Abstract
Complete spinal cord injury (SCI) results in inactivation and unloading of affected skeletal muscles. Unloading causes an increased susceptibility of muscle to contraction-induced injury. This study used magnetic resonance imaging (MRI) to test the hypothesis that isometric contractions would evoke greater muscle damage to the quadriceps femoris muscle (mQF) of SCI subjects than that of able-bodied (AB) controls. MR images were taken of the mQF prior to, immediately post, and 3 days post electromyostimulation (EMS). EMS consisted of five sets of ten isometric contractions (2 s on/6 s off, 1 min between sets) followed by another three sets of ten isometric contractions (1 s on/1 s off, 30 s between sets). Average muscle cross-sectional area (CSA) and the relative areas of stimulated and injured muscle were obtained from MR images by quantifying the number of pixels with an elevated T2 signal. SCI subjects had significantly greater relative area [90 (2)% versus 66 (4)%, P<0.05; mean (SE)] but a lesser absolute area [16 (3) cm2 versus 44 (6) cm2, P<0.05] of mQF stimulated than AB controls. During EMS, peak torque was reduced by 66% and 37% for SCI and control subjects, respectively. Three days post EMS, there was a greater relative area of stimulated mQF injured for the SCI subjects [25 (6)% versus 2 (1)%, P<0.05]. Peak torque remained decreased by 22% on day 3 in the SCI group only. These results indicate that affected muscle years after SCI is more susceptible to contraction-induced muscle damage, as determined by MRI, compared to AB controls. They also support the contention that electrically elicited isometric contractions are sufficient to cause muscle damage after a prolonged period of inactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complete spinal cord injury (SCI) results in inactivation and subsequent unloading of affected skeletal muscle. Fibers of affected muscles have been reported to be small in size (Castro et al. 1999b; Martin et al. 1992), predominantly fast twitch (Grimby et al. 1976; Round et al. 1993), and have a low resistance to fatigue (Castro et al. 2000; Rochester et al. 1995). These factors contribute to the limited ability of muscle in SCI patients to produce and maintain torque, which could make training affected muscles to elicit muscle hypertrophy difficult to obtain. Another confounding factor that could potentially impact a training program for SCI patients, is an increased vulnerability to contraction-induced muscle injury after SCI, due to the extreme unloading and inactivation.
It is generally known that unloading causes affected muscles to be predisposed to contraction induced injury (Ploutz-Snyder et al. 1996; Warren et al. 1994). Studies of lower mammals have shown that hindlimb suspension (Krippendorf and Riley 1993, 1994; Warren et al. 1994), immobilization (Appell 1986), and spaceflight (Riley et al. 1995) put muscles at a higher risk of injury. Tasks that appear rather mild can evoke muscle injury that is uncharacteristic of the activity when performed under normal conditions. For example, reambulation after hindlimb suspension caused sarcomere lesions that were not observed in muscles studied after hindlimb suspension alone (Krippendorf and Riley 1994). Human studies have also illustrated that unloading predisposes the muscle to greater muscle injury. Ploutz-Snyder et al. (1996) found that eccentric contractions with relatively light loads caused muscle injury in the suspended limb of able-bodied individuals after unilateral lower limb suspension (ULLS).
Magnetic resonance imaging (MRI) has been used to measure muscle activation (Adams et al. 1993; Akima et al. 2000; Conley et al. 1995; Fisher et al. 1990; Shellock et al. 1991a) and exercise-induced muscle injury (Baldwin et al. 2001; Dudley et al. 1997; Foley et al. 1999; Ploutz-Snyder et al. 1996, 1998; Prior et al. 2001; Shellock et al. 1991b; Takahashi et al. 1994). Immediately following a bout of contractions there is an increase in signal intensity and T2 relaxation times (Adams et al. 1992, 1993; Fisher et al. 1990). Adams et al. (1992, 1993) demonstrated the unique ability of MRI to map and quantify the pattern of muscle activation after electromyostimulation (EMS). Previously, it has been thought that the increase in muscle T2 immediately following exercise was simply due to increased extracellular fluid volume. As it turns out, if the extracellular fluid is increased without exercise, the effects are not the same as from exercise alone (Ploutz-Snyder et al. 1997).
The same changes in signal and T2 also peak 2–6 days after an exercise bout that induces muscle injury (Mair et al. 1992; Takahashi et al. 1994). Delayed changes in the MR signal correlate with other indicators of muscle fiber injury, such as delayed onset muscle soreness (Takahashi et al. 1994). The time-course of T2 changes has been reported during the early phase of recovery after exercise that induces damage and the general conclusion had been that T2 changes reflect edema. However, studies that have been carried out over longer periods of time have reported increases in muscle T2 up to 80 days after exercise, well after swelling has resolved (Shellock et al. 1991b).
The use of EMS for the training of SCI patients has received considerable attention for many years. The potential for contraction-induced muscle injury in this population should be considered due to the long period of inactivity and unloading that the affected muscles of these patients endure. In the present study, MRI was used to assess the amount of muscle activated and injured after one bout of EMS consisting of 80 isometric contractions. We hypothesized that SCI patients would experience greater muscle injury after a bout of EMS which was not expected to cause injury to the muscles of able-bodied (AB) controls.
Methods
Subjects
Eight AB (two female) and eight SCI (one female) subjects participated in this study. SCI level of injury ranged from C5 to T9 and the average time post injury was 10 (3) years. Descriptive data on all subjects is listed in Table 1. SCI and AB subjects had no history of lower extremity pathology and gave informed consent prior to testing. Both groups were asked to refrain from ingesting non-steroidal anti-inflammatory medications and the AB group was not currently involved in lower extremity resistance exercise. All methods were approved by the Institutional Review Boards of the University of Georgia and Shepherd Center. Briefly, subjects had MR images of the left thigh taken prior to, immediately after, and 3 days post electrical stimulation that evoked 80 isometric contractions.
Quadriceps femoris experimental setup
The quadriceps femoris muscle (mQF) was stimulated essentially as described previously (Adams et al. 1993; Castro et al. 1999b, 2000; Hillegass and Dudley 1999). Subjects were seated in a custom-built chair with the hip and knee secured at approximately 70° of flexion. The leg was firmly secured to a rigid lever arm with an inelastic strap to ensure that the knee extensors could only perform isometric contractions. The moment arm was established by placing a load cell (model 2000A; Rice Lake Weighing Systems, West Coleman Street, Rice Lake, Wis., USA) parallel to the line of pull and perpendicular to the lever arm. Torque was recorded from the load cell using a MacLab A-D converter (model ML 400; ADInstruments, Milford, Mass., USA) sampling at 100 Hz and interfaced with a portable Macintosh computer (Apple Computer, Cupertino, Calif., USA).
Electrical stimulation protocol
Two 8×10-cm surface electrodes (Uni-Patch, 1313 West Grant Boulevard, Wabasha, Minn., USA) were placed on the distal vastus medialis and the proximal vastus lateralis muscle. This electrode placement has been previously shown to allow sufficient recruitment of mQF in both able-bodied and SCI subjects (Adams et al. 1993; Hillegass and Dudley 1999). A commercial stimulator (TheraTouch model 4.7; Rich-Mar Corporation, Inola, Okla., USA) was used for EMS. The initial torque was determined in the following manner. For SCI patients, the maximum torque was determined by increasing current incrementally until torque no longer increased. The AB controls performed a maximum voluntary contraction (MVC) for isometric knee extension prior to EMS. The subjects were highly motivated and all had prior experience with knee extension MVC. Then, the current that elicited approximately 60% of isometric knee extension MVC was determined. The EMS protocol consisted initially of five sets of ten 2-s isometric contractions with 6 s and 1 min rest between contractions and sets, respectively, at the current that was initially determined. The rest between contractions and sets was established in an effort to minimize fatigue. At the completion of the five sets of ten, another three sets of ten 1-s contractions with 1 s and 30 s of rest between contractions and sets were used. The 1-s contractions were utilized to ensure significant contrast shift on MRI as it has been reported that the T2 increase is directly related to exercise intensity (Fisher et al. 1990). For both groups, contractions were evoked with 50-Hz trains of 450-μs biphasic pulses.
The maximum torque that could be evoked via EMS was measured on day 3 in five of the eight SCI subjects. The stimulation set-up and electrode placement was similar to day 1, current was again increased incrementally until torque reached a plateau. Five of eight AB subjects (n=5) were also re-tested for MVC on day 3, to determine if performance was compromised.
Magnetic resonance imaging
Standard spin-echo images of the thigh were collected using a 1.5 T super-conducting magnet (Signa, General Electric, Milwaukee, Wis., USA) essentially as described previously (Adams et al. 1993; Baldwin et al. 2001; Dudley et al. 1999; Hather et al. 1992; Ploutz-Snyder et al. 1996; Stevenson and Dudley 2001). Sixteen 1-cm-thick transaxial images (TR/TE=2000/30,60) spaced 1-cm apart were collected using a 25-cm-diameter extremity coil. A 256×128 matrix was acquired with one excitation and a 20-cm field of view. The proximal aspect of the patella was aligned with the distal portion of the extremity coil for each image to ensure consistent subject position in the magnet over repeat MR images. A test-retest reliability of r > 0.95 (P<0.05) for measuring mQF CSA has previously been reported for these parameters (Adams et al. 1993).
MRI analysis
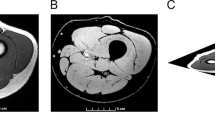
MR images were transferred to computer for calculation of T2 using a modified version of the public domain National Institutes of Health (NIH) Image program (v. 1.52), written by Wayne Rasband at NIH and available from the Internet by anonymous ftp. After spatial calibration (20 cm/256 pixels=0.078 cm/pixel), a region of interest (ROI) was defined by tracing the outline of the mQF. The T2 for each pixel within the ROI was determined from the native images. Pixels with a T2 between 20 ms and 35 ms were assumed to represent muscle at rest in the pre-EMS images. The area that was stimulated was assessed in the post-exercise images and reflected by the pixels with an elevated T2 minus pixels in the pre-EMS images with elevated T2. This was done in order to correct for pixels containing material such as fat, which have longer T2 values than muscle, and would be present in both images. Initially, the mean and SD of the T2 of pixels in each pre-EMS image were calculated. Pixels in matching post-EMS images with a T2 greater than the mean plus 1 SD of the T2 of muscle pre-EMS were considered elevated. The CSA of such pixels was determined. CSA values were averaged over eight slices starting with the first slice not containing gluteal muscle and continuing distally for the next seven slices to determine the average absolute and relative CSA of muscle activated. This region of slices has been reported to represent the maximum CSA of mQF (Castro et al. 1999a; Narici et al. 1989). The CSA of pixels with an elevated T2 was determined from the images taken on day 3 in the same manner to represent damaged muscle.
Statistics
Statistical analyses were run using SPSS (v. 10.0). Variables were analyzed with a one-way ANOVA. Relative CSA of stimulated muscle and resultant torque values were analyzed with simple linear regression. The level of significance was set at P≤0.05. The data are presented as mean (SE).
Results
The SCI subjects were slightly older (7 years, P<0.05) than the AB controls but otherwise were similar in height and weight (Table 1). Fatigue was greater for the SCI subjects over the entire EMS protocol (Fig. 1). On average, torque decreased by ~66% for the SCI group, while the AB controls showed a ~36% reduction in torque after EMS. The AB controls showed nearly complete recovery of torque between sets for the first five sets, while there was clearly incomplete recovery for the same protocol in the SCI subjects (Fig. 1). While there was a substantial decrease in torque for both SCI and AB subjects during the three sets of 1-s contractions, this was expected and desired, to allow a greater contrast shift to be seen on MRI. It has been suggested that the T2 contrast shift associated with muscle activation is directly related to the metabolic activity of the muscle (Adams et al. 1993).
Fatigue during electromyostimulation (EMS). Spinal cord injury (SCI) subjects (open circles) had incomplete recovery between sets resulting in about a 66% decline in torque compared to 36% in able-bodied controls (filled circles). Stim 1 protocol was 2-s contractions with 6 s and 1 min rest between reps and sets, respectively. Stim 2 protocol was 1-s contractions with 1 s and 30 s rest between reps and sets, respectively. n = 8 per group
The absolute average CSA of muscle stimulated in the AB controls was significantly greater than in the SCI patients [44 (6) cm2 versus 16 (3) cm2, P<0.05], due to the atrophied mQF of SCI subjects. However, in a relative sense, there was a higher proportion of the mQF stimulated in the SCI group [90 (2) versus 66 (4)%, P<0.05; Fig. 2]. Despite the small mQF of SCI patients and the long duration since injury, the available motor units responded quite well to stimulation. Overall, EMS torque was predicted by the following equation: torque (Nm) = 3.74 × stimulated CSA (cm2) − 14.37 (R 2 = 0.93, P<0.05, Fig. 3). The relative mQF CSA with an elevated T2 on day 3 was significantly higher for SCI than AB subjects (25% versus 2% of activated muscle, P<0.05, Fig. 4). The damaged area was normalized to the amount of muscle activated on day 1. AB subjects showed no decline in MVC 3–4 days post stimulation (plus/minus 5%), yet SCI subjects’ electrically stimulated torque was reduced by 22%.
Representative single slice, binary T2 map of the quadriceps femoris muscle (mQF) for one able-bodied (upper row) and SCI (lower row) subject pre (left), immediately post (middle), and 3 days post (right) EMS, respectively. Black represents muscle at rest, borders drawn for clarity. In general, SCI subjects had smaller mQF cross-sectional area (CSA) but larger relative amount of muscle stimulated and injured
Torque versus stimulated CSA. The average CSA of stimulated muscle was plotted versus the initial torque prior to the EMS protocol. Simple linear regression shows that torque (Nm) can be predicted from the following equation: torque (Nm) = 3.74 × stimulated CSA (cm2) − 14.37 (r 2=0.93, P<0.05). All subjects included, n = 16
Discussion
The primary finding of this study was that long-term SCI patients experienced increased muscle damage, as determined by MRI, compared to that of able-bodied controls following a single session of electrically-evoked isometric contractions. The unloading and long-term inactivity of the mQF in SCI patients resulted in an increased susceptibility to contraction induced muscle injury compared to able-bodied controls. There was a greater relative area of muscle with increased T2 relaxation times in the SCI patients than AB controls (25% versus 2% of activated muscle) 3 days after 80 isometric contractions. The increased muscle damage also resulted in compromised torque output on day 3 in the SCI subjects. These findings support the hypothesis that the mQF of SCI patients has an increased potential for contraction-induced muscle damage.
These results may help to explain the incomplete torque recovery during EMS that we have observed in our laboratory. In our previous study of chronic SCI patients, incomplete recovery was noted between sets and for at least 60 min after surface EMS, which was probably due to muscle fiber injury (Hillegass and Dudley 1999). We found similar torque declines in a study of SCI patients who had been injured for 6 months and it was concluded that muscle fiber injury might have contributed to their torque declines during isometric actions (Castro et al. 1999b). This present study further supports the notion that SCI muscle can be injured after isometric contractions and incomplete recovery between sets may be indicative of muscle damage in this population (Fig. 1). Previous studies utilizing dynamic actions in AB subjects do suggest that there can be evidence of fiber disruption immediately following exercise (Gibala et al. 1995).
Was the increased muscle damage found in this study due to the greater relative amount of muscle activated in the SCI patients? The answer is “no”. Adams et al. (1993) report that the increased force produced by increasing the amplitude of stimulation is due to a greater amount of muscle mass stimulated. Percutaneous electrical stimulation activates muscle in a synchronous pattern and the stimulus on each motor unit is dependent only on the stimulation parameters. Thus, it should be noted that although the SCI patients had a relatively greater amount of the available muscle stimulated, this was still much less absolute muscle than the AB subjects. Neither of which complicate our findings, as each group received the same stimulus on activated motor units (for example 50 Hz, 2 s on/5 s off). If we were to have activated more muscle in the AB group, we would expect there to be no more or less relative damage.
High-force eccentric actions are not necessary to evoke fiber injury in muscles that have been subjected to unloading (Ploutz-Snyder et al. 1996; Warren et al. 1994). Warren et al.(1994) showed that just 15 tetanic isometric contractions after, but not before, hindlimb suspension in the soleus muscle of a mouse was sufficient to evoke force loss. Others have shown elevated plasma creatine kinase (CK) in human SCI patients after a single bout of functional electrical stimulation leg cycling (Robergs et al. 1993). While plasma CK is not a direct marker of muscle injury, it has been repeatedly shown that elevated CK levels provide support that muscle fibers have been injured (Clarkson and Newham 1995; Clarkson et al. 1992). It is well documented that eccentric actions can cause extensive contraction induced muscle injury and muscle atrophy (10% reported by Foley et al. 1999) in the able-bodied population. Isometric actions were used in this study due to uncertainty of the extent of damage that might have been caused with eccentric actions. In this study, paralyzed muscle was indeed more vulnerable to injury. Consistent with the current literature on muscle injury following eccentric exercise, we would expect even more damage with isotonic exercise. Preliminary observations from this laboratory suggested that isometric actions would cause damage yet be minor enough that it would be repaired within a few weeks (C.S. Bickel et al., unpublished observations). In fact, isometric exercise has been shown to cause small increases in plasma CK levels and muscle soreness in some able-bodied subjects (Triffletti et al. 1988).
Some researchers have suggested that the unloading alone could cause muscle damage, while others argue it is the activity after the unloading that causes the injury to muscle. In this study the muscle contractions alone caused the muscle injury. These patients were 10 years post SCI, time enough for the atrophic response to reach the lowest point. Cross-sectional studies of SCI patients have shown that the affected muscles may atrophy for up to 17 months after injury (Scelsi et al. 1982). Our study of acute patients showed muscle CSA of the mQF to decline the most (~16%) from 6 to 24 weeks of injury and only a 3% decline from 24 to 46 weeks, thereafter (Castro et al. 1999a; Dudley et al. 1999). Although it should be noted that at 6 weeks post injury the SCI mQF is already ~30% smaller than age, height, and weight matched controls (Castro et al. 1999a). Furthermore, short-term bedrest and ULLS do not alter the MR image contrast of muscle, while activities that cause muscle injury do (LeBlanc et al. 1987).
MRI has proven to be an excellent tool for assessing the extent of a damaging bout of exercise on skeletal muscle. This laboratory, and others have shown that increases in T2 signal 2–3 days after exercise are indicative of muscle injury in both young and old subjects (Baldwin et al. 2001; Ploutz-Snyder et al. 1996). To our knowledge this is the first study to document contraction-induced muscle injury with MRI in SCI patients who are years after SCI. To further support our MRI data, torque was also reduced by 22% 3 days after the contractions in the SCI subjects with essentially no change in MVC of AB subjects at the same time point. As expected the muscle damage resulted in compromised performance several days post activity. Taking together the higher percentage CSA with elevated T2 and torque reductions on day 3 after EMS in the SCI group compared to AB controls, it is reasonable to conclude there was indeed muscle injury.
The clinical and practical significance of these findings are important. Skeletal muscle atrophy is a significant problem associated with SCI and is primarily responsible for limiting oxygen consumption during FES exercise (Hopman et al. 1998). This might suggest that in order to establish an FES exercise program to evoke significant exercise responses that stress the cardiovascular system, the muscle needs to be made larger. Muscle hypertrophy is generally evoked through relatively few (<100/week) high-force dynamic (concentric/eccentric) contractions. These types of contractions will cause muscle injury even to the untrained AB individual who begins a resistance training program. Thus, time for recovery and attention to proper progression is important when training SCI patients, as we have shown that even isometric actions evoke substantial damage to them, but not untrained AB people.
In summary, isometric exercise evoked significantly greater muscle injury in SCI subjects compared to AB controls as reflected from MRI and torque measurements. This was probably related to the chronic unloading and inactivity that these patients experience. These results also support previous conclusions that contraction-induced muscle injury may have contributed to decrements in force production of both chronic and acute SCI patients. Clinicians should proceed with caution when developing and implementing EMS training programs whose goal is to evoke muscle hypertrophy.
References
Adams GR, Duvoisin MR, Dudley GA (1992) Magnetic resonance imaging and electromyography as indexes of muscle function. J Appl Physiol 73:1578–1583
Adams GR, Harris RT, Woodard D, Dudley GA (1993) Mapping of electrical muscle stimulation using MRI. J Appl Physiol 74:532–537
Akima H, Ito M, Yoshikawa H, Fukunaga T (2000) Recruitment plasticity of neuromuscular compartments in exercised tibialis anterior using echo-planar magnetic resonance imaging in humans. Neurosci Lett 296:133–136
Appell HJ (1986) Morphology of immobilized skeletal muscle and the effects of a pre- and postimmobilization training program. Int J Sports Med 7:6–12
Baldwin AC, Stevenson SW, Dudley GA (2001) Nonsteroidal anti-inflammatory therapy after eccentric exercise in healthy older individuals. J Gerontol A Biol Sci Med Sci 56:M510–513
Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA (1999a) Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 80:373–378
Castro MJ, Apple DF Jr, Staron RS, Campos GE, Dudley GA (1999b) Influence of complete spinal cord injury on skeletal muscle within 6 months of injury. J Appl Physiol 86:350–358
Castro MJ, Apple DF Jr, Rogers S, Dudley GA (2000) Influence of complete spinal cord injury on skeletal muscle mechanics within the first 6 months of injury. Eur J Appl Physiol 81:128–131
Clarkson PM, Newham DJ (1995) Associations between muscle soreness, damage, and fatigue. Adv Exp Med Biol 384:457–469
Clarkson PM, Nosaka K, Braun B (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24:512–520
Conley MS, Meyer RA, Bloomberg JJ, Feeback DL, Dudley GA (1995) Noninvasive analysis of human neck muscle function. Spine 20:2505–2512
Dudley GA, Czerkawski J, Meinrod A, Gillis G, Baldwin A, Scarpone M (1997) Efficacy of naproxen sodium for exercise-induced dysfunction muscle injury and soreness. Clin J Sport Med 7:3–10
Dudley GA, Castro MJ, Rogers S, Apple DF Jr (1999) A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80:394–396
Fisher MJ, Meyer RA, Adams GR, Foley JM, Potchen EJ (1990) Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Invest Radiol 25:480–485
Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA (1999) MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol 87:2311–2318
Gibala MJ, MacDougall JD, Tarnopolsky MA, Stauber WT, Elorriaga A (1995) Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J Appl Physiol 78:702–708
Grimby G, Broberg C, Krotkiewska I, Krotkiewski M (1976) Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med 8:37–42
Hather BM, Adams GR, Tesch PA, Dudley GA (1992) Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol 72:1493–1498
Hillegass EA, Dudley GA (1999) Surface electrical stimulation of skeletal muscle after spinal cord injury. Spinal Cord 37:251–257
Hopman MT, Dueck C, Monroe M, Philips WT, Skinner JS (1998) Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med 19:98–103
Krippendorf BB, Riley DA (1993) Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve 16:99–108
Krippendorf BB, Riley DA (1994) Temporal changes in sarcomere lesions of rat adductor longus muscles during hindlimb reloading. Anat Rec 238:304–310
LeBlanc A, Evans H, Schonfeld E, Ford J, Schneider V, Jhingran S, Johnson P (1987) Changes in nuclear magnetic resonance (T2) relaxation of limb tissue with bed rest. Magn Reson Med 4:487–492
Mair J, Koller A, Artner-Dworzak E, Haid C, Wicke K, Judmaier W, Puschendorf B (1992) Effects of exercise on plasma myosin heavy chain fragments and MRI of skeletal muscle. J Appl Physiol 72:656–663
Martin TP, Stein RB, Hoeppner PH, Reid DC (1992) Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol 72:1401–1406
Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P (1989) Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59:310–319
Ploutz-Snyder LL, Tesch PA, Hather BM, Dudley GA (1996) Vulnerability to dysfunction and muscle injury after unloading. Arch Phys Med Rehabil 77:773–777
Ploutz-Snyder LL, Nyren S, Cooper TG, Potchen EJ, Meyer RA (1997) Different effects of exercise and edema on T2 relaxation in skeletal muscle. Magn Reson Med 37:676–682
Ploutz-Snyder LL, Tesch PA, Dudley GA (1998) Increased vulnerability to eccentric exercise-induced dysfunction and muscle injury after concentric training. Arch Phys Med Rehabil 79:58–61
Prior BM, Jayaraman RC, Reid RW, Cooper TG, Foley JM, Dudley GA, Meyer RA (2001) Biarticular and monoarticular muscle activation and injury in human quadriceps muscle. Eur J Appl Physiol 85:185–190
Riley DA, Thompson JL, Krippendorf BB, Slocum GR (1995) Review of spaceflight and hindlimb suspension unloading induced sarcomere damage and repair. Basic Appl Myol 5:139–145
Robergs RA, Appenzeller O, Qualls C, Aisenbrey J, Krauss J, Kopriva L, DePaepe J (1993) Increased endothelin and creatine kinase after electrical stimulation of paraplegic muscle. J Appl Physiol 75:2400–2405
Rochester L, Chandler CS, Johnson MA, Sutton RA, Miller S (1995) Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 1. Contractile properties. Paraplegia 33:437–449
Round JM, Barr FM, Moffat B, Jones DA (1993) Fibre areas and histochemical fibre types in the quadriceps muscle of paraplegic subjects. J Neurol Sci 116:207–211
Scelsi R, Marchetti C, Poggi P, Lotta S, Lommi G (1982) Muscle fiber type morphology and distribution in paraplegic patients with traumatic cord lesion. Histochemical and ultrastructural aspects of rectus femoris muscle. Acta Neuropathol (Berl) 57:243–248
Shellock FG, Fukunaga T, Mink JH, Edgerton VR (1991a) Acute effects of exercise on MR imaging of skeletal muscle: concentric versus eccentric actions. AJR Am J Roentgenol 156:765–768
Shellock FG, Fukunaga T, Mink JH, Edgerton VR (1991b) Exertional muscle injury: evaluation of concentric versus eccentric actions with serial MR imaging. Radiology 179:659–664
Stevenson SW, Dudley GA (2001) Dietary creatine supplementation and muscular adaptation to resistive overload. Med Sci Sports Exerc 33:1304–1310
Takahashi H, Kuno S, Miyamoto T, Yoshioka H, Inaki M, Akima H, Katsuta S, Anno I, Itai Y (1994) Changes in magnetic resonance images in human skeletal muscle after eccentric exercise. Eur J Appl Physiol Occup Physiol 69:408–413
Triffletti P, Litchfield PE, Clarkson PM, Byrnes WC (1988) Creatine kinase and muscle soreness after repeated isometric exercise. Med Sci Sports Exerc 20:242–248
Warren GL, Hayes DA, Lowe DA, Williams JH, Armstrong RB (1994) Eccentric contraction-induced injury in normal and hindlimb-suspended mouse soleus and EDL muscles. J Appl Physiol 77:1421–1430
Acknowledgements
The authors would like to thank the subjects for their participation in this study. We also appreciate Chris Elder (UGA) and Carolyn Sharp (SC) for their technical expertise and Gordon Warren, Ph.D. for critical review of the initial draft of this manuscript. Funding was provided, in part, by the Shepherd Center (J.M.S., C.S.B., and G.A.D.), the Foundation for Physical Therapy (CSB) and the National Institutes of Health (HD37439-S1 and HD 39676 to G.A.D.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bickel, C.S., Slade, J.M. & Dudley, G.A. Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol 91, 308–313 (2004). https://doi.org/10.1007/s00421-003-0973-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-0973-5