Abstract
Local muscle fatigue can originate from both peripheral and central factors. The relative contribution of these factors in the course of a fatiguing contraction in 20 healthy subjects was determined. While subjects made a 2-min sustained maximal voluntary contraction (MVC) of the biceps brachii, muscle fibre conduction velocity (MFCV) was determined with surface electromyography (SEMG) as a representation of developing peripheral fatigue. To quantify the amount of peripheral fatigue, the force development following a train of electrical stimuli on the endplate before and after the contraction were compared. To measure force loss caused by central factors, superimposed electrical stimulation was used during the contraction. By two different methods the influence of peripheral fatigue on the superimposed force responses was taken into account. The first method compared the force response with the actual voluntary force, the second –which seemed more valid – used an estimation of peripheral fatigue based on linear interpolation between the force responses during rest before and after sustained contraction. During the contraction, voluntary force decreased to 38%. Peripheral fatigue was responsible for the larger part of this decline (89%). The other part, which was calculated as 12%, was caused by an increase of central activation failure. The decline of MFCV indicated that peripheral fatigue increased predominantly during the first half of the contraction and stayed at a constant level during the latter part. In contrast, central fatigue mainly induced a force decrease in the second part of the contraction. The different mechanisms which could be responsible for this change of emphasis from peripheral to central factors are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Local muscle fatigue is mostly defined as a reduction of the maximal force generating capacity of a muscle induced by exercise (e.g. Gandevia 2001). Peripheral sites of the origin of fatigue include the neuromuscular junction, the sarcolemma and the contractile apparatus, while mechanisms involved may be excitation–contraction coupling, accumulation of metabolites and depletion of fuels (e.g. Kirkendall 1990).
Quantification of peripheral fatigue after a sustained fatiguing contraction has been done by studying the response of a relaxed muscle upon electrical stimulation (e.g. Thomas et al. 1989; Gandevia et al. 1996; Kent-Braun 1999). With the same technique, the development of peripheral fatigue has been examined during periods of relaxation in repetitive contractions (Lloyd et al. 1991).
The appearance of peripheral fatigue during a contraction has mostly been studied by electromyography (EMG). Median or mean power spectrum frequencies were shown to decrease during a sustained maximal voluntary contraction (MVC) (Kranz et al. 1985; Moritani et al. 1986). Zwarts and Arendt-Nielsen (1988) showed that muscle fibre conduction velocity (MFCV) decreased during maximal effort, which contributes to this decline of power frequency (Stegeman and Linssen 1992). The decline of MFCV reflects the accumulation of metabolic byproducts (De Luca 1984; Linssen et al. 1990; Brody et al. 1991; Masuda et al. 1999) and thus can be used as a measure of peripheral fatigue. Thomas et al. (1989) showed that changes in M-wave parameters were not sufficient to detect peripheral fatigue. The reason might be that M-wave duration is an indirect rather than direct measure of conduction velocity. Magnetic resonance spectroscopy has the ability to measure the metabolic situation of the muscle during exercise more directly (Kent-Braun 1999; Houtman et al. 2001).
Besides peripheral factors, a failure of drive from the central nervous system also may contribute to the loss of force induced by exercise (e.g. Kent-Braun and LeBlanc 1996; Gandevia et al. 1996, 1998; Kent-Braun 1999). This central fatigue can have its origin in all structures above the neuromuscular junction (central nervous system and peripheral nerves). It might result from a combination of intrinsic motoneuronal properties, reflex inhibition and disfacilitation, Renshaw cell inhibition, and insufficient drive from supraspinal sites (Gandevia 1998), for example because of a lack of subject motivation (Allen et al. 1995).
Only a few methods have been developed to detect the failure of central drive. Gandevia et al. (1996) showed that central fatigue occurred during prolonged MVC using transcranial magnetic stimulation. However, the non-specificity of this technique complicates quantification. Several authors have therefore used electrical stimulation of motor nerves or motor endplate regions during voluntary contractions (Merton 1954; Bigland-Ritchie et al. 1983; Lloyd et al. 1991; Allen et al. 1995; Gandevia et al. 1996; Kent-Braun and LeBlanc 1996; Gandevia et al. 1998; Kent-Braun 1999). If electrical stimulation resulted in a force response, voluntary activation apparently was less than maximal.
The majority of these studies determined the amount of central fatigue during short MVC or only at the beginning and at the end of sustained MVC (Lloyd et al. 1991; Allen et al. 1995; Kent-Braun and LeBlanc 1996; Gandevia et al. 1998; Kent-Braun 1999). As far as we know, the change of central activation in the course of a sustained contraction has only been studied by Bigland-Ritchie et al. (1983), Thomas et al. (1989) and by Gandevia et al. (1996). The first study did not find any central activation failure (CAF) in adductor pollicis. The second showed that at the beginning of a 5-min sustained MVC of tibialis anterior and first dorsal interosseus muscles central activation was kept optimal, but substantial failure occurred during the latter 3 min. Gandevia's group was the only one that showed the development of central activation failure (CAF) during a sustained maximal contraction in biceps brachii (Gandevia et al. 1996). They presented the superimposed force responses as a percentage of the voluntary force. This method did provide good insight into the development of central fatigue, but did not allow quantification of force loss caused by CAF.
Only a few studies exist investigating the course of both central and peripheral fatigue simultaneously during an isometric sustained MVC. Thomas et al. (1989) did measure both fatigue modalities, but admit that the use of superimposed M-waves does not sufficiently expose the peripheral contribution. Their second technique, electrical stimulation during short interruptions while cuffs preserved the muscle in an ischemic condition, did provide a better measure of peripheral fatigue. These interruptions were made with 1-min intervals. Quantification of peripheral fatigue with a higher time resolution could be valuable.
Quantification of the contributions of both fatigue modalities during maximal effort can be of significance in a clinical setting: many neuromuscular patients complain about fatigue. Knowledge concerning the factors responsible for fatigue in healthy controls could contribute to the development of tools for a better understanding of the phenomena that underlie the various fatigue complaints that are as yet difficult to grasp.
Accordingly, the goal of this study was to find the relative contributions of central and peripheral factors to fatigue during a maximal sustained 2-min voluntary contraction in healthy subjects using electrical stimulation and MFCV measurements simultaneously. The contribution of central factors was calculated by two different methods, whose advantages and disadvantages are discussed.
Methods
Subjects
Twenty (13 male, 7 female) healthy subjects, ranging in age from 19 to 53 years [mean 29.4 (9.2) (SD) years], participated in this experiment. None of these volunteers had a history of neuromuscular problems. In 18 of them surface electromyography (SEMG) measurements were made (11 men, 7 women). The protocol was approved by the local ethics committee. All subjects gave their informed consent.
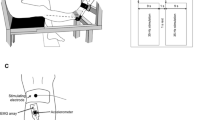
Experimental set-up
Force recording
Subjects sat in a chair, their left arm fixed in an arm flexor dynamometer in the horizontal position with the shoulder in abduction, the elbow in a right angle and the forearm supinated. The trunk was stabilized at the thoracic level. Using strain gauges, the force of elbow flexion was measured at the wrist. Force was sampled at a rate of 2 kHz using an A/D board (Keithly Metrabyte: DAS 1602), low-pass filtered (1 kHz) and stored on hard disk of a standard PC for off-line analysis. The maximal resolution of force measurement was 0.1 N bit−1.
SEMG recording
SEMG measurement was performed with a multi electrode array consisting of five electrodes that were gold coated and placed in line (electrode diameter 2 mm; inter electrode distance 3 mm). The electrode array was placed parallel to the fibre direction of the biceps brachii muscle, distal to the motor points after the skin had been scrubbed lightly and rubbed with electrode cream. A reference electrode was placed at the elbow joint. To investigate the accuracy of the SEMG electrodes placement, cross correlations between the bipolar signals were determined on-line during slight contraction.
Five monopolar signals were amplified, band-pass filtered (3.2–800 Hz) and A/D-converted (16 bits with a resolution of 0.5 μV bit−1 at a rate of 4 kHz/channel) using a 64-channel amplifier system (MARK 6, Biosemi, Amsterdam, The Netherlands). Data were stored on the hard disk of a standard PC for off-line analysis (see below).
A custom-made time code generator was connected between the PC used for force recordings and the one used for SEMG recordings in order to synchronize force and SEMG data.
Electrical stimulation
Electrical endplate stimulation was applied with a general purpose electrical bio-stimulator, designed and manufactured by the local Department of Technical Engineering, via a self-adhesive 93×8 mm (usually shortened to avoid co-stimulation of other muscles) cathode over both motor points of the medial and lateral head of the biceps brachii and an anode (∅ 32 mm) (Teca NCS electrode system 2000) at the proximal muscle belly.
As the basic "unit of stimulation" a 40-ms stimulus train (ST) of five rectangular pulses (each pulse had a duration of 100 μs), with 10-ms intervals (100 Hz) was used (Fig. 1). Five STs were combined to form a stimulus event (SE; inter-train interval 300 ms). So, a SE lasted 1240 ms, leading to five subsequent force responses, which were averaged. This average is referred to as the force response. Stimulated force during rest (see below) was derived from a SE with an inter-train interval of 1 s. From pilot experiments it was concluded that these stimulus characteristics were appropriate for detecting central fatigue without being too uncomfortable with reference to future use in large numbers of patients.
Definition of force parameters. Vertical arrows represent electrical stimulation. (Fs i Initial force response, Fs t superimposed force response during MVC after t s sustained contraction, Fs f final force response, F 0 voluntary force at the beginning of sustained contraction, F t voluntary force after t s sustained contraction, SE stimulation event)
Before the experiment, for every subject stimulus optimization was performed by slowly increasing the intensity, until no further increase of force response resulted from the increase of intensity. All SEs were given at this intensity level (23–92 mA).
Special attention was given to prevent possible co-stimulation of adjacent muscles. If co-stimulation was present, identified by palpating the muscle (triceps or deltoid), the anode was repositioned.
Protocol
While the subject's biceps brachii muscle was relaxed an initial SE was applied (Fig. 1), which caused the initial force response. Then, the subject performed a sustained 2-min MVC of the biceps brachii muscle. SEs were given every 15 s, leading to superimposed force responses. Loud verbal encouragement was given throughout the 2-min maximal effort and force was visually fed back to the subject. Immediately after the sustained contraction, a final SE was applied, resulting in the final force response.
Data analysis
Force
To be able to use superimposed force amplitude as a measure of central fatigue, the amplitude has to be corrected for the peripherally induced force decline. Therefore, the size of the superimposed force during a 2-min sustained contraction was expressed in two different ways:
- Method 1.:
-
Relative to the actual voluntary force. This actual voluntary force was the force averaged from 2-s recordings, measured just before the SE. In this method:
where CAF_1 t is the central activation failure at t s of sustained contraction; Fs t is the amplitude of the superimposed force response during voluntary contraction, which was corrected for changes of voluntary force via linear interpolation between the moment of stimulation and 300 ms after; Fsi is the amplitude of the initial force response (while the arm was relaxed); F t is the actual voluntary force and F 0 is the voluntary force at the beginning of sustained contraction (see Fig. 1).
- Method 2.:
-
As a percentage of an estimated, "expected" stimulated force, obtained by linear interpolation between the amplitudes of the initial and final force responses, thus:
where Fs f is the amplitude of the final force response (while the arm was relaxed; see Fig. 1).
To analyse changes in CAF over time, for each subject linear regression was performed. Then, a Student's t-test was used to test if the average slope of all subjects' regression lines was larger than zero.
From the initial and final force responses, duration was defined as the period for which the force was higher than half maximum. Also maximal contraction and relaxation rates were determined, expressed as the amount of force maximally gained or lost per millisecond (relatively to the force response amplitude). A paired t-test was used to compare initial and final force response parameters.
One subject was excluded from force analysis, because his elbow was not properly fixed in the dynamometer and superimposed force responses deviated more than three standard deviations from the mean responses of all subjects.
SEMG
SEMG values used were calculated from 2 s at the very beginning of exercise and from 2 s of data just before stimulation. Cross correlations from two bipolar signals (inter-signal distance 6 mm) were determined according to Naeije and Zorn (1983). The combination of electrodes showing the highest cross correlation was chosen for further analysis, but only if cross correlation exceeded 0.7. MFCV was calculated from the phase difference spectrum of the two bipolar signals, as described by Linssen et al. (1993). This method determines a linear least-squares fit to the relation between frequency and the phase difference between the two bipolar signals. From this fit the time delay between these signals can be calculated. Relating this delay to the inter-signal distance leads to a MFCV value.
The upper limit of MFCV values was set at 8 m s−1, based on physiological limits. Three subjects were excluded from SEMG analysis since their MFCVs exceeded this value at least three times. Besides, only one measurement showed such a high MFCV value.
Significance
For all analyses, an observation was regarded significant when P≤0.05.
Results
Force
Figure 2 shows a typical example of both voluntary and stimulated forces. For the whole group of subjects, during the 2 min of sustained MVC, voluntary force declined significantly from 214.2 (80.1 SD) N to 79.6 (29.8) N [38.2 (7.8)% of initial MVC] (Fig. 3A). The decrease of the voluntary force in the first minute exceeded that in the second minute.
Typical force profile of a 2-min sustained maximal voluntary contraction in which each circle represents the start of a stimulus event (A). Accompanying stimulated and superimposed forces (B). The upper and lower panels show the stimulated force before and after voluntary contraction respectively. The middle panel shows superimposed forces during sustained contraction, which have been corrected for changes of voluntary force via linear interpolation between the moment of stimulation and 300 ms after
Voluntary force decline, increase of central activation failure (CAF) and decrease of muscle fibre conduction velocity (MFCV) during a 2-min sustained maximal voluntary contraction. Force is represented relatively to MVC values (A). CAF is expressed according to two different methods: as the size of the superimposed force responses relatively to the voluntary force just before stimulation (CAF_1; closed squares), and as the sizes of the superimposed force responses relative to the estimated maximal force response obtained via linear interpolation between the initial and final force response at rest (CAF_2; open squares) (B). MFCV clearly decreases during the first 60 s, but levels off in the last period of contraction (C). All values are means (SEM). For voluntary force and CAF n=19, for MFCV n=16
Force responses decreased significantly from 14.8 (5.9) N before, to 6.8 (3.5) N [45.0 (15.4)% of the initial stimulated force] after sustained MVC, so from that perspective peripheral fatigue was concluded to be responsible for a force decrease to 45.0 (15.4)%.
The initial and final force responses showed a significant increase of duration from 125.1 (9.6) ms to 211.2 (40.8) ms [169.1 (31.7)% of initial duration], caused by both significantly reduced maximal contraction [from 1.03 (0.21)% ms−1 to 0.81 (0.12)% ms−1] and relaxation rates [from 0.60 (0.11)% ms−1 to 0.35 (0.07)% ms−1].
CAF showed a significant increase during 2-min sustained MVC. This was most obvious when CAF was expressed by the first method (Fig. 3B). Calculated via this method, CAF_1 was found to increase from 18.1 (15.2)% after 15 s to 39.8 (39.9)% after a 2-min sustained contraction. When CAF was expressed by the second method (CAF_2) values from 16.9 (13.6)% to 29.0 (21.1)% were found respectively.
It should be noted that values of CAF revealed much spread between subjects. While some subjects showed a clear increase, others showed no increase or even a decrease of CAF.
SEMG
MFCV showed a mean decline from 5.0 (0.9) m s−1 directly after the start of a 2-min MVC to 2.9 (1.0) m s−1 at the end [59.9 (15.9)% of the initial value]. This decline was fully realized during the first minute, while MFCV stayed constant during the last minute of contraction (Fig. 3C).
Discussion
The present study has shown that peripheral and central fatigue do not change in parallel in a sustained 2-min MVC of the biceps brachii. During the first minute the decline of voluntary force can be explained almost exclusively by peripheral factors. After about 1 min, however, peripheral fatigue levels off. Then, the further decrease of voluntary force can almost totally be attributed to central fatigue.
Our method does not distinguish between force produced by the biceps brachii and that produced by other elbow flexors. Although the forearm was supinated, which favours the use of biceps brachii, additional activity of other muscles cannot be excluded. For the quantification of central fatigue only biceps brachii has been stimulated. This is supported by Allen et al. (1995), who reported no differences in the amount of central fatigue between studies stimulating just the biceps brachii and studies additionally stimulating brachioradialis.
Loss of voluntary force
The influence of both fatigue modalities resulted in an MVC after a 2-min sustained contraction which was 38.2% of the initial value. The absolute amount of force loss was largest during the first minute. The force curve's shape was similar to that described by Gandevia et al. (1996), who also investigated 2-min sustained MVC of the biceps brachii, and those of others describing different periods of sustained MVC of the same muscle (Moritani et al. 1986; Zwarts and Arendt-Nielsen 1988).
Peripheral fatigue
In this study, force responses resulted from trains of five stimuli. Therefore, force responses were intermediate between twitch and tetanic force responses. The choice of this stimulus protocol was based on a pilot study which showed that the force elicited by five stimuli was more than twice as high as a twitch response and more than half the response to a train of 20 stimuli. We therefore considered the behaviour of force responses to be comparable to tetanic contractions, while they were not so uncomfortable that they influenced subjects' voluntary performance.
The significant difference in amplitudes of the force responses before and after the fatiguing contractions in our experiment suggests a large peripheral contribution to fatigue: peripheral fatigue accounted for a force loss to 45.0%, compared to a total voluntary force loss to 38.2%. This implies that 89.0% [=(100–45.0)/(100–38.2)] of the force loss can be accredited to peripheral changes. The changes in the contractile properties and the metabolic environment are also expressed by the increase of duration of the stimulated forces, the decreases of the rates of contraction and relaxation and the slowing down of muscle fibre conduction during sustained MVC.
A large contribution of peripheral factors was also found by Gandevia et al. (1996) after a 3-min sustained MVC, who reported that voluntary force decreased to 25.9%, while stimulated force decreased to 29.5%. For the ankle dorsiflexors, Kent-Braun (1999) reported that about 80% of the loss of voluntary force during a 4-min sustained contraction could be attributed to peripheral factors.
Assuming that the changes in MFCV reflect the metabolic situation of the muscle (De Luca 1984; Linssen et al. 1990; Brody et al. 1991; Masuda et al. 1999), it is evident from our data that this strongly worsens in the first part of the exercise. In the last part of the contraction no further changes of the intra-muscular situation occurs. Using magnetic resonance spectroscopy, Kent-Braun (1999) showed the same pattern for changes in metabolites for the ankle dorsiflexors. In the literature, at the end of a 65-s sustained MVC of the biceps brachii an increase of MFCV has been shown (Zwarts and Arendt-Nielsen 1988). Experimental evidence indicated that this was because the declining force allows partial restoration of the blood flow.
One could argue that changes of MFCV may be caused by changes in the motor unit recruitment pattern (e.g. by derecruiting the fast conducting type II fibres), which has a central origin. However, based on Zwarts et al. (1988), it does not seem probable that the MFCV decline caused by central factors exceeds 0.5 m s−1, where we found a total decline of 2.1 m s−1. Besides, the larger part of the decline was found in the first minute of the sustained contraction, where no central activation changes were seen. Thus, peripheral factors lead to a decrease of voluntary force especially in the first minute of contraction. Then, the decline of voluntary force continues although peripheral components stabilize. This ongoing loss of voluntary force can be explained by the increased CAF during this period (see Fig. 3B and below).
Central fatigue
The present study is the first to report the amount of force loss in biceps brachii because of central activation failure during a sustained contraction. Most earlier work was limited to values at the beginning and end of a sustained contraction (Kent-Braun and LeBlanc 1996; Kent-Braun 1999). Gandevia et al. (1996) did present a study with data measured during the contraction, but their method of data presentation did not easily allow interpretation in terms of the amount of force lost. Thomas et al. (1989) showed the amount of CAF in tibialis anterior and first dorsal interosseus muscles during long sustained maximal voluntary contractions.
The earlier studies showed smaller amounts of central fatigue. Since central activation is known to differ between muscles, only a true comparison can be made with other studies investigating biceps brachii. Investigating five healthy subjects during short maximal contractions in several sessions, Allen et al. (1995) presented CAFs of the same muscle between 0 and 22%. Gandevia et al. (1996), who also studied sustained maximal contraction of biceps brachii, found mean values of 0.7% at the beginning and 9.3% after 3 min of sustained MVC in six healthy subjects. Why these values are smaller than the ones showed in the present study is unclear. However, because inter-subject differences are large, confidence intervals still show overlap.
Using superimposed force responses to estimate the amount of failure of central drive during the fatiguing contraction was complicated by the fact that peripheral fatigue caused a decrease of the maximally possible force response. This means that "the ruler to measure the central fatigue changed length during the contraction". To compensate for this disturbing influence of peripheral fatigue, we normalized the superimposed force response: (1) by comparing it with the actual voluntary force and (2) by comparing it with the estimated linearly interpolated stimulated force.
Both methods resulted in a significant increase of CAF during sustained MVC, especially in the last minute (Fig. 3B). During the first minute, central activation was submaximal, but only peripheral fatigue contributed to the actual decline of voluntary force. In the last minute, central fatigue was responsible for a further decrease of MVC. It is interesting to discuss the possible advantages and disadvantages of the two different methods used to calculate CAF.
In the first method, which was used by Thomas et al. (1989), a superimposed force response is compared with the actual voluntary force left in spite of the fatigue that has already occurred. However, both peripheral and central factors have influenced this voluntary force. Therefore, the correction made in this way is not purely a correction for force loss caused by peripheral factors, but is also influenced by CAF itself. This means that the first method will result in an over-estimation of CAF. The size of this over-estimation increases with increasing CAF.
The second method, which uses linear interpolation between the initial and final force responses, avoids this problem. Interpolation allows us to estimate force responses which are only influenced by peripheral fatigue and which would have been obtained at an arbitrary moment during the fatiguing contraction. However, as concluded above from MFCV changes, in fact peripheral fatigue appears not to increase linearly throughout the 2-min sustained contraction. Therefore, apart from the moments at the beginning and at the end of contraction, CAF_2 most probably uses an underestimation of peripheral fatigue during the whole contraction. This means that the force response superimposed on MVC is being compared to an estimated force response that is higher than it actually should be, leading to an underestimation of CAF_2. This underestimation must be highest after about 1 min of contraction, while then the difference between the peripheral fatigue estimated by linear interpolation and the actual peripheral fatigue seems largest. However, especially at the beginning and at the end of contraction the error of CAF_2 will be small, while the error of CAF_1 increases over time.
In conclusion, expressing CAF by the second method appears to give the most valid measure of central fatigue during a sustained MVC. Using this method, it was found that after 15 s of sustained MVC CAF was 16.9 (13.6)%, which increased by 12.1% to 29.0 (21.1)% at the end of the contraction. Together with the peripheral fatigue which already accounted for 89.0%, this 12.1% increase of CAF clearly explained the total loss of force during a 2-min sustained contraction.
As already mentioned above, in the second method of calculating CAF it was assumed that peripheral fatigue would develop linearly. However, from MFCV measurements it was concluded that the course of peripheral fatigue was more complex. Therefore, we are currently investigating whether MFCV can be used directly to quantify the amount of peripheral fatigue during a sustained contraction. Then, central fatigue could be estimated more precisely.
Conclusions
Expressing CAF by comparing the actual superimposed force response with an estimated force response only influenced by peripheral factors gives the most reliable quantification of central fatigue. In this way, we were able to determine the relative contributions of peripheral and central factors to fatigue during a sustained contraction. It can be concluded that in healthy subjects peripheral fatigue dominates, but central factors become more prominent, during longer sustained isometric contractions. This sequence makes sense, because in the first part of the contraction the output of the muscle is highest, combined with a high metabolic demand and occluded blood flow, resulting in maximal demands on the muscle. In the second half of the contraction, it seems that the continuous and repetitive firing of neurons accompanying the central command is increasingly difficult to maintain. The precise neurological level(s) at which this CAF occurs has still to be determined. In this respect it would be interesting to see how this interplay between central and peripheral factors ensues in different types of contraction. Future studies including different patient populations may reveal the impact of both aspects of fatigue in several diseases.
References
Allen GM, Gandevia SC, McKenzie DK (1995) Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 18:593–600
Bigland-Ritchie B, Johansson R, Lippold OCJ, Smith S, Woods JJ (1983) Changes in motoneurone firing rates during sustained maximal voluntary contractions. J Physiol (Lond) 340:335–346
Brody LR, Pollock MT, Roy SH, De Luca CJ, Celli B (1991) pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J Appl Physiol Nov 71(5):1878–1885
De Luca CJ (1984) Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng 11(4):251–279
Gandevia SC (1998) Neural control in human muscle fatigue: changes in muscle afferents, motoneurones and motor cortical drive. Acta Physiol Scand 162:275–283
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789
Gandevia SC, Allen GM, Butler JE, Taylor JL (1996) Supraspinal factors in human muscle fatigue: evidence for a suboptimal output from the motor cortex. J Physiol (Lond) 490:529–536
Gandevia SC, Herbert RDo, Leeper JB (1998) Voluntary activation of human elbow flexor muscles during maximal concentric contractions. J Physiol (Lond) 512:595–602
Houtman CJ, Heerschap A, Zwarts MJ, Stegeman DF (2001) pH heterogeneity in tibial anterior muscle during isometric activity studied by (31)P-NMR spectroscopy. J Appl Physiol 91(1):191–200
Kent-Braun JA (1999) Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol 80:57–63
Kent-Braun JA, LeBlanc R (1996) Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 19:861–869
Kirkendall DT (1990) Mechanisms of peripheral fatigue. Med Sci Sports Exerc 22:444–449
Kranz H, Cassell JF, Inbar GF (1985) Relation between electromyogram and force in fatigue. J Appl Physiol 59(3):821–825
Linssen WHJP, Jacobs M, Stegeman DF, Joosten EMG, Moleman J (1990) Muscle fatigue in McArdle's disease. Brain 113:1779–1793
Linssen WHJP, Stegeman DF, Joosten EMG, van 't Hof MA, Binkhorst RA, Notermans SLH (1993) Variability and interrelationships of surface EMG parameters during local muscle fatigue. Muscle Nerve 16:849–856
Lloyd AR, Gandevia SC, Hales JP (1991) Muscle performance, voluntary activation, twitch properties and perceived effort in normal subjects and patients with the chronic fatigue syndrome. Brain 114:85–98
Masuda K, Masuda T, Sadoyama T, Inaki M, Katsuta S (1999) Changes in surface EMG parameters during static and dynamic fatiguing contractions. J Electromyogr Kinesiol 9(1):39–46
Merton PA (1954) Voluntary strength and fatigue. J Physiol (Lond) 123:553–564
Moritani T, Muro M, Nagata A (1986) Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol 60(4):1179–1185
Naeije M, Zorn H (1983) Estimation of the action potential conduction velocity in human skeletal muscle using the surface EMG cross-correlation technique. Electromyogr Clin Neurophysiol 23:73–80
Stegeman DF, Linssen WHJP (1992) Muscle fiber action potential changes and surface EMG: a simulation study. J Electromyogr Kinesiol 2(3):130–140
Thomas CK, Woods JJ, Bigland-Ritchie B (1989) Impulse propagation and muscle activation in long maximal voluntary contractions. J Appl Physiol 67(5):1835–1842
Zwarts MJ, Arendt-Nielsen L (1988) The influence of force and circulation on average muscle fibre conduction velocity during local muscle fatigue. Eur J Appl Physiol 58:278–283
Zwarts MJ, Van Weerden TW, Links TP, Haenen HTM, Oosterhuis HJGH (1988) The muscle fiber conduction velocity and power spectra in familial hypokalemic periodic paralysis. Muscle Nerve 11:166–173
Acknowledgements
The authors would like to thank all participants who volunteered for this study. Furthermore, we thank Hans van Dijk for technical support and Henny Janssen and Jeannine Hermens for their contributions to the performance of the measurements. This study was supported by a grant from Prinses Beatrix Fonds (grant no. MAR00–121). All experiments complied with the current laws for human studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schillings, M.L., Hoefsloot, W., Stegeman, D.F. et al. Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. Eur J Appl Physiol 90, 562–568 (2003). https://doi.org/10.1007/s00421-003-0913-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-0913-4