Abstract
The study investigated the possible association between nocturnal trapezius muscle activity and shoulder and neck pain. Sixty female subjects participated in the study, 33 were classified as pain-afflicted on the basis of shoulder and neck pain reports for the previous 6 months. Electromyographic (EMG) activity was monitored bilaterally from the trapezius in all recordings. EMG recording of the deltoid, biceps, and hand flexors was included for 26 subjects (17 with pain) to provide a comparative basis for evaluation of the trapezius recordings. There was considerable variation in the amount of muscle activity between subjects; however, some subjects presented a continuous, low-level activity pattern throughout the presumed sleep period. Subjects classified as pain-afflicted had significantly higher activity level and longer duration of trapezius EMG activity than the pain-free subjects. The deltoid had significantly more activity than the trapezius, while the biceps and the hand flexors presented similar activity level and duration as the trapezius. Nocturnal trapezius activity was not associated with pain exacerbation the same night. We suggest nocturnal trapezius muscle activity is a pointer to physiological mechanisms that contribute to some forms of shoulder and neck pain, such as trapezius myalgia, but nocturnal muscle activity may not be casually implicated in the pain-induction process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor sleep is a characteristic of several clinical conditions with muscle pain, e.g., fibromyalgia, myofascial pain and some headache syndromes (Ashburn and Staats 1999; Buskila 2000; Moldofsky 2001). It is generally accepted that sustained muscle activity can elicit pain (e.g., Simons and Mense 1998). Attempts have therefore been made to show an association between muscle activity during sleep and clinical conditions with muscle pain or with night-time pain exacerbation in these patient groups (Fischer and Chang 1985; Hatch et al. 1991; Hyyppä and Kronholm 1995; Lavigne et al. 1991; Lobbezoo et al. 1996). The reported results are mostly negative; however, the assessment of nocturnal motor activity can be relatively crude, by electromyographic (EMG) recordings scored by visual inspection or detecting body movement by use of instrumented beds. A low level of muscle activity can thus be classified as negligible or absent, even though “residual” muscle activity is often observed.
Nocturnal muscle activity is of interest for researchers attempting to identify physiological mechanisms causing putative work-related muscle pain at low physical exertion levels (Sjøgaard et al. 2000; Westgaard 1999). Occupational groups are found with high prevalence of shoulder and neck pain, despite low biomechanical demands imposed by their work situation (Vasseljen et al. 2001; Westgaard et al. 2001). Pain development in these occupational groups is frequently associated with work stress, and with perceived tension acting as an intermediate response variable (Holte et al. 2003; Vasseljen and Westgaard 1996). As the development of trapezius myalgia occurs over time periods of months and years, the muscular response pattern over 24 h is clearly relevant in any conceptualization of mechanisms for muscle pain development that incorporates muscle activity patterns.
A recent study of service workers, recording trapezius EMG activity and subjective pain reports over 24 h, showed that shoulder and neck pain augmented during working hours and was at its lowest level late at night and in the early morning (Holte and Westgaard 2002a). Long periods of EMG activity with an unusual, low frequency modulation were observed during sleep, pointing to a different muscle activation mechanism than those operating when awake (Westgaard et al. 2002). The present study, in part based on the same material, aims to determine whether subjects with shoulder and neck pain are distinguished from pain-free subjects with respect to nocturnal trapezius activity. A second aim is to examine whether the nocturnal activity pattern of trapezius is distinguished from that of other, usually pain-free muscles. The deltoid, biceps, and hand flexor muscles were therefore included additional to the upper trapezius muscles in a subset of recordings.
Methods
Subjects
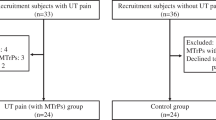
Sixty female subjects (mean age 44.6 years, SD 9.9 years), recruited from the administrative staff of the university (secretaries, n=26), two health care centers (health care personnel, n=18), and two shopping centers (retail personnel, n=16) were included. Body mass ranged from 51 to 85 kg (mean 65.7 kg), with body mass index (BMI) ranging from 19.2 to 31.6 kg m−2 (mean 23.9 kg m−2). Subjects with shoulder and neck pain potentially due to injury or systemic disease were excluded, as were pregnant women and workers with diagnosed fibromyalgia. None of the subjects had diagnosed sleep disorder. The EMG recordings, interviews, questionnaire responses, and observations indicated that the work situation entailed low biomechanical strain for these subjects; however, psychosocial stress through client or customer contact was considered a significant strain (Holte and Westgaard 2002b; Westgaard et al. 2002). A description of work duties is presented elsewhere (Holte and Westgaard 2002a).
Trapezius EMG recordings failed on both sides for five subjects, who were excluded from the data analyses. Trapezius EMG failed on only the right or the left side for further five and two subjects, respectively. Successful recording channels were retained in the material. In case of the secretaries, who were EMG recorded also from upper arm muscles, both trapezius recordings were lost for one subject. Another two subjects lost either right or left trapezius recordings. Finally, three deltoid, five biceps, and nine hand flexor recordings were lost. The cause of failure was either involuntary removal of the electrodes or instrument failure.
The subjects were classified as pain-afflicted in the shoulder and neck or pain-free, respectively, on the basis of self-reports with intensity and frequency of shoulder and neck pain during the last 6 months scored on a scale from 0 to 6 (Westgaard and Jansen 1992). Pain-afflicted subjects scored 2 or higher, representing pain reports ranging from moderate to severe. A total of 33 subjects were classified as pain-afflicted, including 17 secretaries, 9 health care personnel, and 7 shop assistants. BMI (23.6 vs 24.2 kg m−2) and age (43.8 vs 45.2 years) did not differ between the pain-afflicted and the pain-free groups.
In an earlier study that included 32 of the subjects in the present study, a clinical examination was carried out (Vasseljen et al. 2001). A slight reduction in the range of head movement for subjects with shoulder and neck pain was found. Pressure-sensitive trigger points (Simons et al. 1999) in the trapezius were detected more frequently in the pain-afflicted subjects. None of these differences were statistically significant. The shoulder and neck pain may therefore be considered a sub-clinical condition in terms of clinical findings, but was of sufficient magnitude to periodically interfere with the ability to carry out tasks of daily living. The subjects were all working at the time of recording. In view of the findings in the previous study, a formal clinical evaluation was not carried out, but a test with maximal force development in 90° arm abduction for physiological calibration purposes did not provoke shoulder or neck pain. Diagnoses such as subacromeal bursitis and rotator cuff tendonitis should thereby be excluded. All subjects gave their informed consent before inclusion. The study protocol was approved by the regional ethics committee.
Physiological recordings
Surface EMG and electrocardiograph (not reported) were recorded over 24 h, including the full workday, the subsequent leisure and sleep period, until start of work the following day. Results for the work and leisure periods are reported elsewhere (Holte and Westgaard 2002a, b). EMG activity was recorded (Physiometer PHY-400, Premed, Norway), using silver–silver chloride bipolar electrodes (Neuroline, Medicotest, Denmark) with an active diameter width of 6 mm. A center-to-center distance of 20 mm was used in all EMG recordings. The EMG activity was sampled at 1,600 Hz and digital band-pass filtered at 20–800 Hz. An artifact detection procedure sensitive to sharp transients and slow deviations from the signal baseline (400-ms periods) was carried out on the raw EMG. The EMG signals were thereafter A/D converted, the root mean square value calculated (100-ms window) and transmitted at 10 Hz on a pair of serial interfaces to two palmtop PCs carried by the subject (HP 200LX, Hewlett-Packard, USA). The processed signals were later analyzed in the laboratory by use of the Physiometer software. A time resolution of 0.2 s was used in the analysis.
EMG was recorded bilaterally from the upper trapezius in all subjects, with additional recordings from the middle deltoid, the biceps, and the hand flexors of the 26 university secretaries. The dominant arm was selected for the three latter muscles (two subjects were left-handed). The skin was rubbed lightly with alcohol before application of the EMG electrodes. For the upper trapezius the electrodes were placed at a point two-thirds of the distance from the spinous process of the seventh cervical vertebra (C7) towards the lateral edge of the acromion (Jensen et al. 1993). For the middle deltoid the first electrode was placed 15 mm above tuberositas deltoidea and the second electrode placed centrally on the muscle belly 20 mm above the first electrode. Electrodes for the biceps were placed on the lower two-thirds of the muscle belly towards the elbow joint. For the hand flexors, the electrodes were placed on the proximal end of the forearm two-thirds towards the elbow joint and 20 mm in palmar direction in parallel to a reference line drawn between the medial epicondyles on the humerus and the ulna.
The subjects performed three or more isometric maximal voluntary contractions (MVCs) until a consistent EMG response was obtained both at the start and the end of the recording session, i.e., successive EMG responses during MVC deviated by less than 20%. The contractions were separated by 1-min pauses. The MVCs were held for approximately 3 s. All calibration contractions were performed with the subjects in an erect seated posture. The maximal EMG response (EMGmax) for the upper trapezius was obtained from a posture with both arms 90° abducted in the scapular plane and resistance applied just proximal to the elbow joint. The EMGmax for the middle deltoid muscle was obtained with subjects adopting a posture with arms abducted to 45°, the elbow joint flexed to 90°, and resistance applied just proximal to the elbow joint. The EMGmax for the biceps was obtained with the lower arm held supine and the elbow joint flexed to 90°, flexing the elbow joint against resistance applied just proximal to the wrist. For the hand flexors the arm was held supine, the elbow flexed to 90°, and the wrist held in a neutral position. EMGmax was obtained by flexing the wrist against resistance applied to the palm.
The criterion for an acceptable calibration response was that the difference between the highest response obtained at the beginning and at the end of the 24-h recording period should not exceed 20% of the mean of the two responses. Two subjects exceeded this limit by 1–2% for the upper left trapezius and one subject for the deltoid and the hand flexors. We chose to retain these recordings in the material. Most subjects showed consistent calibration responses, with less that 10% difference between the two responses and no tendency of the highest response on the first or the second day of recording (Table 1). The highest EMG response, regardless of whether it was obtained at the beginning or the end of the recording period, was used to normalize the EMG signal. There was no significant difference in EMGmax between pain-afflicted and pain-free workers. Some of the EMGmax responses of the deltoid and biceps muscles were, however, quite low, presumably because the bipolar electrode straddled the insertion of the distal tendon into the muscle. The EMG results were therefore examined to check whether the responses, calibrated in percentage EMGmax, for any of the EMG variables correlated with the EMGmax response, which were not the case.
Determination of sleep period
The study was based on ambulatory monitoring of subjects through 24 h, with an interest also in the daytime recording of muscle activity and subjective responses. The sleep period was in the subjects’ own homes, without access to EEG equipment. The sleep period was therefore not quantified by traditional methods. Instead, we used a sudden change in the EMG activity pattern from irregular phasic activity when awake to no activity or EMG activity with a stable mean amplitude and minimal temporal variation (except for a low-frequency oscillatory modulation; Westgaard et al. 2002) as a proxy indicator of sleep to determine the onset and the end of a sleep period. The sharp transition of muscle activity pattern at a time when sleep onset was expected provided a well-defined timing indicator. A distinct, rhythmic EMG activity pattern during sleep is previously described in sleep studies where also EEG recordings were performed (e.g., Arduini et al. 1963; Glenn and Dement 1981; Marchiafava and Pompeiano 1964).
When removing the equipment the following day, the subjects were questioned regarding the previous night’s sleep. Fifteen reported being awake once during the night, three had been awake twice and one had been awake three times. An independent inspection of the nocturnal recordings identified 14 subjects with sustained phasic activity of more than 2 min duration that interrupted the tonic activity pattern, as exemplified in Fig. 1A and B. The longest period of sustained phasic EMG activity lasted 18 min (3.5% of the estimated sleep period). Eleven of the 14 subjects showing periods of phasic activity were among the 19 subjects reporting being awake during the night; the periods of phasic activity may thus represent periods of being awake. The effect of including or eliminating these periods when quantifying the EMG responses was examined and only negligible effects on the results were found. The periods of phasic activity of more than 2 min duration were excluded in the subjective assessment of the duration of EMG activity during sleep (cf. next section), but were included in the automated assessments of EMG responses (i.e., mean EMG activity level and duration of EMG activity periods above a threshold level).
An example of sustained EMG activity pattern from upper right (A) and left (B) trapezius (Trap.) during sleep, interrupted by phasic activity. Each data point represents root mean square (RMS)-detected EMG activity in 0.2-s periods. The phasic activity is interpreted to indicate a period with the subject awake. C A 30-min section of an EMG recording from the upper left trapezius during sleep. The figure illustrates the various sources of interference with the EMG activity pattern: non-physiological noise (gray shading below zero level, subtracted from recordings before analysis), EMG band at zero activity and the influence of heart beat potentials. The detection level for muscle activity, using the 1.5-μV-threshold criterion, is indicated. EMG max maximal EMG response
EMG analysis
Variables used to quantify muscle activity during sleep were: (1) the mean EMG response, calculated from the start to the end of the sleep period, (2) the highest EMG response of at least 10 min duration, and (3) the duration of periods with EMG activity above a threshold level. Prior to the calculation of these variables, instrument noise was determined and subtracted from each recording. The level of instrument noise was defined as the offset of the lower edge of the EMG band for the lowest stable response during the 24-h recording (Fig. 1C). Instrument noise was equally present for the different muscles (mean 0.7–1.0 μV, SD 0.2 μV; Table 1). Instrument noise was not correlated with the EMG response in MVC or with any other EMG variable. Thus, it would not influence correlation values in the analyses. We chose nevertheless to remove this bias in order to report correct muscle activity amplitudes and facilitate the assessment of muscle activity periods (cf. criterion for determining muscle activity periods by visual inspection).
After subtraction of the instrument noise, the width of the EMG band was determined by visual inspection for each recording (mean ~1 μV, SD ~0.3 μV; Table 1). The upper border of the EMG band, as a maximum value, was in most cases easily determined by this method (cf. insets B and C in Fig. 2). The width correlated positively with EMGmax (r=0.4–0.8) and negatively with BMI (r=−0.4 to −0.6), indicating a biological influence on this variable. This may be due to the electrical conductivity of the subcutaneous tissue, or any other tissue property that influences signal conduction to the skin surface. Two criteria for detection of EMG activity were used. First, a set threshold level of 1.5 µV, corresponding approximately to the mean width of the EMG band plus 1 SD (Fig. 1C). In case of the left upper trapezius the influence of the heartbeat was clearly visible as a secondary band in 23 of 53 successful recordings (range 1.3–4.4 μV after correcting for instrument noise). For these recordings, assuming a heartbeat of 60 min−1 and an EMG time resolution of 0.2 s, four of five EMG values will register below threshold, as a first approximation. A correction factor of 1.24 to 1.27, determined by the recorded heart rate, was applied to the time periods of no EMG activity. The adjusted periods of no EMG activity were thereafter subtracted from the overall estimated duration of the sleep period to calculate the time with EMG activity during sleep.
A Time course of the RMS value of the EMG signal recorded from the right upper trapezius muscle during sleep to illustrate a typical activity pattern during sleep. The arrows on the top of the plot, marked start and stop, indicate the estimated start and end of the sleep period. Several intervals of almost constant amplitude of the EMG signal, corresponding to RMS values between 1 and 3% EMGmax, are observed. Transient activity spikes mark instances of presumed body movement, often initiating an abrupt change in the constant EMG amplitude. B, C Details of the recording at higher resolution. Thresholds to detect periods with EMG activity are indicated. The dashed line at 0.28% EMGmax corresponds to EMG amplitude of 1.5 µV, which is the first threshold criterion. The second criterion uses visual inspection to detect periods of activity where the EMG band is considered not to be elevated above resting level, identified by horizontal bars underneath the recording. D Time plot from the period with highest EMG activity (EMGhigh in Tables) on an expanded time scale to illustrate low-frequency oscillatory activity pattern. This phenomenon is described in detail in a previous publication (Westgaard et al. 2002)
The second criterion to determine the duration of periods with EMG activity relied on visual inspection of the EMG recordings. This is illustrated in the insets of Fig. 2B and C, with horizontal bars indicating periods considered to be periods with no EMG activity. This method detects EMG activity at higher sensitivity and thereby registers longer periods of EMG activity than using the 1.5-µV-threshold criterion.
Subjective data
The subjects scored their level of shoulder and neck pain, tension, perceived stress from the environment, and physical and mental fatigue every hour on visual analog scale with end points “very low” and “very high” during work and leisure. This included scoring late at night and the following morning. Average pain intensity same day was quantified as the mean of the hourly pain scores during work.
The subjects further responded to a questionnaire (Vasseljen et al. 1995, 2001) covering biographical data (weight, stature, number of children), general health (exercise, sleep), psychological profile (perceived general tension, general mood, psychological health), subjectively reported physical and psychosocial exposure factors at work (job satisfaction, social support, job control, self-realization, job instructions, work load/pace, work place design, load variation, indoor environment) and general psychosocial stress factors (off-work duties, personal economy, family relations).
Statistical analyses
One-way ANOVA by ranks (Kruskal-Wallis) with a Bonferroni post hoc test was used to test the hypothesis that the EMG responses did not differ between muscles. Spearman’s rho (ρ) was used for correlation analysis due to the non-normal distribution of data. The Mann-Whitney U test was used to test the hypothesis that EMG responses did not differ between pain-afflicted and pain-free subjects. All comparisons were performed two-tailed and a probability level of P<0.05 was used to determine significant differences.
Results
The duration of the sleep period for subjects with successful EMG recordings ranged from 4.7 to 9.2 h (mean 7.1 h). A recording from the right trapezius is shown in Fig. 2A. Long periods of EMG activity with stable amplitude are interrupted by short sequences of phasic EMG activity, presumably representing body movement. The EMG band, the width increasing at higher activity levels, contains oscillatory activity in the low frequency range of 0.05–0.3 Hz, described in another publication (Westgaard et al. 2002). In this example the EMG amplitude was relatively high in more than half the sleep period, reaching ~3.5% EMGmax for about half an hour towards the end of the night. Two sections of the EMG recording are shown in expanded view, to illustrate the two methods for detection of EMG rest periods (Fig. 2B, C). The horizontal dashed line indicates the 1.5-μV threshold level, corresponding to 0.28% EMGmax for this recording. The horizontal bars underneath the recording mark the time periods where the EMG recording by visual inspection is judged to not deviate from the resting EMG level. Figure 2D shows the typical low-frequency oscillatory pattern of EMG activity observed during sleep.
Figure 3 shows the distribution of mean EMG activity the full sleep period for the right and left trapezius (A, B) and activity level the 10-min period with highest EMG response (C, D). The overall duration of EMG activity during sleep, using the 1.5-μV threshold criterion, varied from no activity to sustained activity throughout the sleep period (Fig. 4A, B). The median duration of EMG activity for the two trapezius muscles during sleep increased from about 33 to 53% when the more sensitive visual-inspection criterion was used (Table 2), but the full range of activity periods was still observed (Fig. 4C, D). Subjects with shoulder and neck pain the last 6 months showed significantly more trapezius activity than subjects reporting no pain (Table 3).
In the secretaries group, which included recordings of upper extremity muscles, deltoid showed the most EMG activity both with respect to mean activity level and duration of EMG activity periods (Table 2). Biceps and hand flexors presented similar EMG activity level and pattern to the trapezius muscles. The EMG activities of the trapezius muscles and the deltoid were compared for secretaries with shoulder and neck pain (15 subjects). The EMG activity of the deltoid was higher and of longer duration than for the trapezius muscles of this subgroup (Table 4). There was no indication of differential activity levels in the deltoid, biceps, and hand flexors for secretaries with versus without shoulder and neck pain.
Trapezius EMG activity during sleep was not associated with mean pain intensity the same day (P values ranging from 0.06 to 0.58 for eight unilateral comparisons of pain with EMG variables from right and left trapezius), pain exacerbation during the nighttime (seven comparisons had P values ranging from 0.20 to 0.87; one comparison had a P value of 0.04). Finally, there was no association of trapezius EMG activity during sleep with perceived tension or any other subjectively reported variable from the questionnaires.
Discussion
This is to our knowledge the first study that quantifies muscle activity during sleep by methods that focus low-level, sustained activity pattern. The observed muscle activity has characteristics that point to pre-motor input to motoneurons from the autonomic nervous system and in particular from the sympathetic nervous system, considering the low frequency range in the modulation of the motor activity (Westgaard et al. 2002). Thus, it may be speculated that the subjects with shoulder and neck pain have an elevated level of autonomic arousal, manifested by elevated trapezius motor activity.
There were two contrasting observations of relevance for the putative role of sustained low-level trapezius motor activity as a causal factor in the development of shoulder and neck pain (Hägg 1991; Melin and Lundberg 1997; Sjøgaard et al. 2000). First, subjects with shoulder and neck pain the 6 months prior to the recording activate the trapezius to a considerably higher level and with a more sustained activity pattern than pain-free subjects during sleep. The numeric difference in EMG responses between the two groups was considerable, especially the duration of EMG activity. Interestingly, there was no association between EMG variables and pain intensity same day, or pain exacerbation during the night. This latter result is in agreement with the similar observation in a study of tension headache patients, utilizing ambulatory monitoring of posterior neck and frontal muscles (Hatch et al. 1991). The second, contrasting observation is that the commonly pain-free deltoid presented higher EMG amplitude and more sustained activity than trapezius. This was true also for pain-afflicted secretaries with relatively high level of trapezius activity during sleep. Biceps and hand flexors presented EMG activity at the same level as trapezius.
The two observations allow for contrasting conclusions: elevated trapezius activity for pain-afflicted workers can be interpreted to support low-threshold motor unit overexertion as a mechanism for pain induction. However, the higher activity level in deltoid should make this muscle more susceptible to pain development than the trapezius. Two possible ways of resolving the contrasting findings are, first, the elevated activity level in trapezius for the pain-afflicted subjects is a consequence and not a cause of shoulder and neck pain. A strong argument against this explanation is the lack of association between trapezius EMG activity and shoulder and neck pain on the day of recording or with pain exacerbation during the same night. Low-level trapezius activity may alternatively be part of a more complex physiological response with activation of other physiological systems more critical to pain development. This was suggested as a possible explanation of the finding that shoulder and neck pain is consistently associated with the perception of tension, expressed as an average over a 2-month period (Vasseljen and Westgaard 1996; Westgaard 1999). Perceived tension is described in interviews as a sensation of elevating shoulders together with a variety of autonomic responses that included elevated heart rate, respiratory, and perspiration responses (Holte et al. 2003).
This study can be criticized on the basis of sleep not being monitored, but proper monitoring of sleep was not compatible with the overall design of the study, which included daytime monitoring of trapezius activity in a naturalistic environment (Holte and Westgaard 2002a, b). It would be of interest to know the relationship between muscle activity pattern and sleep stages and we aim for follow-up studies to elucidate this issue further. The lack of EEG recordings introduces an element of uncertainty in the determination of the sleep period. Our testing of the data analysis procedure indicated that this uncertainty has negligible influence on the results. Furthermore, any errors are likely to be non-specific with respect to the association with pain.
In conclusion, sustained low-level muscle activity during sleep is not associated with pain development in a simple manner. The night-time trapezius activity pattern may nevertheless serve as an indicator of processes, including sustained muscle activity pattern, which cause shoulder and neck pain. If confirmed in future studies, this may open up for the development of physiological indicator variables that signal risk of future pain development.
References
Arduini A, Berlucchi G, Strata P (1963) Pyramidal activity during sleep and wakefulness. Arch Ital Biol 101:530–544
Ashburn MA, Staats PS (1999) Management of chronic pain. Lancet 353:1865–1869
Buskila D (2000) Fibromyalgia, chronic fatigue syndrome, and myofascial pain syndrome. Curr Opin Rheumatol 12:113–123
Fischer AA, Chang CH (1985) Electromyographic evidence of paraspinal muscle spasm during sleep in patients with low back pain. Clin J Pain 1:147–154
Glenn LL, Dement WC (1981) Membrane potential, synaptic activity, and excitability of hindlimb motoneurons during wakefulness and sleep. J Neurophysiol 46:839–854
Hägg GM (1991) Static work loads and occupational myalgia—a new explanation model. In: Anderson PA, Hobart DJ, Danoff JV (eds) Electromyographical kinesiology. Elsevier, Amsterdam, pp 141–143
Hatch JP, Prihoda TJ, Moore PJ, Cry-Provost M, Borcherding S, Boutros NN, Seleshi E (1991) A naturalistic study of the relationship among electromyographic activity, psycological stress, and pain in ambulatory tension-type headache patients and headache-free controls. Psychosom Med 53:576–584
Holte KA, Westgaard RH (2002a) Daytime trapezius muscle activity and shoulder-neck pain of service workers with work stress and low biomechanical exposure. Am J Ind Med 41:393–405
Holte KA, Westgaard RH (2002b) Further studies of shoulder and neck pain and exposures in customer service work with low biomechanical demands. Ergonomics 45:887–909
Holte KA, Vasseljen O, Westgaard RH (2003) Exploring perceived tension as a response to psychosocial work stress. Scand J Work Environ Health 29:124–133
Hyyppä MT, Kronholm E (1995) Nocturnal motor activity in fibromyalgia patients with poor sleep quality. J Psychosom Res 39:85–91
Jensen C, Vasseljen O, Westgaard RH (1993) The influence of electrode position on bipolar surface electromyogram recordings of the upper trapezius muscle. Eur J Appl Physiol 67:266–273
Lavigne GJ, Velly-Miguel AM, Montplaisir J (1991) Muscle pain, dyskinesia, and sleep. Can J Physiol Pharmacol 69:678–682
Lobbezoo F, Thon MT, Rémillard G, Montplaisir JY, Lavigne GJ (1996) Relationship between sleep, neck muscle activity, and pain in cervical dystonia. Can J Neurol Sci 23:285–290
Marchiafava PL, Pompeiano O (1964) Pyramidal influences on spinal cord during desynchronized sleep. Arch Ital Biol 102:500–529
Melin B, Lundberg U (1997) A biopsychosocial approach to work-stress and musculoskeletal disorders. Int J Psychophysiol 11:238–247
Moldofsky H (2001) Sleep and pain. Sleep Med Rev 5:387–398
Simons DG, Mense S (1998) Understanding and measurement of muscle tone as related to clinical muscle pain. Pain 75:1–17
Simons DG, Travell JG, Simons LS (1999) Myofascial pain and dysfunction. The trigger point manual, vol 1, Upper half of body. Williams and Wilkins, Baltimore
Sjøgaard G, Lundberg U, Kadefors R (2000) The role of muscle activity and mental load in the development of pain and degenerative processes at the muscle cell level during computer work. Eur J Appl Physiol 83:99–105
Vasseljen O, Westgaard RH (1996) Can stress-related shoulder and neck pain develop independently of muscle activity? Pain 64:221–230
Vasseljen O, Westgaard RH, Larsen S (1995) A case-control study of psychological and psychosocial risk factors for shoulder and neck pain at the workplace. Int Arch Occup Environ Health 66:375–382
Vasseljen O, Holte KA, Westgaard RH (2001) Shoulder and neck complaints in customer relations: individual risk factors and perceived exposures at work. Ergonomics 44:355–372
Westgaard RH (1999) Effects of physical and mental stressors on muscle pain. Scand J Work Environ Health 25 [Suppl 4]:19–24
Westgaard RH, Jansen T (1992) Individual and work related factors associated with symptoms of musculoskeletal complaints. I. A quantitative registration system. Br J Ind Med 49:147–153
Westgaard RH, Vasseljen O, Holte KA (2001) Trapezius muscle activity as a risk indicator for shoulder and neck pain in female service workers with low biomechanical exposure. Ergonomics 44:339–353
Westgaard RH, Bonato P, Holte KA (2002) Low-frequency oscillations (<0.3 Hz) in the electromyographic (EMG) activity of the human trapezius muscle during sleep. J Neurophysiol 88:1177–1184
Acknowledgement
This work was supported by a grant from the Norwegian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mork, P.J., Westgaard, R.H. The association between nocturnal trapezius muscle activity and shoulder and neck pain. Eur J Appl Physiol 92, 18–25 (2004). https://doi.org/10.1007/s00421-003-1039-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-1039-4