Abstract
An intermittent exposure to artificial hypergravity with physical exercise by a human centrifuge may provide a countermeasure against various physiological problems after space flight. To test the effects of hypergravity with ergometric exercise on dynamic regulation of heart rate during weightlessness, we quantified autonomic cardiovascular control before and after head-down-tilt bed rest (HDBR) with and without the countermeasure. Twelve male subjects underwent a 14-day period of HDBR. Six of them were exposed to a hypergravity (+1.2 Gz acceleration at heart level) for 30 min with ergometric exercise (60 W, n=4; 40 W, n=2) as a countermeasure on day 1, 2, 3, 5, 7, 9, 11, 12, 13 and 14, during HDBR (CM group). The remaining six were not exposed to a hypergravity exercise during HDBR (control group). Blood pressure and ECG were recorded at a supine position before and after HDBR. The high frequency power of R–R interval (HFRR; 1,008±238 to 353±56 ms2 P<0.05) as an index of cardiac parasympathetic activity, and transfer function gain between BP and R–R interval in the high frequency range (GainHF; 21.9±5.4 to 14.5±4.2 ms/mmHg, P<0.01) as an index of vagally mediated arterial-cardiac baroreflex, decreased significantly after HDBR in the control group. However, these changes were not statistically significant in the CM group (HFRR, 1,150±344 to 768±385 ms2; GainHF, 21.5±3.3 to 18.6±3.4 ms/mmHg). Moreover, baroreflex gain by sequence analysis showed similar results. This observation suggests that the intermittent exposure to hypergravity with ergometric exercise may attenuate the decreases in the parasympathetic activity and the spontaneous arterial-cardiac baroreflex function after weightlessness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Space flight or simulated microgravity (head-down-tilt bed rest; HDBR) leads to various physiological and/or histological changes (Levine et al. 1997). For example, cardiac parasympathetic activity and spontaneous arterial-cardiac baroreflex function (Cooke et al. 2000; Custaud et al. 2002; Hughson et al. 1994; Iwasaki et al. 2000, 2001; Pavy-LeTraon et al. 1997; Sigaudo et al. 1998) are reduced after space flight or bed rest, associated with a reduction in plasma volume (Alfrey et al. 1996; Buckey et al. 1996; Custaud et al. 2002; Hughson et al. 1994; Iwasaki et al. 2000, 2001). These changes may reduce the ability to control cardiovascular systems of astronauts.
This adaptation to the microgravity environment is the opposite of that observed in subjects who underwent intermittent exposure to low or high sustained +Gz acceleration (Convertino 1998, 2001; Iwasaki et al. 1998), such as increased cardiac-baroreflex function, blood volume, and cardiac output. Therefore, it is considered that intermittent exposure to artificial hypergravity might provide a countermeasure against changes in the cardiovascular system when it is used in space (Convertino 1998; Iwasaki et al. 1998). In fact, our preliminary study (Iwasaki et al. 2001), and the other (Korolkov et al. 2001), showed the positive effects of an intermittent exposure to artificial hypergravity on cardiovascular changes during simulated microgravity. Also, artificial hypergravity may be effective in various other physiological problems induced by microgravity (e.g. bone demineralization). Furthermore, a combination of hypergravity and physical exercise is suggested as the better countermeasure (Greenleaf et al. 1999; Vil-Viliams 1994). However, no study clearly shows that an intermittent exposure to artificial hypergravity with physical exercise via centrifuge reduces any physiological changes induced by real or simulated microgravity. Hence, there is no clear evidence that such a countermeasure is, at least effective to some extent in deconditioning after exposure to microgravity.
To evaluate the effects of an intermittent exposure to hypergravity with physical exercise as a countermeasure against changes in dynamic regulation of heart rate after bed rest, we quantified autonomic cardiovascular control before and after a 14-day period of HDBR with or without the countermeasure.
Materials and methods
Subjects
Twelve young male subjects with a mean (±SD) age of 20.7±1.9 years, underwent a 14-day period of 6° head-down bed rest. All candidates were thoroughly briefed regarding the objectives, significance, content, and potential adverse effects of the study. In addition, subjects were informed that they were free to withdraw from the study at their own discretion at any time whatsoever. Tests and measurements were initiated only after obtaining informed consent. All subjects were evaluated as physically healthy by the detailed medical history, physical examination, complete blood count, resting electrocardiogram, blood chemistry analysis, and psychological testing. None of the subjects smoked, used recreational drugs, or had significant medical history. The protocol was approved by The Ethical Committee on Human Research, Research Institute of Environmental Medicine, and by the Ethical Committee of National Space Development Agency of Japan.
Study protocol
A 14-day period of 6° head-down bed rest study protocol was implemented. All subjects received identical meals, with a daily intake of 2,000–2,100 kcal/day (55% carbohydrate, 25% fat, 20% protein) including ~6,000 mg/day of sodium from 2 days before bed rest to the end of its 2 week duration. Fluid intake was allowed ad libitum, but the subjects were encouraged to take at least 1,100 ml fluid per day. Urination and defecation were performed while inclined at −6° on the bed. Changes of position in bed, including supine, lateral, and prone positions, were permitted, but at no time was the head allowed to exceed the height of the heart.
Six of them were exposed to a +1.2 Gz acceleration at heart level for 30 min with simultaneous ergometric exercise training (60 W, n=4; 40 W, n=2) on the cycle ergometer in a human centrifuge as a countermeasure, on days 1, 2, 3, 5, 7, 9, 11, 12, 13 and 14, during head-down bed rest as a countermeasure group (CM group) (a mean±SD; age of 21.2±2.4 years; height 171.4±4.9 cm, and body weight 69.6±8.2 kg). We chose “+1.2 Gz for 30 min” to prevent the development of presyncopal symptoms or all-out exhaustion based on a pilot study and thatof Vil-Viliams (1994). Although 60 W was a prescribed exercise load (Iwase et al. 2002), two subjects could not tolerate to the 30 min of centrifuge with 60 W ergometric exercise. At their request, 40 W was used for two subjects.
The remaining six were not exposed to a +Gz acceleration, the control group (a mean±SD; age of 20.2±1.2 years; height 168.6±5.2 cm; and body weight 62.1±5.0 kg).
Human centrifuge
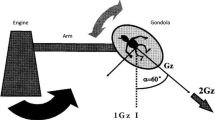
The centrifuge device consists of a rotating rod with a diameter of 4 m, weighing 400 kg (Fig. 1). The maximum bearing weight is 80 kg for a subject and 40 kg for the measurement devices. The heart of the subject is 70 cm from the center of rotation in the supine position. It has the capacity of a max 2 G-load at the heart level and is equipped with a detachable bicycle ergometer with a maximum workload of 150 W. The eyes are covered with a head-mount display (Sony Glasstron), and instructions are provided through this display.
The centrifuge. This consists of a rotating rod with a diameter of 4 m. The heart of the subject is 70 cm, from the center of rotation, in the supine position. When 1.2 G of the centrifugal force from head to feet is generated at the subject’s heart level in the supine position, the centrifugal force at the feet level is approximately 3.5 G. The subject pedals a detachable cycle ergometer in the cabin in the supine position
Measurements
In order to evaluate the basal resting autonomic circulatory control function, measurements were performed in the morning in the supine position one day before head-down bed rest, at least 2 h after a light breakfast and more than 12 h after the last caffeinated beverage or alcohol, in a quiet, environmentally controlled laboratory with an ambient temperature of 25–26°C and the humidity of 30–40%. The post head-down bed rest measurements were also performed in the supine position just before standing after head-down bed rest, in the same laboratory. Following the establishment of a quiet, resting, hemodynamic steady state (at least 30 min of supine rest), arterial blood pressure waveform and ECG were recorded during 6 min of spontaneous respiration on a digital data recorder (PC204, Sony Precision Technology, Tokyo, Japan) for heart rate variability spectral analysis and baroreflex function analysis. An analog ECG was obtained from a CM5 lead, and beat-by-beat arterial blood pressure was obtained at the finger by photoplethysmography using the Penaz principle (Portapres, Otsuka Electronics). Intermittent blood pressure was measured in the arm by electrosphygmomanometry. Respiratory rate and end tidal CO2 concentration were monitored continuously via a nasal cannula by using a capnometer (NPB-75, Tyco Healthcare, Japan). After this data collection period, hematocrit was measured in the supine position. The magnitude of reduction of plasma volume after bed rest was calculated from the changes in hematocrit using the van Beaumont formula (Van Beaumont 1972). Finally, 60° headup tilt (HUT) test for 15 min was performed on a tilting bed with a foot support, just before ambulation.
Spectral and transfer function analysis
The analog ECG and arterial pressure were analyzed as previously reported (Iwasaki et al. 2000). High frequency power of R–R interval and SBP in the range of 0.15–0.5 Hz and low frequency power in the range of 0.04–0.15 Hz were calculated from the integration of the autospectra. This data acquisition and processing strategy conforms to recommendations of international consensus panels for the assessment of cardiovascular variability (Task Force 1996). The transfer function gain, phase, and coherence between SBP and R–R interval were estimated using the crossspectral method (Iwasaki et al. 2000). These were estimated as mean values in the high frequency range (0.15–0.35 Hz) and in the low frequency range (0.04–0.15 Hz). These frequency ranges were chosen because of the high coherence function. The transfer function gain between rhythmic changes in the SBP and R–R interval was used to reflect spontaneous arterial-cardiac baroreflex function.
Sequence analysis
Time-dependent changes in the baroreflex function was determined from spontaneous changes in systolic blood pressure and R–R interval using a sequence method established by Bertinieri et al. (1988). Spontaneously occurring ramps of at least three consecutive heart beats in which SBP and the following R–R interval either decreased or increased, were first detected. The threshold for an SBP and R–R interval change was set at 1 mmHg and 4 ms, respectively. When the relationship of the ramp between SBP and R–R interval was considerably linear (correlation coefficient >0.85), the slope of the linear regression line was taken as an estimate of the baroreflex gain. The spontaneous baroreflex gain values were calculated as the mean value of these slopes for 6 min.
Statistical analysis
A two-factor repeated-measures analysis of variance (ANOVA) was performed with time (pre and post) and interventions (head-down bed rest with and without countermeasure) as factors. When significance occurred, Student’s paired t test was used between pre and post in each group. A comparison between groups was made using a nonparametric Mann–Whitney test in pre-data and post-data respectively. A P value <0.05 was considered statistically significant. The analysis was performed using a PC-based software (ABstat, Anderson Bell). Data are presented as mean ± SE.
Results
Hemodynamic variables
Respiratory and hemodynamic data are listed in Table 1. Respiratory rate and end tidal CO2 concentration did not change after head-down bed rest in either of the groups. No significant changes in SBP and DBP were observed in either of the groups. There was a significant difference in heart rate between pre and post HDBR (a two-factor repeated ANOVA, with time as a factor). Therefore, student’s paired t test was used between pre and post in each group. Increase in heart rate was significant (R–R interval decreased significantly) in the control group. However, it was not significant in the CM group.
Spectral analysis
Cardiovascular variability data are listed in Table 2. There were significant differences in power in the high frequency of R–R variability (HFRR), and the ratio of low to high frequency power (LF/HF), between pre and post head-down bed rest (a two-factor repeated ANOVA, with time as a factor). In the control group, decrease in HFRR and increase in LF/HF were significant by post hoc tests. However, these were not significant in the CM group. Moreover, LF/HF in the CM group was significantly lower than that in the control group after head-down bed rest (a two-factor repeated ANOVA, with interventions as a factor). In both groups, power in the low frequency of SBP variability tended to decrease, although these were not statistically significant.
Baroreflex function by transfer function analysis
Transfer function data are listed in Table 3. Coherence was above 0.5 under all conditions. A negative phase between the systolic blood pressure and R–R interval was observed in all cases and the phase did not change after bed rest.
There was a significant difference with high frequency transfer function gain between pre and post head-down bed rest (a two-factor repeated ANOVA, with time as a factor). High frequency transfer function gain decreased significantly in the control group. However, this index showed no significant changes after head-down bed rest in the CM group. Similar tendencies were observed in low frequency transfer function gain, although these changes were not statistically significant (Table 3).
Baroreflex gain by sequence analysis
There was a significant difference with gain by sequence analysis between pre and post head-down bed rest (a two-factor repeated ANOVA, with time as a factor). Gain by sequence analysis decreased significantly in the control group (18.4±3.6 to 11.5±2.0 m/mmHg). However, this index showed no significant changes after head-down bed rest in the CM group (19.9±4.4 to 15.0±3.6 m/mmHg) (Fig. 2).
Mean changes in gain by sequence method as an index of spontaneous arterial-cardiac baroreflex function after head-down-tilt bed rest. The “control group”, HDBR without a countermeasure. The “CM-group”, HDBR with hypergravity exercise. Significant difference between pre and post bed rest are noted. *P<0.05
Plasma volume reduction
The Magnitude of plasma volume reduction after bed rest calculated from the changes in hematocrit is significantly larger in the control group (−16.2±4.8%) than in the CM group (−6.8±8.1%).
HUT test
All subjects of both the control and hypergravity exercise groups completed the HUT test without any signs or symptoms of orthostatic hypotension before bed rest. After bed rest, two of the six control subjects experienced orthostatic hypotension (SBP <80 mmHg), with symptoms of presyncope that terminated HUT testing. Also, two of the six subjects in CM-group experienced orthostatic hypotension after bed rest.
Discussion
The present study aimed to evaluate the effects of an intermittent exposure to hypergravity with ergometric exercise as a countermeasure against changes in dynamic regulation of heart rate after bed rest. Therefore, we quantified autonomic cardiovascular control before and after a 14-day period of HDBR with and without the countermeasure. High frequency power of R–R interval variability as an index of cardiac parasympathetic nervous activity, the transfer function gain between blood pressure and R–R interval in the high frequency range as an index of vagally mediated spontaneous arterial-cardiac baroreflex function, and baroreflex gain by sequence analysis decreased significantly after HDBR in the control group. However, these changes in heart rate variability and baroreflex control of heart rate were not statistically significant in the CM group. Moreover, plasma volume reduction in the CM group is significantly smaller compared with that of the control group. These findings indicate that the intermittent exposure to hypergravity, when exercise is performed simultaneously, attenuates reductions in the cardiac parasympathetic activity, the vagally mediated spontaneous arterial-cardiac baroreflex function, and plasma volume during 14-day simulated weightlessness.
Heart rate variability spectral analysis
Spectral analysis of R–R interval variability quantifies the dynamic, frequency dependent changes in heart period, which reflect autonomic modulation of sinus node activity (Iwasaki et al. 2000). It is widely accepted that high frequency power of R–R interval is mediated predominantly by changes in vagal activity, while low frequency power is determined by changes in both sympathetic and vagal activity. In the present study, high frequency power of R–R interval variability decreased significantly after HDBR in the control group. Hence, cardiac parasympathetic activity may be reduced during 14-day simulated weightlessness. These results are consistent with previous reports (Cooke et al. 2000; Custaud et al. 2002; Hughson et al. 1994; Iwasaki et al. 2000, 2001; Pavy-LeTraon et al. 1997; Sigaudo et al. 1998). Associated with that, the ratio of low to high frequency power increased after bed rest in the control group, suggesting relative sympathetic dominance of the heart. However, these indexes showed no significant changes after head-down bed rest in the CM group. These results may suggest that an artificial hypergravity with physical exercise might attenuate the changes in autonomic modulation of cardiac period.
Blood pressure variability
Arterial pressure Mayer waves, occurring at about 0.1 Hz, are presumed to be related to sympathetic vasomotor activity. Therefore, the low frequency (0.04–0.15 Hz) power of BP variability may reflect the magnitude of sympathetic vasomotor variability (Pagani et al. 1997). However, although heart rate and the ratio of low to high frequency power increased after bed rest in the control group, there was no increase in vasomotor activity, as indicated by decreases rather than increases in the low frequency power of BP variability in the present study. The contradictory results in heart rate and blood pressure variability are consistent with a previous bed rest study (Iwasaki et al. 2000). This discrepancy between the relative sympathetic dominance of the heart and the tendency toward decreases in vasomotor activity may be explained by two possible mechanisms. First, vascular remodeling during bed rest may occur through adaptation of the endothelium and/or smooth muscle. Thus, the responsiveness of peripheral vessels to sympathetic nervous modulation may decrease, and mask the increases in BP variability. Second, Sympathetic modulation to peripheral vessels and heart adapt differently during head down bed rest. The protocol for artificial hypergravity exercise application in the present study did not change the bed rest induced tendency of decreases in BP variability.
Spontaneous arterial-cardiac baroreflex
Transfer function analysis of spontaneous variations between arterial pressure and R–R interval has been employed for the evaluation of dynamic properties of spontaneous arterial-cardiac baroreflex (Iwasaki et al. 2000). This analysis emphasizes the frequency dependence of baroreflex control of cardiac periods, allowing the assessment without the injection of vasoactive drugs, or artificial mechanical stimulation of the receptor. Transfer function gain between blood pressure and R–R interval in high frequency is thought to be an index of the vagally mediated arterial-cardiac baroreflex function. In the present study, transfer function gain between blood pressure and R–R interval in the high frequency range decreased significantly after HDBR in the control group. Hence, associated with decreased cardiac parasympathetic activity, the vagally mediated spontaneous arterial-cardiac baroreflex function may be reduced during 14-day simulated weightlessness. These results are also remarkably consistent with previous reports (Cooke et al. 2000; Custaud et al. 2002; Hughson et al. 1994; Iwasaki et al. 2000, 2001; Pavy-LeTraon et al. 1997; Sigaudo et al. 1998). However, there are some possible limitations and concerns of this method (Iwasaki et al. 2000, 2001). To minimize these concerns, we estimated spontaneous baroreflex function by not only transfer function gain, but also sequence analysis (Bertinieri et al. 1988). The results in spontaneous baroreflex function were virtually identical between the gain in high frequency range by transfer function analysis and the gain by sequence analysis. In the present study, an intermittent +Gz acceleration with mild ergometric exercise on short radius centrifuge, attenuated changes in these cardiovascular physiological parameters after 14-day simulated microgravity. The present results confirm and extend findings from our previous 4-day bed-rest study on artificial hypergravity without physical exercise (Iwasaki et al. 2001). The present results suggest that an artificial hypergravity with physical exercise via an onboard centrifuge attenuates, at least, the changes in the vagally mediated spontaneous arterial-cardiac baroreflex function in space.
Plasma volume change
In a previous study (Iwasaki et al. 2000), we demonstrated that both chronic (2 weeks) HDBR and acutely induced hypovolemia led to similar impairments in cardiac parasympathetic activity and spontaneous arterial-cardiac baroreflex function. We suggested that reductions in plasma volume, rather than a unique cardiac autonomic nervous adaptation to bed rest, are largely responsible for the observed changes in autonomic arterial baroreflex regulation of the cardiac period after bed rest. Therefore, we speculated that artificial hypergravity with ergometric exercise reduced the plasma volume reduction first, and then, attenuated the decreases in cardiac parasympathetic activity and the spontaneous arterial-cardiac baroreflex. The effect of the attenuation in the plasma volume reductions may be the key mechanism to reverse the changes in autonomic reflex control of the heart. In fact, the magnitude of plasma volume reduction is significantly larger in the control group than in the CM group in the present study, suggesting that hypergravity with ergometric exercise partially prevented a plasma volume reduction after bed rest.
Perspectives
Endurance exercise training has the effect of increasing heart rate variability and spontaneous arterial-cardiac baroreflex function (Iwasaki et al. 2003). This effect of physical exercise might also be a key mechanism of reversing the changes in dynamic regulation of heart rate after bed rest.
The concomitant use of physical exercise and +Gz loads has more advantages for the cardiovascular system. Antigravity effects of leg exercise (Greenleaf et al. 1999) allow for longer and more intensive +Gz acceleration, although the effect of simultaneous exercise with centrifuge on G load tolerance would be complicated (Iwase et al. 2002). The concomitant use combines the stresses of physical exercise and hypergravity, providing greater cardiovascular stress (e.g., greater share stress and/or greater variability of venous return) than single load (Convertino et al. 1982). Moreover, it is suggested that a combination of +Gz acceleration and physical exercise may be useful not only for changes in cardiovascular regulatory systems but also against muscle atrophy, decreases in aerobic power, and/or bone demineralization. In fact, our preliminary data from this bed-rest study showed that this countermeasure attenuated the increase of deoxypyridinoline as a bone resorption marker from 72% in the control group to 22.9% in the CM group (unpublished observations ).
Limitations
In the present study, a 60° HUT test for 15 min was performed. After bed rest, two of the six subjects in both groups experienced orthostatic hypotension. Because of the small number of subjects and a discrete variable with a tilt test, differences between two groups on orthostatic tolerance might not be detected by the tilt test. Evaluating autonomic circulatory control at the upright position gives us the information about responsiveness of the autonomic nervous system and may reveal more detailed effects of the countermeasure. However, spectral analysis of heart rate variability by the standard methods (FFT in the present study) cannot be applied for progressively changing heart rate and BP data during the HUT test. Nonstandard analysis for the investigation of nonstationary/unstable oscillations, such as complex demodulation, may be useful for further analyses.
Furthermore, if the more stable upright position (e.g. seated position) had been used as reference instead of the supine in the present study, the effects of hypergravity exercise might have been much more pronounced. Therefore, in future studies, subjects should also be investigated in the seated position also.
The protocol for artificial hypergravity with ergometric exercise in the present study did not fully reverse cardiovascular changes. The HUT tests could not reveal the difference on the orthostatic tolerance between two groups. Further studies will be necessary with more insightful approaches, for better and appropriate protocol.
Conclusions
Although a study in the more stable upright position might reveal the more pronounced effects of hypergravity exercise, the present study showed that the intermittent exposure to +1.2 Gz acceleration at heart level with mild ergometric exercise for 30 min attenuated the decreases in parasympathetic activity and the spontaneous arterial-cardiac baroreflex function in the supine position and a reduction in plasma volume during a 14-day simulated weightlessness. The present result suggests that artificial hypergravity with physical exercise via an onboard short radius centrifuge is useful to reduce the changes in dynamic regulation of heart rate and plasma volume in space.
References
Alfrey CP, Udden MM, Leach-Huntoon C et al (1996) Control of red blood cell mass in spaceflight. J Appl Physiol 81:98–104
Bertinieri G, Di Rienzo M, Cavallazzi A et al (1988) Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol 254:H377–H383
Buckey JC, Lane LD, Levine BD et al (1996) Orthostatic intolerance after spaceflight. J Appl Physiol 81:7–18
Convertino VA (1998) High sustained +Gz acceleration: physiological adaptation to high-G tolerance. J Gravitat Physiol 5:53–56
Convertino VA (2001) Mechanisms of blood pressure regulation that differ in men repeatedly exposed to high-G acceleration. Am J Physiol Regul Integr Comp Physiol 280: R947–R958
Convertino VA, Goldwater DJ, Sandler H (1982) Effect of orthostatic stress on exercise performance after bedrest. Aviat Space Environ Med 53:652–657
Cooke WH, Ames JE IV, Crossman AA et al (2000) Nine months in space: effects on human autonomic cardiovascular regulation. J Appl Physiol 89:1039–45
Custaud MA, de Souza Neto EP, Abry P et al (2002) Orthostatic tolerance and spontaneous baroreflex sensitivity in men versus women after 7 days of head-down bed rest. Auton Neurosci 30:66–76
Greenleaf JE, Chou JL, Stad NJ et al (1999) Short-arm (1.9 m) +2.2 Gz acceleration: isotonic exercise load-O2 uptake relationship. Aviat Space Environ Med 70:1173–1182
Hughson RL, Maillet A, Gharib C et al (1994) Reduced spontaneous baroreflex response slope during lower body negative pressure after 28 days of head-down bed rest. J Appl Physiol 77:69–77
Iwasaki K, Hirayanagi K, Sasaki T et al (1998) Effects of repeated long duration +2 Gz load on human’s cardiovascular function. Acta Astronaut 42:175–183
Iwasaki K, Zhang R, Zuckerman JH et al (2000) Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. Am J Physiol Regul Integr Comp Physiol 279:R2189–R2199
Iwasaki K, Sasaki T, Hirayanagi K, Yajima K (2001) Usefulness of daily +2 Gz load as a countermeasure against physiological problems during weightlessness. Acta Astronaut 49:227–35
Iwasaki K. Zhang R, Zuckerman JH, Levine BD (2003) Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: How much training for what benefit? J Appl Physiol 95:1575–1583
Iwase S, Fu Q, Narita K, Morimoto E, Takada H, Mano T (2002) Effects of graded load of artificial gravity on cardiovascular functions in humans. Environ Med 46(1–2):29–32
Korolkov VI, Kozlovskaya IB, Kotovskaya AR et al (2001) Efficacy of periodic centrifugation of primates during 4-week head-down tilt. Acta Astronaut 49:237–242
Levine BD, Zuckerman JH, Pawelczyk JA (1997) Cardiac atrophy after bed-rest deconditioning. A nonneural mechanism for orthostatic intolerance. Circulation 96:517–525
Pagani M, Montano N, Porta A et al (1997) Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95:1441–1448
Pavy-LeTraon A, Sigaudo D, Vasseur P et al (1997) Orthostatic tests after a 4-day confinement or simulated weightlessness. Clin Physiol 17:41–55
Sigaudo D, Fortrat JO, Allevard AM, et al. (1998) Changes in the sympathetic nervous system induced by 42 days of head-down bed rest. Am J Physiol 274:H1875–H1884
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065
Van Beaumont W (1972) Evaluation of hemoconcentration from hematocrit measurements. J Appl Physiol 32:712–713
Vil-Viliams IF (1994) Principle approaches to selection of the short-arm centrifuge regimens for extended space flight. Acta Astronaut 33:221–229
Acknowledgements
We would like to thank Dr. Makoto Igarashi for his critical review of the manuscript. This study is carried out as a part of “Ground-based Research Announcement for Space Utilization” promoted by Japan Space Forum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwasaki, Ki., Shiozawa, T., Kamiya, A. et al. Hypergravity exercise against bed rest induced changes in cardiac autonomic control. Eur J Appl Physiol 94, 285–291 (2005). https://doi.org/10.1007/s00421-004-1308-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1308-x