Abstract
We hypothesized that oxygen consumption (V̇O2) rises incrementally in very heavy and fatiguing exercise where the slow component gain increases with higher work rates. Eight trained males completed a graded exercise test and bouts of square-wave cycle ergometry at 40% and 60% of the difference between the estimated lactate threshold (LT) and V̇O2peak (designated 40%D and 60%D). Exhaled gases were collected and analyzed every breath using models that allowed for a linear slow component or a slow component with one or more exponential increments. All subjects were able to complete 30 min at 40%D but not at 60%D. The slow component was generally best fit with two increments at 40%D and two or three increments at 60%D. In further (<Emphasis Type=”Italic”>, our results question the reliability of determining parameters of multiple slow component increments when repeated bouts are averaged together. This study demonstrates that V̇O2 can continue to rise incrementally beyond the onset of the slow component in very heavy and fatiguing exercise. These results support the concept of a recurring mechanism underlying the slow component of V̇O2 kinetics during square-wave exercise and suggest that the dynamics (time of onset, rate of development, magnitude) of this mechanism may vary from day to day.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary V̇O2 kinetics in square-wave exercise transitions are characterized by three phases (Whipp and Wasserman 1972). Phase 1 is the product of an immediate rise in pulmonary blood flow and generally referred to as the cardiodynamic phase (Krogh and Lindhard 1913). Phase 2 is the “non-steady state” where pulmonary V̇O2 accelerates toward a higher value and reflects the gain (Poole et al. 1992) and time-course (Grassi et al. 1996; Rossiter et al. 1999) for V̇O2 in the active muscle. Phase 3 is the steady state where V̇O2 remains relatively stable over time. There is no phase 3 if the intensity is supra-maximal or the bout is stopped prior to its attainment.
When the exercise work rate is light or moderate (below the lactate threshold, LT), phase 2 is mono-exponential (Paterson and Whipp 1991; Whipp and Wasserman 1972) with a gain of ~9–10 ml/min per watt and a time constant of ~15–40 s (Barstow et al. 1996; Brittain et al. 2001; Carter et al. 2000; Grassi et al. 1996; Whipp and Wasserman 1972). Above the LT, the gain and time constant of the overall response become progressively larger (Whipp and Wasserman 1972). This is firmly established to be the result of a distinct “slow” component in series with the initial fast component (Barstow and Molé 1991; Bearden and Moffatt 2000; Paterson and Whipp 1991). The fast component has a gain and time constant similar to that for moderate exercise (Barstow and Molé 1991). The slow component raises the overall gain to 11–17 ml/min per watt regardless of fitness level (Henson et al. 1989) and delays the attainment of a steady state. The mechanisms underlying the slow component are unknown, though many have been refuted (see Discussion).
Whipp and Wasserman (1972) noted that the slow component appeared “somewhat delayed” in onset. Barstow and Molé (1991) and Paterson and Whipp (1991) confirmed this by demonstrating that V̇O2 kinetics in heavy square-wave exercise are modeled best with the slow component as a delayed onset phenomenon that begins approximately 80–180 s into the bout. We recently advanced these mathematical reports by showing that the slow component is indeed a delayed physiological increase in O2 requirement (Bearden and Moffatt 2000). Nevertheless, the character of the slow component itself has not been further investigated. Defining the dynamics of pulmonary oxygen uptake at intensities eliciting a slow component of V̇O2 is an essential step in elucidating the underlying physiological correlates of V̇O2 kinetics and of exercise tolerance since these dynamics reflect the sum processes of many intermediate steps in the cascade of events from muscle contraction through whole-body V̇O2. Identifying the major parameters of the overall response is integral to uncovering its mechanistic components. Of interest is the observation that the slow component gain is not invariant but generally becomes larger at higher work rates (Barstow and Molé 1991; Casaburi et al. 1989a; Özyener et al. 2001) with a time constant that may be 50 or more times larger than that for the fast component. As the intensity of square-wave exercise is increased, the slow component becomes an increasing percentage of the overall response and may be greater than 1 l/min in amplitude (Barstow and Molé 1991; Casaburi et al. 1989a; Özyener et al. 2001; Whipp and Wasserman 1972). We (Bearden and Moffatt 2000) and others (e.g., Barstow and Molé 1991; Barstow et al. 1993; Kindig et al. 2001) have previously described the rise in V̇O2 beyond the fast component as a single linear or mono-exponential phenomenon. This practice has been widely used to derive parameters for convenience of describing the overall nature of the response rather than as an attempt to assign specific mechanistic events to each parameter.
One potential strength of a model lies in its ability to aid in the identification and quantification of underlying physiological processes. It is difficult to hypothesize a mechanism that could account for such a large and sustained increase in V̇O2 beginning at a single time delay some minutes into the bout. It is more plausible that the slow component is composed of progressive increases in V̇O2 rather than a single and abrupt increase in oxygen demand (Whipp et al. 2002). Such a finding would be more consistent with dynamic maintenance of energy balance than a single large error signal implied by a mono-exponential slow component. Interestingly, Henson et al. (1989, Fig. 3) provides a precedent for our investigation by showing V̇O2 increasing exponentially beginning 3–4 min after the onset of the slow component.
The purpose of this study was to test the hypothesis that the slow component is not a single mono-exponential event but can develop in serial increments during prolonged heavy exercise. Square-wave bouts of cycle ergometry to work rates of 40% and 60% of the difference between the estimated LT and V̇O2peak were used to test the hypothesis. Additionally, we investigated the reliability of determining parameters of multiple slow-component increments in single bouts and following the averaging of responses from repeated exercise bouts.
Methods
Subjects
Eight healthy male volunteers experienced with cycling on a stationary ergometer gave written informed consent to participate in this study. The procedures were approved by the Florida State University Human Subjects Review Board, and the study was conducted in accordance with the Declaration of Helsinki. Subjects were [mean (SD)] 24 (3) years of age, 178 (3) cm tall, 74.0 (5.6) kg in mass with a V̇O2peak of 58.4 (5.6) ml/kg per min.
Preparation
Subjects were prohibited from alcohol and strenuous activity for 24 h and from caffeine for 15 h prior to arrival in the laboratory. No one reported taking dietary supplements or ergogenic aids aside from vitamin/mineral supplements. Subjects consumed a light carbohydrate meal 2–3 h prior to arrival in the laboratory and repeated this meal on each test day. Testing was at the same time of day (± 2 h) for each subject. Subjects were not permitted to cycle to the laboratory and remained sedentary in the testing area for at least 30 min prior to each test.
Testing
Subjects cycled at 90 rpm on an electrically braked leg ergometer (Lode Excalibur, Groningen, The Netherlands) on 3 separate days. This cadence was chosen because it was the average preferred cadence for these subjects during pilot study data collection as expected for fit subjects at high work rates (Marsh and Martin 1993). The 1st day was a graded exercise test (1 W/6 s) for V̇O2peak and continued until the cadence could not be maintained despite verbal encouragement. From this test, the lactate threshold was estimated from the plot of V̇CO2/V̇O2 (Beaver et al. 1986).
Square-wave exercise bouts of 30 min length were completed in subsequent sessions to work rates of 40% and 60% of the difference between the estimated LT and V̇O2peak (designated 40%D and 60%D, respectively). Only one test was completed on a given day, and tests were separated by at least 2 days. Test order was assigned randomly within a counterbalanced design so that the 40%D bout was the first test for four subjects, and the 60%D bout was the first test for the other four subjects. Subjects began each test by cycling at 60 W for 5 min (very light for these fit subjects), after which the work rate was abruptly imposed without warning. Subjects were told they would cycle for 30 min and were not allowed cues as to time during the bout. Subjects were not informed that they were expected to fatigue on one of the days prior to 30 min, and only the principal investigator was aware of which test a subject was completing on a given day. Subjects were verbally encouraged to continue if they appeared to be fatiguing and were stopped only if cadence could not be recovered for 10 s; subjects were otherwise encouraged to continue until they refused. Time of fatigue was recorded if they did not complete the 30 min. Four subjects were randomly chosen to perform repeated bouts; these subjects returned on 2 additional days and completed repeated tests identical to the first two bouts at 40%D and 60%D with the exception that no blood was collected during these tests.
Blood collection and analysis
Capillary blood was drawn into micro-Scholander tubes from a warm fingertip (hot lamp) at the following times: (1) 1–2 min after fatigue in the graded exercise test, (2) 1 min prior to transition onset during the square-wave tests, and (3) every 10 min during the square-wave tests. A sample was also taken at fatigue if a sample had not been taken within the previous 2 min; otherwise, the recent blood sample was considered the “fatigue sample”. Each blood sample was analyzed immediately for [La−] (Accusport, Indianapolis, Ind., USA), pH, [HCO3 −], and base excess (AVL Omni, Roswell, Ga., USA).
Gas analysis
Throughout each test V̇O2 was measured every breath with a Parvomedics MMS-2400 system (Consentius Technologies, Salt Lake City, Utah, USA). A seven-point flow-meter calibration was completed prior to each test with a 3-l syringe (Hans-Rudolph, Kansas City, Mo., USA) at rates that spanned the expected measurements. Gas calibration was made immediately before each test with gases spanning the range of O2 and CO2 expected during data collection.
To remove extreme datum points resulting from coughing and sneezing, for example, which might erroneously distort the modeling results, any point more than four standard deviations away from the mean of the surrounding six values (three before and three after) were deleted. The decision to remove these points was confirmed visually to ensure only clear outliers were deleted; these averaged approximately 2 breaths every 3 min for all tests. The phase 1 component of the data was removed by identifying the onset of the phase 2 component as a fall in the respiratory exchange ratio (V̇CO2/V̇O2) and corresponding increase in the V̇O2 marking the end of the leg-to-lung transit delay (Casaburi et al. 1989b; Krogh and Lindhard 1913), as described by Whipp et al. (1982). The data for each bout were then interpolated second by second. Data from repeated tests were modeled individually and following time-aligned averaging. V̇O2 was modeled as a function of time. The first two models incorporated a mono-exponential fast component followed by a single increment slow component, linear in model 0 and exponential in model 1 (Barstow and Molé 1991; Bearden and Moffatt 2000):
Subsequent models included an increasing number of slow component increments:
In model 0, S is the slope of the linear portion. In each model, V̇O2 (t) is the V̇O2 at time t, B is the baseline (warm-up) value calculated as the average in the last minute of warm-up, A is amplitude, τ is the time constant, and TD stands for time delay. Numbers with each parameter designate separate segments of the response. Note that the model and equation numbers correspond to the number of exponential slow component increments in the model.
Control 1
Computer simulations were run to determine if natural Gaussian noise in the gas exchange responses could have led to an erroneous impression of serial increments in V̇O2 as a result of model increments curving into or “catching” the noise. Initially, 20 smooth V̇O2 responses of 10–30 min duration were entered into a computer; each of these contained an initial delay and fast component followed by either a mono-exponential (n=10) or linear (n=10) slow component. Components of the smooth response (amplitudes, delays, time constants) were chosen to span the range of values for subjects in this study (from model 1 results), values obtained in our laboratory, and those reported in the literature. Specifically, values used ranged A 2=0.2–1.5 l/min, TD2=60–240 s, and τ2=50–1,500 s for the monoexponential simulations, and TD2=60–240 s, slope=0.05–0.3 l/min for the linear simulations. These 20 smooth responses then underwent 10 transformations each (GraphPad Prism, San Diego, Calif., USA); the transformations imposed Gaussian (uncorrelated) random error with a standard deviation of 0.150 l/min to each datum point of the underlying smooth response (~4–9% of the response amplitudes). This level of imposed random error was chosen as a conservative value based on the collected data. The actual breath-to-breath variability in the data modeled for this study, measured as the standard deviation of the variability about the mean during 2 min of the 60-W steady-state warm-up, was 0.057 (0.028) l/min [mean (SD)] with a range of 0.024–0.118 l/min. This noise was 1.7 (0.8)% of the response amplitudes. The resultant 200 simulated responses (20×10) were then modeled in the identical manner as the data from the eight subjects. If noise was capable of producing the erroneous impression of serial increments in the slow component, then it was expected that at least one of these 200 simulations would have produced a statistically better fit with a more complex model.
Control 2
For this second control we borrowed from the logic of Paterson and Whipp (1991) in demonstrating that the slow component is a delayed onset phenomenon. The first one-third of each data set was modeled with the usual one-increment slow component equation (Eq. 1). An end-exercise predicted V̇O2 was then extrapolated from the result and compared with the actual end-exercise V̇O2. If the slow component is a single increment, then these two values should not differ. However, if the slow component is comprised of sequential increments, then the extrapolation should fall below the actual V̇O2. We chose to model the first one-third of the data to normalize procedures across all bouts since half of the trials led to fatigue prior to the prescribed 30 min. The results of this control were not different when we repeated the procedures using one-half bout length, one-quarter bout length, or the first 3 min.
Monte Carlo analyses
Error in model parameters was estimated by Monte Carlo analyses as detailed by Motulsky and Ransnas (1987) for tests from the four subjects randomly selected to complete repeated tests. The best-fit model response was used as a template to generate 100 simulated responses with the same amount of Gaussian noise as observed in each original data set followed by modeling of each of the 100 generated responses. The standard deviation of the results for each parameter across the 100 simulations is the estimate of the parameter error and is analogous to the confidence interval in linear regression.
Statistics
Alpha was set equal to 0.05 for all statistical comparisons. The V̇O2 responses, actual and simulated, were modeled with successively more complex models, as described above. The F-test was used for each subject for each transition to determine if the more complex model was a significantly better fit of the data over the previous model. This test evaluates the reduction in residual summed squared error by the more complex model at the expense of losing degrees of freedom with a model that contains more parameters. If the F-test was significant, then the more complex model was considered a superior fit over the simpler model. When the F-test was not significant, the simpler model was recorded as optimal. Extrapolated and actual end-exercise V̇O2 were compared in the second control experiment by dependent samples t-test. Individual blood measurements were compared using two-way and one-way repeated measures ANOVA with Tukey’s HSD post-hoc tests as appropriate; since average time to fatigue at 60%D was close to 20 min, fatigue values were lumped as a 20-min value for statistical comparisons. All blood measurements were compared to the corresponding value attained at the end of the V̇O2peak test by ANOVA with Dunnett’s post-hoc test. The response gain (ΔV̇O2/ΔW) and percentage contribution of the slow component to the overall response were compared by dependent samples t-test between the 40%D and 60%D bouts. All values are reported as mean (SD).
Results
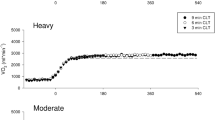
The LT estimated from gas exchange was 56.4 (4.0)% V̇O2peak. Work rates for the two bouts were 233 (31) W and 258 (34) W for the 40%D and 60%D bouts, respectively. All subjects completed the 30-min bouts at 40%D but none could complete the 30-min at 60%D. Average time to fatigue at 60%D was 16.9 (5.7) min. Figure 1 is a plot of the blood variable responses. Blood variables at fatigue in the 60%D bouts were not different from peak (measured at the end of the graded exercise test) but remained significantly different from peak throughout the 40%D bouts.
Blood measurements [mean (SD); n=8] throughout square-wave transitions to 40%D (squares) and 60%D (circles). Asterisks indicate significant (P<0.05) differences between 40%D and 60%D at each time point (fatigue values in 60%D were lumped as 20-min values for statistical comparisons). Open symbols represent values not significantly different from those attained at fatigue during V̇O2peak test
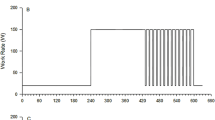
Parameters for the pulmonary V̇O2 models are given in Table 1 for the two exercise intensities. In no case did a linear slow component (Eq. 0) fit the data best; therefore, the linear model was not evaluated further. Table 2 presents the results of the F-test comparisons among exponential models. In the 40%D bouts, three responses were well fit with a mono-exponential slow component (Eq. 1) while a two-increment slow component (Eq. 2) fit the other five responses significantly better. Further complexity did not improve any of the model fits in the 40%D bouts. In the 60%D bouts, a mono-exponential slow component model (Eq. 1) best fit two responses, a two-increment slow component model (Eq. 2) best fit one response, and a three-increment slow component model (Eq. 3) best fit five responses. Figure 2 shows the response for one subject modeled with the standard fast and slow components (Eq. 1) and the best-fit three-increment slow component (Eq. 3) along with residuals plots. As can be seen, the data are poorly fit using the single increment slow component.
Modeled V̇O2 response to 60%D with associated residuals plots (subject no. 2). A Mono-exponential fast and slow components (Eq. 1) as typically used in the literature. B Best-fit function for the same data; a three-increment slow component (Eq. 3). Residuals plots have both been fit with a third-order polynomial to emphasize the trends. Note how the residuals in A vary substantially from zero, reflecting a poor fit of the data, while the plot in B demonstrates a much better fit
A 1/W was 10.7 (1.3) ml/min per watt and 10.6 (1.4) ml/min per watt at 40%D and 60%D, respectively (P=0.82). By the end of the bouts, the slow component contributed 22.9 (9.2)% and 29.2 (10.1)% (P=0.043) to the increase in V̇O2 from baseline in the 40%D and 60%D bouts, respectively. The slow component added 3.2 (1.4) ml/min per watt and 4.5 (2.0) ml/min per watt (P=0.048) to the initial fast component gain in the 40%D and 60%D bouts, respectively. These observations of an increase in the amplitude of the slow component with respect to response amplitude and work rate are consistent with previous reports (Barstow and Molé 1991; Casaburi et al. 1989a; Özyener et al. 2001). The overall V̇O2 gain was 13.9 (1.4) ml/min per watt and 15.1 (1.8) ml/min per watt (P=0.033) in the 40%D and 60%D bouts, respectively.
Control 1
Computer simulations were modeled to establish the probability that the present findings were the result of more complex models “catching” normal Gaussian noise. Two-hundred responses with the underlying characteristics of a fast component followed by a one-increment slow component were simulated with parameters spanning the values reported here and in the literature, including 0.15 l/min of Gaussian noise (Lamarra et al. 1987). All of the 200 responses were best fit by a one-increment slow component model (Eq. 1) with an F-test P value greater than 0.13 in all cases for the comparison between a one-increment and two-increment slow component model. This analysis provides confidence that the modeling results are truly physiologic and not a result of “noise” error.
Control 2
In the bouts that were best fit by a one-increment slow component (n=3 at 40%D and n=2 at 60%D), modeling one-third of the collected data with the traditional one-increment slow component and extrapolating this value to end-exercise predicted the actual end-exercise V̇O2 with a difference of 0.00 (0.05) l/min (P=0.89). In the bouts that were best fit by multi-increment slow component models (n=5 in 40%D and n=6 in 60%D), the extrapolated value significantly under predicted the actual end-exercise V̇O2 with a difference of 0.15 (0.09) l/min (P<0.001). As explained in Methods, these findings were reproducible with different modeling time periods and thus are not a result of the modeling time frame. This control demonstrates that the slow component is often not a single increment at these high work rates, since its initial projection falls below values ultimately attained in longer bouts (Paterson and Whipp 1991).
Monte Carlo anlyses
Four subjects completed repeated bouts to determine the accuracy of parameter estimates and to investigate whether the responses vary from day to day. Table 3 gives parameters along with the standard deviation of the error generated by Monte Carlo analysis which averaged 7.98 (8.96)%. The largest errors tended to be in the parameters of the last increment of a given response. For each subject and exercise intensity, there was no difference in the optimal model fit among the two single tests and averaged response; e.g., if model 2 best fit a response it was also the optimal model for the repeated single bouts and when the two bouts were averaged. Therefore, the best-fit model was reliable for single tests from day to day and for an averaged response created by averaging repeated identical tests. For the majority of parameters, day-to-day differences were small and the responses were generally reproducible. However, differences can be found in a large number of cases (Table 3). The good estimates of error shown from Monte Carlo analyses (high accuracy of parameter estimation) are smaller than the day-to-day differences in parameter estimation. Since the differences in these parameters from day-to-day are greater than the error of estimation on a given day, we conclude that there is true day-to-day variability. Therefore, these findings do not support the practice of averaging multiple bouts if parameters of multiple increments are to be determined accurately in future studies.
Discussion
The original finding of this investigation was that V̇O2 rises in serial increments throughout prolonged heavy exercise. Thus, the slow component of V̇O2 kinetics can often be described in terms of a multiple increment response when exercise is prolonged. These data are the first to support the hypothesis that the mechanism of the V̇O2 slow component is not a single event occurring near a particular time delay (e.g., TD2) but can be recurrent as long as the heavy exercise is continued, reflecting step-wise increments in oxygen demand to sustain a constant work rate.
We have investigated the dynamics of the slow component itself which has until now been modeled as a single component of the overall response. Modeling procedures may be used either to predict an outcome from a set of input data or to empirically describe the outcome response so that predictions and hypotheses may be made concerning the input. At present we have taken the second approach since the underlying physiologic mechanisms of the slow component are unknown. In doing so, we have analyzed the output (i.e., V̇O2) data and it is important to recognize that these data will most likely reflect a multitude of underlying events. Therefore, V̇O2 kinetics from lung gas exchange will be, to some extent, an approximation of the underlying mechanisms and not necessarily reflect the precise timing of any specific event. Our observations, however, show that the slow component is not confined to a slowly evolving increase in V̇O2 but can have periods of more rapid increase similar to its initial onset around TD2.
Since it is possible that the mechanisms underlying the slow component vary in their magnitude, rate of development, and/or time of onset from test-to-test, four subjects completed identical repeat bouts to examine the reliability of single tests and averaged responses in determining multiple increment parameters. These repeat bouts were modeled individually and following time-aligned averaging. Variability has been reported in slow component parameters, especially its time constant, when modeling single transitions from subjects less fit than in this study (Özyener et al. 2001). Experienced cyclists were chosen in this study for their expected large response amplitudes (high work rates) and low breath-to-breath variability due to well-established and comfortable breathing patterns on the cycle ergometer. Overall, modeled parameters for the repeat bouts were, more often than not, similar when modeled separately (Table 3) with no discrepancy in the number of increments in the optimal model. This finding suggests that the individual increments are, for the most part, temporally, quantitatively, and qualitatively reproducible. However, with regard to the accuracy of each parameter, we found that the difference between repeated bouts was larger than can be explained by error in the estimate for 20 of the 75 repeated single-bout parameters given in Table 3; i.e., in 20 of 75 cases, the difference between parameters was greater than the 95% confidence interval. The conclusion from this is that these parameters were truly different on repeated testing days. This calls into question whether averaging multiple bouts is an accurate method for removing noise in an effort to accurately uncover the underlying response parameters, at least when multiple increments are present. Lamarra et al. (1987) demonstrated that averaging repeated bouts serves to reduce Gaussian noise and clarify the underlying response in moderate intensity (<LT) exercise; in these cases the underlying response is believed to be the same from test to test and the random noise can be reduced by averaging repeated bouts. The data presented here do not support the same assumptions for exercise where multiple increments are observed. Therefore, we believe errors in multiple increment parameter estimates may result from averaging bouts in these cases. The reason for differences in some parameters from test to test will have to await future study but the finding suggests that the mechanisms underlying the V̇O2 response to very heavy and fatiguing exercise are malleable on a test-to-test basis.
It has been proposed that many small increments with rapid time constants overlap and give the appearance of a smooth increment response, as traditionally modeled with a single slow component increment, when measured from expired gases at the mouth (Whipp et al. 2002). Our data provide partial evidence for this. At 60%D, additional increments in the slow component occurred as early as ~280 s; 8 of the 16 increments found beyond the onset of the slow component (TD2) were within the 6- to 10-min bout length that we and others typically use. Further, the time constant for developing each increment of the slow component in this study was reduced substantially from that for a single mono-exponential response and to values closer to that for the fast component. Most importantly, these data demonstrate that the slow component is not a singular event and thus is not the result of a single large error signal. Therefore, the as yet undetermined mechanisms underlying the slow component may recur in stepwise fashion throughout prolonged exercise at high intensities. However, our results can not fully support the hypothesis that the entire slow component is comprised of many small increments (Whipp et al. 2002); studies relying on pulmonary oxygen consumption measurements are probably not sensitive enough to uncover such subtle intramuscular kinetics if they exist. If each of the TD points found in this study represents a critical period of development or increased magnitude of the mechanisms underlying the slow component, then longer bout lengths at exercise intensities eliciting multiple increments should provide useful mechanistic insight in future studies.
It is widely accepted that the majority of the slow component manifests in the active musculature (Carra et al. 2003; Poole et al. 1991) and appears unrelated to temperature (Koga et al. 1997), lactate oxidation (Gaesser and Poole 1996; Poole et al. 1994), or epinephrine levels (Gaesser et al. 1994; Womack et al. 1995). The most popularly touted mechanism is shifting motor unit recruitment patterns. The present data could be explained by such a mechanism if sustained heavy exercise leads to progressive fatigue whereby groups of new motor units are sequentially recruited as the contracting fibers fatigue over time rather than a slow consistent recruitment which would lead to a more uniform V̇O2 profile. Yet, recent EMG studies have been unable to establish a link between motor unit recruitment and the slow component (Lucia et al. 2000; Scheuermann et al. 2001). In the studies that suggest a relationship between EMG and the slow component, the EMG signal did not rise until 1–2 min after the onset of the slow component (Perrey et al. 2001; Shinohara and Moritani 1992), which is inconsistent with a cause-effect relationship. An alternative scenario was proposed by Pringle et al. (2003) where type II fibers recruited at the onset of exercise would progressively fatigue; these fibers would no longer contribute to force production but would continue to consume oxygen as they recover. The EMG signal might be little affected if similar fibers are recruited as the old ones drop out. Interestingly, Grassi et al. (2000) demonstrated that a slow component could be generated in isolated, electrically stimulated dog gastrocnemius muscle. In this preparation, maximal tetanic contraction elicits the full involvement of all motor units at a constant frequency from exercise onset. Therefore, a change in motor unit recruitment or rate coding appears unnecessary for generating a slow component, at least under some experimental conditions. Changes in recruitment patterns as the mechanism of slow component V̇O2 development would be expected to result in many small increments that should blend into an apparent smooth curve with progressively lengthening time constant until a steady state or fatigue is reached. Our data do not argue against this; however, the appreciable 2–4 sequential increments demonstrate bursts of acceleration in the rising V̇O2 pattern. This would be consistent with intermittent periods of relatively rapid shift in the motor unit recruitment profile or with similar periods of rapid changes in a metabolic component reflecting reduced exercise economy (i.e., increased V̇O2 for maintaining the same work rate).
Consistent with the V̇O2 slow component as a portent of fatigue, HCO3 −, base excess, and pH fell while blood lactate and V̇O2 rose throughout the 60%D bouts, all reaching end-exercise fatigue values not different from those obtained at the end of a V̇O2peak test (Fig. 1). During the 40%D bouts, V̇O2 and blood measurements remained significantly different from their V̇O2peak values. Fatigue will ensue earlier at higher intensities, and the fast component will dominate a greater percentage of the overall V̇O2 response. Ultimately the projected gain of the fast component for a severe work rate can be equal to or greater than V̇O2peak. While the present data show an increasingly complex V̇O2 response when exercise intensity increases, this will reach a critical work rate above which the kinetics are less complex as fatigue reduces bout duration. For example, slow component data for subject no. 3 was best fit by 2 increments at 40%D but 1 increment at 60%D (Table 3) where the heavy work rate caused fatigue in 790 s. In some cases this may cause the slow component to take on a linear appearance. None of the responses presented here reduced to a mono-exponential function and none of the slow components were linear.
In summary, the increasing gain and long slow-component time constants in very heavy and fatiguing exercise were shown, at least in some cases, to be the result of multiple increments in V̇O2 during the development of the slow component. Further, our results from multiple repeated bout analyses demonstrate that averaging repeated bouts in an effort to remove Gaussian noise and enhance the underlying response may lead to errors in multiple increment parameter estimates and thus obscure the ability to correlate the kinetic parameters with putative mechanisms. Therefore, we conclude that V̇O2 kinetics during high-intensity exercise demonstrate ongoing and dynamic changes that reflect sequential increments in metabolic demand. The physiological correlates of the sequential increments are likely to play a major role in limiting exercise tolerance.
References
Barstow TJ, Molé PA (1991) Linear and nonlinear characteristics of oxygen uptake kinetics during heavy exercise. J Appl Physiol 71:2099–2106
Barstow TJ, Casaburi R, Wasserman K (1993) O2 uptake kinetics and the O2 deficit as related to exercise intensity and blood lactate. J Appl Physiol 75:755–762
Barstow TJ, Jones AM, Nguyen PH, Casaburi R (1996) Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol 81:1642–1650
Bearden SE, Moffatt RJ (2000) VO2 kinetics and the O2 deficit in heavy exercise. J Appl Physiol 88:1407–1412
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Brittain CJ, Rossiter HB, Kowalchuk JM, Whipp BJ (2001) Effect of prior metabolic rate on the kinetics of oxygen uptake during moderate-intensity exercise. Eur J Appl Physiol 86:125–134
Carra J, Candau R, Keslacy S, Giolbas F, Borrani F, Millet GP, Varray A, Ramonatxo M (2003) The addition of inspiratory resistance increases the amplitude of the slow component of the O2 uptake kinetics. J Appl Physiol 94:2448–2455
Carter H, Jones AM, Barstow TJ, Burnley M, Williams CA, Doust JH (2000) Oxygen uptake kinetics in treadmill running and cycle ergometry: a comparison. J Appl Physiol 89:899–907
Casaburi R, Barstow TJ, Robinson T, Wasserman K (1989a) Influence of work rate on ventilatory and gas exchange kinetics. J Appl Physiol 67:547–555
Casaburi R, Daly J, Hansen JE, Effros RM (1989b) Abrupt changes in mixed venous blood gas composition after the onset of exercise. J Appl Physiol 67:1106–1112
Gaesser GA, Poole DC (1996) The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev 24:35–71
Gaesser GA, Ward SA, Baum VC, Whipp BJ (1994) Effects of infused epinephrine on slow phase of O2 uptake kinetics during heavy exercise in humans. J Appl Physiol 77:2413–2419
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80:988–998
Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden LB (2000) Role of convective O2 delivery in determining VO2 on-kinetics in canine muscle contracting at peak VO2. J Appl Physiol 89:1293–1301
Henson LC, Poole DC, Whipp BJ (1989) Fitness as a determinant of oxygen uptake response to constant-load exercise. Eur J Appl Physiol 59:21–28
Kindig CA, McDonough P, Erickson HH, Poole DC (2001) Effect of L-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol 91:891–896
Koga S, Shiojiri T, Kondo N, Barstow TJ (1997) Effect of increased muscle temperature on oxygen uptake kinetics during exercise. J Appl Physiol 83:1333–1338
Krogh A, Lindhard J (1913) The regulation of respiration and circulation during the initial stages of muscular work. J Physiol (Lond) 47:112–136
Lamarra N, Whipp BJ, Ward SA, Wasserman K (1987) Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 62:2003–2012
Lucia A, Hoyos J, Chicharro JL (2000) The slow component of VO2 in professional cyclists. Br J Sports Med 34:367–374
Marsh AP, Martin PE (1993) The association between cycling experience and preferred and most economical cadences. Med Sci Sports Exerc 25:1269–1274
Motulsky HJ, Ransnas LA (1987) Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J 1:365–374
Özyener F, Rossiter H, Ward S, Whipp B (2001) Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol (Lond) 533:891–902
Paterson DH, Whipp BJ (1991) Asymmetries of oxygen uptake transients at the on- and offset of heavy exercise in humans. J Physiol (Lond) 443:575–586
Perrey S, Betik A, Candau R, Rouillon JD, Hughson RL (2001) Comparison of oxygen uptake kinetics during concentric and eccentric cycle exercise. J Appl Physiol 91:2135–2142
Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD (1991) Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol 71:1245–1260
Poole DC, Gaesser GA, Hogan MC, Knight DR, Wagner PD (1992) Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol 72:805–810
Poole DC, Gladden LB, Kurdak S, Hogan MC (1994) l-(+)-Lactate infusion into working dog gastrocnemius: no evidence lactate per se mediates VO2 slow component. J Appl Physiol 76:787–792
Pringle JS, Doust JH, Carter H, Tolfrey K, Campbell IT, Jones AM (2003) Oxygen uptake kinetics during moderate, heavy and severe intensity ‘submaximal’ exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol 89:289–300
Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ (1999) Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol (Lond) 518:921–932
Scheuermann B, Hoelting BD, Noble ML, Barstow TJ (2001) The slow component of O(2) uptake is not accompanied by changes in muscle EMG during repeated bouts of heavy exercise in humans. J Physiol (Lond) 531:245–256
Shinohara M, Moritani T (1992) Increase in neuromuscular activity and oxygen uptake during heavy exercise. Ann Physiol Anthropol 11:257–262
Whipp BJ, Wasserman K (1972) Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol 33:351–356
Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K (1982) Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol 52:1506–1513
Whipp BJ, Rossiter HB, Ward SA (2002) Exertional oxygen uptake kinetics: a stamen of stamina? Biochem Soc Trans 30:237–247
Womack CJ, Davis SE, Blumer JL, Barrett E, Weltman AL, Gaesser GA (1995) Slow component of O2 uptake during heavy exercise: adaptation to endurance training. J Appl Physiol 79:838–845
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bearden, S.E., Henning, P.C., Bearden, T.A. et al. The slow component of V̇O2 kinetics in very heavy and fatiguing square-wave exercise. Eur J Appl Physiol 91, 586–594 (2004). https://doi.org/10.1007/s00421-003-1009-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-1009-x