts on immunoendocrine responses. Eur J Appl Physiol 95, 391–399 (2005). https://doi.org/10.1007/s00421-005-0024-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-0024-5