Abstract
Although the metabolic transition speed for forward exercise has already been determined, the walk–run transition speed for backward exercise has not been investigated before. The aim of this study was to determine the speed at which it becomes metabolically more efficient to run backwards than to walk backwards. Eighteen healthy volunteers, who successfully completed three backward exercise practice sessions, participated in the study. All subjects randomly performed two exercise tests: backward walking and backward running. Both protocols started at a treadmill speed of 5 km.h−1. Every minute the speed was increased by 0.5 km.h−1 until 8 km.h−1 was reached. Cardiorespiratory variables were continuously measured and blood lactate concentration [La] was determined every 2 min, using the Accusport lactate analyser. At each work load subjects rated their perceived exertion (RPE), using the Borg scale. There were no statistically significant differences in oxygen consumption, minute ventilation and heart rate between 6 and 7 km.h−1, for backward walking and backward running (P>0.05). There was no statistically significant difference in blood [La] between walking and running at 7.5 km.h-1 (P>0.05). According to the RPE values, subjects rated running at speeds less than 6 km.h−1 more difficult than walking at similar speeds. We conclude that the metabolic transition speed between backward walking and running is between 6 and 7 km.h−1, which is lower than the metabolic transition speed for forward locomotion (7.2–7.9 km.h−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Walking is often included in rehabilitation programs of several lower limb pathologies or injuries as it has been shown that early mobilisation and weight bearing promotes the healing process (Brukner and Kahn 2001). Backward walking, as opposed to forward walking, reduces the compression forces at the patellofemoral joint and decreases the force absorption at the knee. This is mainly because of the reduced eccentric function of the quadriceps muscle (Flynn and Soutas-Little 1991, 1993). Backward walking also prevents overstretching of the anterior cruciate ligament due to decreased quadriceps activity (Mackie and Dean 1984). While the eccentric function of the quadriceps muscle is reduced during backward walking, isometric and concentric quadriceps strength could be maintained (Flynn and Soutas-Little 1993; Kramer and Reid 1981; Mackie and Dean 1984; Threlkeld et al. 1989). Theoretically, this makes backward walking a suitable component of rehabilitation and exercise training programs.
Additional benefits of backward locomotion include the higher cardiopulmonary demand during backward walking and running, compared to forward walking and running at the same speed (Cavanagh et al. 1973; Flynn et al. 1994; Myatt et al. 1995). It has been shown that concentric muscle contraction is associated with higher metabolic cost than eccentric muscle contraction (Abbott et al.1952) and that the muscles of the lower limbs are active for a longer period during backward walking, compared to forward walking (Flynn et al. 1994; Kramer and Reid 1981). Furthermore, for most people backward locomotion will be a novel activity, which also explains the higher energy demand compared to forward locomotion. Childs et al. (2002) have shown that at least 12 exercise sessions (20 min per session) are needed to decrease the energy expenditure of a novel task such as backward walking.
The cardiorespiratory fitness of patients with injuries, who cannot follow their normal training program, rapidly deteriorates during periods of inactivity. Signs of deconditioning are observed within 12 days after cessation of a training program and may include a decrease in oxygen consumption (V̇O2) and stroke volume and an increase in heart rate (HR) (Coyle et al. 1984). Athletes with injuries could therefore possibly maintain a certain degree of fitness during periods of rehabilitation or where forward locomotion is contra-indicated.
Margaria et al. (1963) found that the transition speed between forward walking and forward running is between 7.2 km.h−1 and 7.9 km.h−1. This means that the metabolic cost of forward walking and running is the same at speeds between 7.2 and 7.9 km.h−1 (Hreljac 1993; Margaria et al. 1963). Before the transition speed, the metabolic cost [i.e. V̇O2, HR and minute ventilation (V̇ E)] of running is higher than for walking, whereas when the transition speed is exceeded, the metabolic cost of walking is higher than for running.
It is suggested that the transition speed between forward walking and running is determined by the energy cost of locomotion, i.e. V̇O2, HR and blood lactate concentration [La] (Alexander 1980; Cavagna and Kaneko 1977). Theoretically, however, the transition speed can also be determined by biomechanical aspects, such as articular limitations and lever advantages (Minetti et al. 1994), as well as amplitude of movement, limb length, stride length, stride frequency (Flynn et al. 1994; Mercier et al. 1994), previous experience, purpose of locomotion, and sensory aspects, for instance perception of exercise exertion (Minetti et al. 1994).
The biomechanics and kinematics of backward locomotion (both walking and running) are extensively described in the literature (Devita and Stribling 1991; Kramer and Reid 1981; Thorstensson 1986; Vilensky et al. 1987). However, it is not known at what speed it becomes metabolically more efficient to run than to walk backwards. This may have important implications for the design and application of rehabilitation and exercise programs that include backward walk–run training.
Methods
Healthy subjects (men and women) of varying fitness levels volunteered to participate in the study. All subjects were free of cardiopulmonary and musculo-skeletal abnormalities. Only subjects who successfully completed three backward walking–running practice sessions (similar to the final exercise protocol) were included in the study. Eighteen subjects (eight men and ten women) met all the inclusion criteria. The study was approved by the Ethics Committee of the Faculty of Health Sciences, Stellenbosch University. All subjects voluntarily signed an informed consent form after the test procedures and risks involved in the study were explained to them.
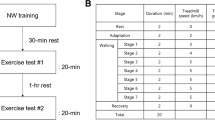
All subjects randomly completed two exercise tests (walking backwards and running backwards) on the treadmill. The exercise protocol consisted of seven work loads, starting at 5 km.h−1 and ending at 8 km.h−1. The treadmill speed was increased by 0.5 km.h−1 every minute. Within 3–8 days after the first test, the second test (either walking or running) was completed. A minimum rest period of 3 days was chosen to ensure that subjects had fully recovered from the first test session. A maximum of 8 days was chosen to prevent subjects from losing their newly acquired backward locomotion skills and familiarity on the treadmill. Before and after each training and test session the subjects performed stretching exercises to lower the risk of injury and muscle soreness.
At the beginning of the first test session the following anthropometric measurements were made: body mass (to the nearest 0.1 kg) measured with a digital, calibrated electronic scale, and standing height measured with an anthropometer (to the nearest 1 mm, without shoes).
During both exercise tests cardiorespiratory variables [V̇O2, V̇ E and respiratory exchange ratio (R)] were measured continuously, using the Cosmed Quark b2 metabolic system (Rome, Italy). HR was measured with a Polar heart rate monitor (Kempele, Finland), which was interfaced with the metabolic system. Blood [La] was measured at the end of four different work loads, namely 5.5, 6.5, 7.5 and 8 km.h−1. Blood samples were obtained with a finger prick and the [La] was measured with an Accusport lactate analyser (Boeringer Mannheim, Germany). After each work load subjects rated their level of perceived exertion (RPE) using the Borg scale (Borg 1970).
The metabolic system was calibrated before each exercise test. The volume transducer was calibrated with a 3-L calibration syringe, and the gas analysers with a gas mixture of known concentrations (20.93% O2 and 0.03% CO2; 15.5% O2 and 5.8% CO2).
Statistical analysis
Descriptive statistics are reported as mean (SD). A two-way analysis of variance (protocol ×treadmill speed) was used to test for differences in V̇O2, HR, V̇ E, R, [La] and Borg scale between the two exercise tests (walking and running). Tukey post hoc analysis was done to determine significant differences between walking and running at a specific treadmill speed. Transition points for each variable were determined for each individual separately. From the graphs, the four data points surrounding the transition point were connected with straight lines. The intersection point was identified and both the x-coordinate (treadmill speed, km.h−1) and the y-coordinate (the physiological variable) were determined. The level of statistical significance was set at P=0.05.
Results
A total of 18 subjects successfully completed both exercise protocols. Their anthropometric data is shown in Table 1.
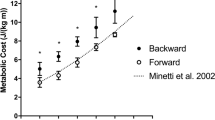
For both walking and running, V̇O2, HR, V̇ E, R and blood [La] changed significantly with increasing treadmill speed (P<0.001). V̇O2, V̇ E and R (Fig 1a, b and e, respectively) were significantly higher during running than walking at and below a treadmill speed of 6 km.h−1 (P<0.001). The opposite was true for treadmill speeds at and above 7 km.h−1 (P<0.001). At 6.5 km.h−1 there were no significant differences in V̇O2, HR, V̇ E and R between walking and running (P>0.05).
Figure 1c shows that there were no significant differences in HR at 6 and 6.5 km.h-1 (P>0.05), while for blood [La] (Fig 1d) there was no significant difference between walking and running at 7.5 km.h−1 (P>0.05).
Participants subjectively rated the running test significantly less stressful above 6.0 km.h−1 than the walk test at the same speeds (P<0.05). At 5.0 and 5.5 km.h−1 there were no statistically significant differences in the subjects' RPE (SEM) between walking and running [7 (1.0) vs 8 (1.0) and 9 (1.0) vs 9 (1.0), respectively; P>0.05).
Table 2 summarises the individual transition points for each variable measured. This analysis shows that for V̇O2, HR, V̇ E and R the transition point between backward walking and running varies between 6.4 and 6.7 km.h−1, while for RPE (Borg scale) the transition point is lower, namely 5.6 km.h−1. The transition point for blood [La] was the highest for all variables, namely 7.1 km.h−1.
In Table 3 the level of metabolic responses at which the transition speed occurs during forward locomotion (Mercier et al 1994) and backward locomotion (this study) is compared. In all cases the metabolic cost of forward locomotion was less than for backward locomotion, even though the transition speed for forward locomotion was higher than for backward locomotion (7.71 km.h−1 vs 6.41–6.65 km.h−1).
Discussion
The transition speed is defined as the speed at which a person spontaneously elects to start to run, rather than to walk. Many arguments have been put forward to explain the walk–run transition during forward locomotion (Prilutsky and Gregor 2001). The metabolic cost hypothesis is most often cited in this regard and proposes that the walk–run transition takes place at a speed at which walking becomes less economical than running (Hreljac 1993; Mercier et al. 1994).
The increased metabolism (V̇O2, V̇ E and HR) during forward running, compared to forward walking, before the transition speed, can possibly be attributed to the increased mechanical work associated with lifting the body with each stride and the increased energy cost as a result of internal work (Margaria et al. 1963). Internal work refers to the work done by internal forces, that is forces acting within the subject system. Internal work does not lead to a displacement of the centre of gravity, but is required to overcome muscle viscosity and to sustain muscle isometric contractions involved in making the body rigid and in fixating the joints (Cavagna et al. 1963). External work leads to a displacement of the centre of gravity.
The results of our study show that, according to the metabolic cost of backward locomotion, the transition speed for backward walking to backward running is between 6.41 and 6.65 km.h−1 (Table 2). The metabolic variables, V̇O2, V̇ E, HR and R indicated that the energy cost for walking at speeds slower than 6.41 km.h−1 is lower than for running, while the opposite is true for speeds faster than 6.65 km.h−1. Therefore, at speeds above 6.65 km.h−1 it is more economical to run backwards than to walk backwards. Our results therefore show that the transition speed for backward locomotion is lower than for forward locomotion (Margaria et al. 1963: 7.2–7.9 km.h−1; Mercier et al. 1994: 7.71 km.h−1).
Furthermore, the level of metabolic responses (V̇O2, V̇ E, HR and R) at which the transition speed occurs, is also higher for backward locomotion compared to forward locomotion (Table 3). This finding is in agreement with the literature, showing that the cardiorespiratory demands and metabolic costs of backward walking are consistently higher compared to forward walking and forward running at the same speed (Flynn et al 1994).
The transition speed for blood [La] occurred at 7.1 km.h−1, a higher speed than for V̇O2, V̇ E and HR. This is almost 2 speed increments, (equal to 2 min), later than for all the other metabolic variables. This finding can possibly be explained by normal lactate kinetics during progressive, incremental exercise. Nichols et al. (1997) showed that the blood [La] increases for at least 2 min after cessation of strenuous exercise. Therefore, in our study, the blood [La] produced at 6.5 km.h−1 is only measured in the blood at 7.0 or 7.5 km.h−1.
Flynn et al. (1994) also found that the blood [La] was statistically significantly higher during backward compared to forward walking (P<0.001), and the difference was even more pronounced when backward running was compared to forward running. The higher blood [La] during backward exercise is a reflection of the greater concentric work that is done by the leg muscles during backward locomotion than during forward locomotion. The muscles probably also rely more on O2-independent metabolism during backward walking and running.
Overall, the higher cardiopulmonary demands of backward exercise, compared to forward exercise, can be attributed to the different actions of the quadriceps muscle group . Backward walking causes both isometric and concentric activity of the quadriceps femoris muscle, while the muscle's action during forward walking is mainly eccentric (Flynn and Soutas-Little 1993; Kramer and Reid 1981; Mackie and Dean 1984; Thorstensson 1986; Threlkeld et al. 1989; Vilensky et al. 1987). Abbott et al. (1952) have shown that the metabolic cost for concentric muscle contraction is higher than for eccentric muscle contraction.
The higher metabolic cost of backward exercise can also be attributed to the execution of an unfamiliar task (Flynn et al. 1994; Myatt et al. 1995). Of the subjects initially recruited for this project, 42% could not complete both protocols. This is in agreement with the study of Flynn et al. (1994) where 40% of the participants failed to complete the exercise test. This could be due to a lack of coordination, which leads to unfamiliar patterns of motor unit recruitment and high blood [La]; both factors may contribute to muscle fatigue and cessation of exercise. Schwane et al. (1983) also suggested that an unfamiliar task will demand the recruitment of a greater number of motor units, thus increasing the energy and oxygen demand on tissue level.
From the literature it is apparent that one should distinguish between the spontaneous transition speed (the speed at which a person elects to start running) and the metabolic transition speed. Minetti et al. (1994) reported a significant difference of 0.5–0.9 km.h−1 (P<0.00002) between the spontaneous and metabolic transition speeds during forward exercise. These results imply that one will start running when it is still metabolically more effective to walk. A possible reason for this finding is that the peripheral mechanoreceptors detect the discomfort due to inefficient muscle function, and this leads to gait transition (Thorstensson and Roberthson 1987). In this study only the metabolic transition speed was directly determined, while the Borg scale was used to determine the subjects' RPE. The latter could possibly be viewed as an indirect measurement of the spontaneous transition speed.
While the subjects' RPE was the same for backward walking and running at 5 and 5.5 km.h−1, their effort levels were significantly lower for running above 6 km.h−1, compared to walking. One may therefore deduce from these results that, above 6 km.h−1, subjects probably would have preferred to run.
It should also be noted that although the lactate threshold for backward locomotion is around 7 km.h−1, the RPE (Borg scale) for running never exceeded 12, while for walking it rose above 13. The reasons for this apparent discrepancy is probably twofold. First, despite increase in V̇O2, V̇ E, HR, R and blood [La] during running above the transition point, subjects experienced a decreased sense of effort while running at the higher treadmill speeds. Secondly, the studies of Noble et al. (1973) and Hreljac (1993) suggested that the RPE is not related to V̇O2 and HR (and therefore possibly also [La]) when the exercise mode is changed from walking to running.
The results from these studies therefore suggest that the walk–run transition during forward locomotion is not related to the metabolic cost hypothesis but rather to the perceived sense of effort during walking and running at higher speeds. Our study also suggests that one will start to run backwards before it is actually more economical to run than to walk.
A major limitation of this study is that the exercise intervals were limited to 1 min each and therefore it is unlikely that steady-state conditions were achieved during each speed interval. However, due to the novelty of the task it is unlikely that steady-state conditions will be reached in a practical setting, particularly if backward exercise is used in short-term rehabilitation programs. Although not the perfect protocol to determine the transition speed, we are of the opinion that our results are a true reflection of what happens in practice. It should therefore be applicable to individuals who are unfamiliar and unaccustomed to backward exercise and who may be using it for the first time for rehabilitation or training purposes.
A further limitation of this study is that, due to practical constraints, the exact transition speed during backward locomotion could not be determined. Since the speed interval during which the transition takes place is now known, it will be easier to design protocols for future studies to determine more specifically the speed at which the metabolic and spontaneous transition between walking and running takes place, as well as for studies investigating backward training as part of rehabilitation following injury.
Conclusions
The metabolic transition between backward walking and backward running takes place between 6.4 and 6.7 km.h−1. This speed interval is higher than the apparent spontaneous transition speed for backward exercise (5.6 km.h−1), but lower than the metabolic transition speed for forward locomotion (7.2–7.9 km.h−1). The results of our study may therefore be helpful for those involved in the design and application of backward locomotion training programs for rehabilitation purposes.
References
Abbott BC, Bigland B, Ritchie JM (1952) The physiological cost of negative work. J Physiol 117:380–390
Alexander RM (1980) Optimum walking techniques for quadrupeds and bipeds. J Zool Soc Lond 192:97–117
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand. J Rehabil Med 2:92–98
Brukner P, Kahn K (2001) Clinical sports medicine, 2nd edn. McGraw Hill, New York, pp 466, 468–469
Cavagna GA, Kaneko M (1977) Mechanical work and efficiency in level walking and running. J Physiol (Lond) 268:467–481
Cavagna GA, Saibene FP, Margaria R (1963) External work in walking. J Appl Physiol 18(1): 1–90
Cavanagh PR, Davies CTM, Grieve DW, Sargeant AJ (1973) Electromyographic, kinesiological, and metabolic examination of running on a treadmill. J Physiol (Lond) 223:7P
Childs JD, Gantt C, Higgins D, Papazis JA, Franklin R, Metzler T, Underwood FB (2002) The effect of repeated bouts of backward walking on physiologic efficiency. J Strength Cond Res 16: 451–455
Coyle EF, Martin WH, Sinacore DR, Joyner MJ, Hagberg JM, Holloszy JH (1984) Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Phys 57:1857–1864
Devita P and Stribling J (1991) Lower extremity joint kinetics and energetics during backward running. Med Sci Sports Exerc 23:602–610
Flynn TW, Soutas-Little RW (1991) Patello-femoral joint compressive forces during forward and backward running (abstract). Med Sci Sports Exerc 23:32S
Flynn TW, Soutas-Little RW (1993) Mechanical power and muscle action during forward and backward running. J Orthop Sports Phys Ther 17:108–112
Flynn TW, Connery SM, Smutok MA, Zeballos RJ, Weisman IM (1994) Comparison of cardiopulmonary responses to forward and backward walking and running. Med Sci Sports Exerc 26:89–94
Hreljac A (1993) Preferred and energetically optimal gait transition speeds in human locomotion. Med Sci Sports Exerc 25:1158–1162
Kramer JF, Reid DC (1981) Backward walking: a cinematographic and electromyographic pilot study. Physiother Can 33:77–86
Mackie JW, Dean TE (1984) Running backward training effects on upper leg musculature and ligamentous instability of injured knees (abstract). Med Sci Sports Exerc 16: S151
Margaria R, Cerretelli P, Aghemo P, Sassi G (1963) Energy cost of running. J Appl Physiol 18:367–370
Mercier J, Le Gallais D, Durand M, Goudal C, Micallef JP, Prèfaut C (1994) Energy expenditure and cardiorespiratory responses at the transition between walking and running. Eur J Appl Physiol, 69:525–529
Minetti AE, Ardigo LP, Saibene F (1994) The transition between walking and running in humans: metabolic and mechanical aspects at different gradients. Acta Physiol Scand 150:315–323
Myatt G, Baxter R, Dougherty R, Williams G, Halle J, Stetts D, Underwood F (1995) The cardiopulmonary cost of backward walking at selected speeds. J Orthop Sports Phys Ther 21:132–138
Nichols JF, Phores SL, Buono MJ (1997) Relationship between lactate response to exercise and endurance performance in competitive female master cyclists. Int J Sports Med 18:458–463
Noble B, Metz K, Pandolf KB, Bell CW, Cafarelli E, Sime WE (1973) Perceived exertion during walking and running – II. Med Sci Sports 5:116–120
Prilutsky BI, Gregor RJ (2001) Swing- and support-related muscle actions differentially trigger human walk-run and run-walk transitions. J Exp Biol 204:2277–2287
Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB (1983) Delayed-onset muscular soreness and plasm CPK and LDH activities after downhill running. Med Sci Sports Exerc 15:51–56
Thorstensson A (1986) How is the normal locomotor program modified to produce backward walking? Exp Brain Res 61:664–668
Thorstensson A and Roberthson H (1987) Adaptations to changing speed in human locomotion: speed of transition between walking and running. Acta Physiol Scand 131:211–214
Threlkeld AJ, Horn TS, Wojtowicz GM, Rooney JG, Shapiro R (1989) Kinematics, ground reaction forces, and muscle balance produced by backward running. J Orthop Sports Phys Ther 11:56–62
Vilensky JA, Gankiewicz E, Gehlsen G (1987) A kinematic comparison of backward and forward walking in humans. J Hum Mov Stud 13:29–50
Acknowledgement
The authors would like to thank Jacolene Kroff for excellent laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terblanche, E., Cloete, W.A., du Plessis, P.A.L. et al. The metabolic transition speed between backward walking and running. Eur J Appl Physiol 90, 520–525 (2003). https://doi.org/10.1007/s00421-003-0890-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-0890-7