Abstract
Hyperventilation prior to breath-hold diving increases the risk of syncope as a result of hypoxia. Recently, a number of cases of near-drownings in which the swimmers did not hyperventilate before breath-hold diving have come to our attention. These individuals had engaged in prolonged exercise prior to breath-hold diving and it is known that such exercise enhances lipid metabolism relative to carbohydrate metabolism, resulting in a lower production of CO2 per amount of O2 consumed. Therefore, our hypothesis was that an exercise-induced increase in lipid metabolism and the associated reduction in the amount of CO2 produced would cause the urge to breathe to develop at a lower P O2, thereby increasing the risk of syncope due to hypoxia. Eight experienced breath-hold divers performed 5 or 6 breath-holds at rest in the supine position and then 5 or 6 additional breath-holds during intermittent light ergometer exercise with simultaneous apnoea (dynamic apnoea, DA) on two different days: control (C) and post prolonged sub-maximal exercise (PPE), when the breath-holds were performed 30 min after 2 h of sub-maximal exercise. After C and before the prolonged submaximal exercise subjects were put on a carbohydrate-free diet for 18 h to start the depletion of glycogen. The respiratory exchange ratio ( RER) and end-tidal P CO2, P O2, and SaO2 values were determined and the data were presented as means (SD). The RER prior to breath-holding under control conditions was 0.83 (0.09), whereas the corresponding value after exercise was 0.70 (0.05) ( P <0.01). When the three apnoeas of the longest duration for each subject were analysed, the average duration of the dynamic apnoeas was 96 (14) s under control conditions and 96 (17) s following exercise. Both P O2 and P CO2 were higher during the control dynamic apnoeas than after PPE [PO2 6.9 (1.0) kPa vs 6.2 (1.2) kPa, P <0.01; P CO2 7.8 (0.5) kPa vs 6.7 (0.4) kPa, P <0.001; ANOVA testing]. A similar pattern was observed after breath-holding under resting conditions, i.e., a lower end-tidal P O2 and P CO2 after exercise (PPE) compared to control conditions. Our findings demonstrate that under the conditions of a relatively low RER following prolonged exercise, breath-holding is terminated at a lower P O2 and a lower P CO2 than under normal conditions. This suggests that elevated lipid metabolism may constitute a risk factor in connection with breath-holding during swimming and diving.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a study by Craig (Craig 1961) elucidating the mechanisms of syncope during underwater swimming, the risk of hyperventilation prior to breath-hold swimming was highlighted. Hyperventilation reduces the arterial CO2 without increasing the O2 content in the blood to the same extent. The initially reduced CO2 level makes it easier to prolong the breath-hold period. This allows the arterial O2 pressure to drop to dangerously low levels. Craig (Craig 1961) showed that prolonged breath-hold times after hyperventilation were associated with lower arterial O2 levels; levels that were low enough to cause hypoxic syncope. This was especially evident if the breath-holding was carried out during exercise such as underwater swimming.
Hyperventilation prior to breath-hold diving is now strongly discouraged by most swimming schools and diving clubs, and warnings about this are often displayed in the vicinity of public swimming pools. Nonetheless, accidents do still occur in connection with underwater breath-hold swimming. Although the typical victim of such an accident is a young male trying to compete with his peers, accidents involving more experienced underwater swimmers also occur. These accidents are often attributed to hyperventilation (Craig 1976), although it is usually difficult to establish whether the victim actually did hyperventilate.
In connection with recent studies involving a group of experienced breath-hold divers (Lindholm 2002; Lindholm and Linnarsson 2002), a number of near-drowning incidents during underwater swimming have come to our notice. A similarity between these incidents is that the swimmers deny any deep hyperventilation prior to the dives, but all the breath-hold swims involved physical work and were carried out after extended periods of physical exercise, such as long distance swimming or underwater rugby games.
It is well known that lengthy periods of physical work deplete the carbohydrate stores (glycogen) in the body, while increasing the rate of lipid metabolism. As a consequence the respiratory quotient is reduced, and the ratio of the amount of CO2 produced to the amount of O2 consumed is lowered. It is also well established that exercise cause an excess post exercise O2 consumption (EPOC), due to among other factors lactic acid removal, and increase in muscle temperature (Borsheim and Bahr 2003).
Thus, it was hypothesised that breath-holding following a bout of exercise long enough to result in increased lipid metabolism is associated with an increased rate of O2 consumption and a concomitant reduction in the relative rate of CO2 production.
Methods
Subjects
Eight healthy male volunteers, all experienced in breath-hold diving, between 20 and 37 years old, weighing 68–87 kg, and with a height of 179–188 cm were recruited for this study. Four of these subjects had had previous incidents of syncope in connection with breath-hold diving. The experimental procedure was conducted in conformity with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Karolinska Institutet. All subjects gave their written informed consent prior to participation.
Experimental procedures
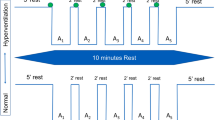
Each subject was tested on two consecutive days (see the timeline in Table 1 for overview).
Day 1: Control
The subjects arrived at the laboratory on the morning of the first day. After being informed about the procedures to be carried out, the vital capacity of each subject was determined. They were then allowed to rest for 30 min before sitting on the ergometer bicycle for 10 min in order to obtain a measurement of their respiratory exchange ratio ( RER).
Thereafter, the subjects performed 5 or 6 static apnoeas (SA) while resting supine on a couch, with a 3-min rest between each two consecutive apnoeas. Following the last SA, the subjects rested for 15 min before beginning the dynamic apnoeas (DA), which involved intermittent exercise on the ergometer bicycle at a workload of 50 W. Starting and stopping at the same time as the exercise, 5 or 6 DAs were performed with 2–3 min of rest between each two consecutive apnoeas. Following these DAs, the RER was measured again with the subject sitting at rest on the ergometer cycle. In all cases, only the 3 longest apnoeas were used for analysis.
On this first day, the lunch break was followed by 2 h of rest, after which the maximal O2 uptake was determined by an incremental cycle ergometer test, followed by measurement of capillary lactate levels. Thereafter, the subjects were placed on a carbohydrate-free diet, i.e., allowed to eat only meat (chicken) or eggs and drink only water for dinner and for breakfast the following day with the exception of a cup of coffee in the morning.
Day 2: Similar measurements following prolonged exercise
Day 2 commenced with a 10 min measurement of the RER, followed by 2 h of sub-maximal exercise, during which the workload was adjusted continuously in order to maintain a heart rate of 115–130 beats/minute. The total amount of this sub-maximal work was not determined. Three minutes after termination of this exercise, capillary levels of lactate were assayed. Subsequently, approximately 30 min after this prolonged exercise, the procedures involving the apnoeas and the determinations of RER as performed on the first morning were repeated.
Measurement procedures
Upright dynamic leg exercise was performed on an electrically braked cycle ergometer (Type 380 B, Siemens-Elema, Stockholm, Sweden). An electrocardiogram used for supervision of the subjects during the experiment was acquired from three chest electrodes and a combined amplifier and beat-by-beat tachometer (SMK 154–9 Hellige Servomed, Germany).
Ear-lobe arterial O2 saturation (SaO2) was measured with a beat-by-beat pulse oximeter (Satlite Trans, Datex Engstrom, Finland). The subject’s earlobe was rubbed with an ointment containing capsaicin in order to enhance local blood flow. In a comparison with invasive measurements, and using a technique which was identical to that used in the present study, Benoit et al. (1997) found an agreement within 2% in the range of 57–100% SaO2 (Benoit et al.1997). The manufacturer does not guarantee the accuracy of the instrument below a saturation level of 50%.
For the determination of the RER and V̇O2max, the subjects breathed through a low-resistance, non-return valve (Hans Rudolf, Mo.), with the inspiratory volume per minute being measured with a turbine ventilation module (KL Engineering, Northridge, Calif.). The expired air was collected via a hose in an 8-l Plexiglas mixing box, from which samples were drawn continuously for analyses of O2 (Applied Electrochemistry S-3A/I O2 analyser, Pittsburg, Pa.) and CO2 (Beckman model LB-2, CO2 analyser, Fullerton, Calif.). Subjects breathed into the mixing box for a few minutes until the readings of O2 and CO2 stabilised. Thereafter data was collected for 12–13 min. Each minute was analysed separately and then the data was averaged over the last 10 min period. During the breath-holding procedures, the same low-resistance valve was used, but the respiratory hosing was disconnected and a bag designed to supply a predetermined volume of air was connected to the inspiratory port of the breathing valve. A thin capillary tube connected the mouth-piece of the valve to a Datex Normocap 200 apparatus (Dansjo medical, Sundbyberg, Sweden) in order to allow measurement of the end-tidal P CO2 (with an IR sensor) and of the P O2 (using a paramagnetic sensor). All apnoeas were performed at 80% of the subject’s vital capacity. Subjects were asked to exhale to residual volume and then allowed to inhale the predetermined volume of air in the bag. Thereafter, they were ordered to hold their breath for as long as possible, without being told how long this period was for any of the trials. The subjects were not permitted to hyperventilate prior to these apnoeas, but they always took 3 deep breaths before exhaling to residual volume. End-expiratory P CO2 and P O2 were measured for the last breath prior to breath-holding in order to be sure that no extended hyperventilation had occurred.
The subjects had continuous access to water for drinking throughout the tests. In order to prevent hypoxic syncope, the apnoeas were interrupted by the medical supervisor when the SaO2 fell below 50%. In one subject, one episode of breath-holding during DA under control conditions and two static apnoeas during PPE were terminated for this reason. Similarly, one control DA and two PPE DA were stopped in another subject.
Lactate concentrations in capillary blood were measured with an Accusport apparatus (Boehringer Mannheim). All measurements were recorded at 200 Hz per channel in a computer-based system and analysed employing the AcqKnowledge 3.7.3 software (Biopac Systems, Goleta, Calif.).
Statistical analyses
In order to test for statistically significant differences in end-tidal P CO2 and P O2 values pre- and post-apnoea under control and PPE conditions a 3×2 factor ANOVA test with repeated measures on both factors (http://faculty.vassar.edu/lowry/anova202corr.html) was used. Differences in V̇CO2, V̇O2 and RER in connection with respiratory exchange determinations were tested for statistical significance using repeated measures ANOVA (StatView 5.0, SAS Instiute, Cary, N.C.). Bonferroni corrections were applied for the post hoc tests. The data were presented as means (SD) and all differences with a P value of <0.05 were considered significant.
Results
The average V̇O2max value for all 8 subjects during exercise on the bicycle ergometer with incrementally increasing load was 4.8 (0.8) l/minSTPD. Capillary levels of lactate following the V̇O2max tests were 14 (3.5) mmol/l and 2.7 (0.73) mmol/l ( n =7) after the prolonged exercise; none of the latter values was >4 mmol/l.
Determination of the RER revealed that the subjects metabolised more lipid and less carbohydrate following their restricted diet and the 2-h exercise session. Thus, the RER prior to PPE breath-holding was 0.70 (0.05), compared to a control value of 0.83 (0.09) ( P <0.01, Table 2).
The SaO2 determinations have not been included in the tabulated statistical analysis since the lowest values exhibited by several of the subjects reached below 50%, where the measurements were unreliable. Despite the fact that the apnoeas were not allowed to continue once the SaO2 had declined to 50%, the nadir O2 saturation often declined below this figure post-apnoea. However, during the DAs the lowest SaO2 averaged 70 (10)% under control conditions and 60 (13)% PPE ( n =7, P <0.01 according to the paired t -test, see also Fig. 1). The corresponding nadir of SaO2 during the SAs averaged 72 (9)% under control conditions and 61 (17)% during PPE ( n =8, P <0.05, paired t -test).
Recording from an individual subject during apnoea in connection with simultaneous exercise at a load of 50 W. The black line represents the control (apnoea duration 124 s) and the grey line represents the corresponding values for a test following 2 h of aerobic exercise (apnoea duration 120 s). This figure illustrates that O2 was depleted more rapidly and to a greater extent lowest value (56% vs 67% SaO2) during apnoea following prolonged exercise
Carbon dioxide levels prior to breath-holding tended to be lower PPE than under control conditions. However, this difference was statistically significant only in connection with SA ( P <0.05, n =8, Table 3).
There were no significant differences in the average duration of either the static or the dynamic apnoeas under control conditions or following prolonged exercise (Table 3). After the 2-h aerobic exercise, the end-tidal level of CO2was reduced following both the static ( P <0.01) and the dynamic ( P <0.001) apnoeas, as was the end-tidal partial pressure of O2 (SA P <0.05 and DA P <0.01, Table 3).
Discussion
The principal finding of the present study is that following a low carbohydrate diet and prolonged physical exercise that results in a reduction in the respiratory quotient (as reflected in a decrease in the RER), the post apnoea end-tidal partial pressures of CO2 and O2 exhibited by experienced breath-hold divers are significantly lower than the corresponding values recorded under control conditions. It is important to note that our subjects were highly experienced underwater swimmers. Due to technical and temporal constraints, it was not possible to vary the order in which the subjects were exposed to the different conditions. However, this is not likely to have introduced a significant bias since the effects of further training on well-trained individuals are limited.
Exposure to repeated episodes of apnoea has been shown to involve a “warming-up” effect, with the initial apnoeas being of shorter duration than those that follow (Hentsch and Ulmer 1984). This effect has been demonstrated to coincide with a temporary increase in the number of circulating erythrocytes as a consequence of contraction of the spleen (Schagatay et al. 2001). Therefore, we had the subjects perform 5 or 6 apnoeas in each session, thereby inducing this “warming-up” effect, and the 3 apnoeas of the longest duration for each subject were chosen for analysis.
When the human body utilises lipids instead of carbohydrates as an energy source, the ratio between the amount of CO2 produced and the amount of O2 consumed decreases by almost 30%. The respiratory quotient ( RQ) for glucose is 0.995, while the corresponding value for fat is 0.71 (Greger and Bleich 1996). Since the arterial level of CO2 constitutes the primary respiratory drive, predominant lipid metabolism should theoretically reduce this respiratory drive. Indeed, this hypothesis is supported by a number of studies on healthy individuals and on patients with chronic obstructive pulmonary disease (COPD) (Jansson 1982; Angelillo et al. 1985; Sue et al. 1989), in which an increased ratio of lipid to carbohydrate metabolism was associated with a decrease in the respiratory minute ventilation, but no change in the arterial P CO2. Subsequently, lipid-rich diets have been employed clinically to reduce hypercapnia and dyspnoea in COPD patients (Kuo et al. 1993; Cai et al. 2003).
The concept that similar metabolic changes may affect the performance and safety of breath-hold divers has, to our knowledge, never been discussed previously. A metabolic state dominated by lipid turnover would lengthen the period of time required for the P CO2 to reach the break-point, a prolongation which is also observed when breath-holding is performed following a period of hyperventilation. However, in this case it is not the initial level of CO2, but rather the attenuated rate of increase in the level of this gas during the period of apnoea that causes the delay.
When performing a breath-hold after hyperventilation, the amount of extra O2 taken aboard does not compensate for the increased breath-hold duration, and this makes it possible to hold the breath for longer than it takes to reach critical hypoxemia (Craig 1961). Every year accidents are caused by this phenomenon, mainly in swimmers who are unaware of this risk (personal observations). Similarly, metabolically induced prolongation of the time required to attain the critical level of CO2 would reduce the level of arterial O2 remaining at the breath-hold break-point and thereby constitute a risk for the underwater swimmer.
From the present experiments involving comparison of breath-holds of similar durations, it was evident that the CO2 levels present at the apnoea break-point were significantly lower when the breath was held during a state of low RQ. However, the P O2 in the alveolar air was also depressed, despite the fact that the breath-holds with a low RQ were not prolonged compared to the control situation. This highlights yet another risk associated with breath-holding during a state of predominantly lipid metabolism. When carbohydrates are being metabolised, roughly 5.1 mol ATP are produced /mol O2 consumed, compared to the 4.7 mol ATP/mol O2 (about 8% lower) formed in connection with lipid metabolism (Borsheim and Bahr 2003). Thus, during the expenditure of a given amount of energy, the O2 stores will be depleted more rapidly when the RQ of the breath-hold diver is relatively low. The actual increase in consumption of O2 in association with breath-holding may not be large, depending on the rate of work and the duration of the period of apnoea, but the reduction in arterial P O2 may nonetheless be significant, in particular when the O2 levels are close to the threshold for unconsciousness.
After exercise there is a period of “excess post-exercise O2 consumption” (EPOC) (Borsheim and Bahr 2003). This could explain part of the increase in O2 consumption in our subjects under the PPE conditions after the 2-h exercise session. EPOC consists of rapid components such as replenishment of ATP and phosphocreatine stores, removal of lactate etc. The prolonged EPOC component is not as clearly elucidated, but it has been shown that part of this component is dependent on a shift in metabolism from carbohydrates to lipids (Bahr et al. 1990). Both the prolonged exercise, which results in depletion of the carbohydrate stores, and the late EPOC component will reduce the RQ, resulting in an increase in O2 consumption versus CO2 production. The increased O2 consumption that persists during apnoea is clearly shown in Fig. 1.
EPOC, together with the relatively low efficiency of lipid metabolism and the consequent increase in O2 consumption may explain the observation that in some underwater swimming accidents, loss of consciousness appears to occur after a period which is shorter than the normal endurance time of the diver (personal communications from near-drowning victims). Obviously, in some cases part of the explanation may also be provided by the fact that the swimmers began their breath-hold swims immediately after an exercise session, before their metabolic rates had regained their basal levels.
In the present experiments the periods of breath-holding were of similar duration during the control and PPE trials. However, the alveolar break point CO2 levels were lower under the PPE conditions. The respiratory stimulation due to CO2 was thus lower in the PPE trials than during the control trials, assuming that the sensitivity of the central nervous system to CO2 stimulation was not increased during PPE. Of relevance in this context are our observations that re-breathing tests did not reveal any change in CO2 sensitivity during PPE compared to the control conditions (unpublished observations). However, the alveolar O2 concentration was significantly reduced during the breath-holds PPE compared to the control breath-holds. Therefore it appears that during the PPE apnoeas the breath-hold break point was determined to a greater extent by the hypoxic drive. It is well known that the respiratory drive is dependent on the interaction between hypoxic and hypercapnic stimuli, and this has also been shown to be the case during apnoea (Hesser 1965).
To further illustrate the effect of the experimental intervention, individual results have been included in the O2-CO2 diagram (Rahn and Fenn 1955), with SaO2 isopleths calculated for humans at sea level taken from Feretti et al (Ferretti et al. 1991). Figure 2 also includes curved lines defining the regions of normal or impaired visual performance, as determined in humans breathing air at high altitude (Otis et al. 1946). These regions show the effects of hypoxia in humans, and have been included due to the lack of reference data for performance during apnoeic hypoxia. For our study the apnoeic period for each subject with the most severe end-apnoea hypoxia was plotted (four conditions for each subject) in Fig. 2. It can be clearly seen in the graph that more subjects were closer to the zone of anoxic collapse during the PPE apnoeas, as was also shown by mean values (Table 3). Comparison of Fig. 2 with published breath-hold data in the O2-CO2 diagram (Ferretti et al. 1991; Ferretti 2001) indicates that our subjects (PPE) were within the same range vis-á-vis end-tidal P O2 and P CO2 as elite divers that had hyperventilated prior to maximal diving or breath-holding. Our subjects were not permitted to hyperventilate before apnoeas, and thus this plot also illustrates that the situation of combined exercise and reduced RER prior to apnoea may create the same risk as prior hyperventilation, a known cause of loss of consciousness (Craig 1976).
The O2-CO2 diagram (Rahn and Fenn 1955) with SaO2 isopleths calculated for humans at sea level taken from Feretti et al (Ferretti et al. 1991) included. The data plotted includes the P ET,O2 and P ET,CO2 from each subject’s most hypoxic static apnoea (▲) or dynamic apnoea (■), performed after a prolonged submaximal exercise. Unfilled symbols SA (△) and DA (□) represent corresponding data from apnoeas performed during control conditions
In our opinion the risk for hypoxic syncope in connection with a state of increased lipid turnover is greatest for experienced breath-hold divers, since these persons are used to experiencing and combatting severe respiratory stimulation due to high levels of CO2. In a situation where the overall respiratory stimulus is dependent to a greater extent than usual on a hypoxic respiratory drive, there is a risk that an effort that can be performed safely under normal metabolic conditions will lead to syncope. Of course, even less well-trained breath-hold divers may run such a risk, especially when the motivation to remain underwater is high, e.g., during competitions.
In conclusion, we would like to draw attention to this risk for hypoxic syncope in connection with underwater swimming, which to our knowledge has never been previously discussed. Subjects who are primarily metabolising lipids, either due to the intake of a lipid-rich diet and/or carbohydrate depletion as a consequence of a long period of aerobic work, are at increased risk for loss of consciousness during voluntary breath-holding. Thus, breath-hold swims should not be performed following long periods of exhausting physical work, and people involved in underwater sports should take care to replenish their carbohydrate stores, for instance, during long competitions or a day of recreational spear-fishing.
References
Angelillo VA, Bedi S, Durfee D, Dahl J, Patterson AJ, O’Donohue WJ Jr (1985) Effects of low and high carbohydrate feedings in ambulatory patients with chronic obstructive pulmonary disease and chronic hypercapnia. Ann Intern Med 103:883–885
Bahr R, Hansson P, Sejersted OM (1990) Triglyceride/fatty acid cycling is increased after exercise. Metabolism 39:993–999
Benoit H, Costes F, Feasson L, Lacour JR, Roche F, Denis C, Geyssant A, Barthelemy JC (1997) Accuracy of pulse oximetry during intense exercise under severe hypoxic conditions. Eur J Appl Physiol 76:260–263
Borsheim E, Bahr R (2003) Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med 33:1037–1060
Cai B, Zhu Y, Ma Y, Xu Z, Zao Y, Wang J, Lin Y, Comer GM (2003) Effect of supplementing a high-fat, low-carbohydrate enteral formula in COPD patients. Nutrition 19:229–232
Craig AB Jr (1961) Causes of loss of consciousness during underwater swimming. J Appl Physiol 16:583–586
Craig AB Jr (1976) Summary of 58 cases of loss of consciousness during underwater swimming and diving. Med Sci Sports 8:171–175
Ferretti G (2001) Extreme human breath-hold diving. Eur J Appl Physiol 84:254–271
Ferretti G, Costa M, Ferrigno M, Grassi B, Marconi C, Lundgren CE, Cerretelli P (1991) Alveolar gas composition and exchange during deep breath-hold diving and dry breath holds in elite divers. J Appl Physiol 70:794–802
Greger R, Bleich M (1996) Normal values for physiological parameters. In: Greger R, Windhorst U (eds) Comprehensive human physiology. Springer, Berlin Heidelberg New York, pp 2427–2449
Hentsch U, Ulmer HV (1984) Trainability of underwater breath-holding time. Int J Sports Med 5:343–347
Hesser CM (1965) Breath holding under high pressure. In: Rahn H (ed) Physiology of breath-hold diving and the Ama of Japan. National Academy of Science-National Research Council, Washington DC, pp 165–181
Jansson E (1982) On the significance of the respiratory exchange ratio after different diets during exercise in man. Acta Physiol Scand 114:103–110
Kuo CD, Shiao GM, Lee JD (1993) The effects of high-fat and high-carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest 104:189–196
Lindholm P (2002) Severe hypoxemia during apnea in humans: influence of cardiovascular responses. Dissertation, Karolinska Institutet
Lindholm P, Linnarsson D (2002) Pulmonary gas exchange during apnoea in exercising men. Eur J Appl Physiol 86:487–491
Otis AB, Rahn H, Epstein MA, Fenn WO (1946) Performance as related to composition of alveolar air. Am J Physiol 146:207–221
Rahn H, Fenn WO (1955) A graphical analysis of the respiratory gas exchange. The O2-CO2 diagram. American Physiology Society, Washington DC
Schagatay E, Andersson JP, Hallen M, Palsson B (2001) Selected contribution: role of spleen emptying in prolonging apneas in humans. J Appl Physiol 90:1623–1629
Sue CY, Chung MM, Grosvenor M, Wasserman K (1989) Effect of altering the proportion of dietary fat and carbohydrate on exercise gas exchange in normal subjects. Am Rev Respir Dis 139:1430–1434
Acknowledgements
We would like to thank Daniel Öberg and Hélène Englund for their assistance in carrying out the experimental procedures. This study was supported financially by the FOI, the Swedish Defence Research Agency, (project no E4450).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindholm, P., Gennser, M. Aggravated hypoxia during breath-holds after prolonged exercise. Eur J Appl Physiol 93, 701–707 (2005). https://doi.org/10.1007/s00421-004-1242-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1242-y