Abstract
We hypothesized that after maximal short-term isometric exercise, when O2 demand is still high and O2 supply is not fully activated, higher oxidative capacity muscle may exhibit slower muscle reoxygenation after the exercise than low oxidative capacity muscle. Seven healthy male subjects performed a maximal voluntary isometric handgrip exercise for 10 s. The reoxygenation rate after the exercise (Reoxy-rate) in the finger flexor muscle was determined by near infrared continuous wave spectroscopy (NIRcws) while phosphocreatine (PCr) was measured simultaneously by 31P magnetic resonance spectroscopy. Muscle oxygen consumption (muscle V̇O2) and muscle oxidative capacity were evaluated using the rate of PCr resynthesis post-exercise. The forearm blood flow (FBF) index at the end of exercise was measured using NIRcws. There was a significant positive correlation between the Reoxy-rate, which ranged between 0.53% s−1 and 12.47% s−1, and the time constant for PCr resynthesis, which ranged between 17.8 s and 38.3 s (r 2=0.939, P<0.001). At the end of the exercise, muscle V̇O2 exceeded the resting level by approximately 25-fold, while the FBF index exceeded the resting level by only 3-fold on average. The Reoxy-rate closely correlated with muscle V̇O2 (r 2=0.727, P<0.05), but not with the FBF index. Also, the estimated O2 balance (muscle V̇O2 index/FBF index) was negatively correlated with the Reoxy-rate (r 2=0.820, P<0.001). These results support our hypothesis that higher oxidative capacity muscle shows slower muscle reoxygenation after maximal short-term isometric exercise because the Reoxy-rate after this type of exercise may be influenced more by muscle V̇O2 than by O2 supply.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the methods for assessing muscle oxidative capacity in vivo includes muscle biopsy (Harris et al. 1974; Esbjornsson et al. 1993; Chilibeck et al. 1998; Mizuno et al. 1999). Although muscle biopsy may be useful and regarded as the gold standard, it is cumbersome and inconvenient to apply in many physiological and clinical conditions because of its invasive nature. During both steady state and non-steady-state conditions, phosphorus magnetic resonance spectroscopy (31P-MRS) can provide a non-invasive and repeated method of measuring muscle energy metabolism. The MRS device, however, is rather expensive and requires careful maintenance for precise measurements. Furthermore, this methodology has its own limitations in practical use.
During the 1990s, near-infrared spectroscopy (NIRS) was used in studying of exercising skeletal muscle in humans (Chance et al. 1992). Over the past several years, many more groups have applied this technique (McCully et al. 1994; Hamaoka et al. 1998; Higuchi et al. 2002). The near-infrared continuous wave spectroscopy (NIRcws) methodology is a useful tool because it is both non-invasive and inexpensive. The parameters commonly measured by NIRcws are oxygenated hemoglobin and/or myoglobin (Oxy-Hb/Mb), deoxygenated hemoglobin and/or myoglobin (Deoxy-Hb/Mb), and total hemoglobin (Hbtot). The NIRcws technique, however, has its own difficulties in measuring energy metabolism during exercise. This is because absolute concentration changes of Hb/Mb cannot be determined due to unquantifiable biophysical quantities (i.e., the optical path length). More recently, some studies have reported that different NIRS technologies (time-resolved spectroscopy, NIRTRS; spatially resolved spectroscopy, NIRSRS) can monitor absolute tissue saturation. However, it is difficult to apply NIRTRS during exercise because of its low time resolution (Patterson et al. 1989; Hamaoka et al. 2000a). On the other hand, NIRSRS cannot accurately calculate absolute saturation because it uses optical signals of different but close measurement areas (Quaresima et al. 2001).
Although NIRcws has a higher time resolution (up to 50 Hz) than NIRTRS, NIRcws can only measure the relative changes of Oxy-Hb/Mb and Deoxy-Hb/Mb as the balance between muscle oxygen consumption (muscle V̇O2) and O2 supply. Because of this limitation, several studies have investigated the relationship between the post-exercise recovery rate of Oxy-Hb/Mb, and muscle oxidative capacity (Chance et al. 1992; McCully et al. 1994; Hamaoka et al. 1998; Mizuno et al. 1999). The results, however, are not consistent. Some investigators reported that the muscle reoxygenation rate after isometric plantar flexion exercise does not correlate with muscle oxidative capacity (Hamaoka et al. 1998; Mizuno et al. 1999). In contrast, however, other studies have shown fast Hb/Mb recovery in high oxidative capacity muscle (HOCmuscle) (Chance et al. 1992; McCully et al. 1994). In particular, McCully et al. (1994) reported that phosphocreatine (PCr) recovery and Oxy-Hb/Mb showed similar behavior after dynamic plantar flexion exercise with muscle pH being 7.0. These divergent results, which appear to be due to slight differences in the exercise paradigm, suggest that post-exercise Oxy-Hb/Mb may be influenced more by O2 supply during dynamic exercise than during isometric exercise.
Again, because the Hb/Mb reoxygenation rate (Reoxy-rate) reflects the balance between muscle V̇O2 and O2 supply in localized muscle, the Reoxy-rate determined by NIRcws can be interpreted in various ways. When the O2 supply is limited, post-exercise Reoxy-rate may be more influenced by muscle V̇O2. Blood flow after static muscular exercise is lower than after dynamic exercise (Huonker et al. 1996). Also, blood flow is the lowest at the beginning of exercise and increases exponentially during exercise (Hughson et al. 1996; Saltin et al. 1998). We hypothesized that during post-maximal short-term handgrip isometric exercise, when O2 demand is high and O2 supply is not sufficient, HOCmuscle would show a greater increase in muscle V̇O2 than low oxidative capacity muscle (LOCmuscle) during the recovery phase. In contrast, the O2 supply would not increase as much as muscle V̇O2 after the exercise. Therefore, it is expected that after a short duration, maximal isometric exercise, HOCmuscle would exhibit slower muscle oxygenation recovery. The primary goal of this study was to test the hypothesis that higher oxidative capacity muscle exhibits a slower muscle oxygenation recovery after short-term maximal isometric exercise. We further evaluated muscle V̇O2 and O2 supply separately to validate the O2 balance determined by NIRcws (Reoxy-rate) after this type of exercise.
Methods
Subjects
Seven healthy male volunteers [five moderately active, one well-trained triathlete, and one former Japanese national windsurfing champion, mean age (SD): 29 (3) years] participated in this study. All subjects were briefed about the experimental protocol, and informed consent was obtained before the test.
Experimental design
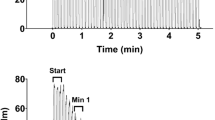
The muscle oxygenation level was measured by NIRcws while the PCr concentration was measured simultaneously by 31P-MRS (Sako et al. 2001). The rate of Oxy-Hb/Mb recovery immediately after exercise up until 6 s after exercise was defined as the Reoxy-rate. Subjects performed the same experiment twice to examine the Reoxy-rate, the time constant for PCr recovery (PCrTc) and muscle V̇O2 at the end of exercise (muscle V̇O2EX) (experiment 1) and the forearm blood flow (FBF) index (experiment 2). These were measured once using 31P-MRS and NIRcws (experiment 1) and once using NIRcws only (experiment 2) (Fig. 1). This dual measurement was performed because the FBF index from the NIR signal required venous occlusion at the end of exercise. To ensure familiarity with the exercise modality, each subject engaged in two to three bouts of isometric handgrip practice before the start of the exercise protocol.
A The Reoxy-rate, PCrTC, and muscle V̇O2EX values (experiment 1). Muscle V̇O2EX was calculated by differentiating the monoexponential curve. B The FBF index value after exercise (experiment 2). Reoxy-rate Reoxygenation rate, PCr TC time constant for phosphocreatine (PCr) resynthesis, muscle V̇O2 EX muscle oxygen consumption at the end of exercise, FBF forearm blood flow, Ex maximal isometric handgrip exercise, Venous Occl venous occlusion
Experimental protocol
Each subject sat on an adjusted chair that allowed the arm to extend to the level of the heart. The forearm was secured to a platform that contained a 3.0 cm diameter circular surface coil for 31P-MRS as well as a light source and optical detector with a distance of 3.0 cm for NIRcws (Sako et al. 2001). The 31P-MRS spectra measurement area was defined by the coil circumference and penetrated to a depth equal to the radius of the coil (McCully et al. 1989; Richardson et al. 1995). The mean penetration depth of near infrared light into the tissue was equal to approximately half the distance between the input and output fibers (Chance et al. 1992). Therefore, both devices could simultaneously take measurements at nearly identical sites. The measurement site was the right finger flexor muscle, the most active site in handgrip contraction. The subjects performed a maximal isometric handgrip exercise for 10 s and the arm was completely relaxed immediately after exercise.
Near-infrared continuous wave spectroscopy
During and after exercise, the oxygenation level in the finger flexor muscles was measured within the magnetic fields using NIRcws (OM100A, Shimadzu, Kyoto, Japan). The basic operation of this NIRcws device has been extensively explained in previously published papers (Chance et al. 1992; Hamaoka et al. 1996; Homma et al. 1996a).
It is well known that the level of oxygenation of Hb/Mb alters the absorption of the light in muscle tissue. For example, as Hb/Mb is oxygenated, the absorbance at 780 nm decreases and the absorbance at 830 nm increases, providing a different signal. Accordingly, the detectable light intensities of the detector change due to the level of Hb/Mb deoxygenation. Both Hb/Mb absorb light equally at the wavelength employed. However, it is reported that Mb concentration is no greater than 25% (Chance et al. 1992) or 20% (Mancini et al. 1994). Therefore, we can conclude that the signals are derived mainly from Hb.
In this study, three-wavelength optical fibers, with wavelengths of 780 nm, 805 nm and 830 nm, were used as the light source. Changes in Oxy-Hb/Mb and Hbtot were calculated with the least-squares method using data from the changes in light absorbance at different wavelength (Homma et al. 1996a). The reliability of this algorithm was previously confirmed in vivo (Homma et al. 1996a; Sako et al. 2001). However, there are some disadvantages in using the NIRcws technique to evaluate muscle energy metabolism during exercise. Most notably, measurement sensitivity is impaired by the influence of adipose tissue (Homma et al. 1996b). The thickness of subcutaneous adipose tissue varies greatly in humans and the optical densities of Oxy-Hb/Mb and Hbtot cannot be compared between individuals (Quaresima and Ferrari 2002). Therefore, to evaluate differences in muscle oxygenation between each subject, arterial occlusion was used (Hamaoka et al. 1996; Sako et al. 2001); this technique involves interrupting arterial blood flow by placing a pneumatic tourniquet on the upper arm at a pressure of 280 mmHg. The pre-occluded resting level of Oxy-Hb/Mb was defined as 100% and the minimum value during ischemia as 0%. The muscle oxygenation level during and after exercise was expressed using the overall change from the resting pre-occluded to the minimum oxygenation level during arterial occlusion.
Due to the fact that the NIRcws machine's time resolution (time resolution=1.5 s), which was simultaneously measured with 31P-MRS, does not accurately estimate the FBF index during venous occlusion, another NIRcws device (HEO-210, OMRON, Kyoto, Japan; time resolution=0.1 s) was used out of the magnetic field. Two-wavelength light-emitting diodes, with wavelengths of 760 nm and 840 nm, were used as the light source. The reliability of this method of calculating signals was previously confirmed with in vitro (Shiga et al. 1997) and in vivo experiments (Hamaoka et al. 1996; Shiga et al. 1997; Hamaoka et al. 1998). The FBF index as determined by venous occlusion is fully described in a previously published paper (Homma et al. 1996a; Van Beekvelt et al. 2001). The resting FBF index was estimated by measuring the peak rate of increase in Hbtot during venous occlusion for 15 s. This procedure was repeated five times every 1 min before the principal exercise; then the mean value was used as the resting FBF index. The FBF index at the end of exercise was measured by venous occlusion immediately after exercise. Before the experiment, the optimal cuff pressure was determined for each subject to obtain the greatest increase in the Hbtot rate during venous occlusion. On average, the cuff pressure used was 70.0 (0.35) [mean (SD)] mmHg.
31Phosphorus magnetic resonance spectroscopy
Phosphorus metabolites during rest, exercise, and recovery were monitored by 31P-MRS (Otsuka Electronics Inc.) with a 2.0-T superconducting 26-cm-bore magnet. A 3.0 cm diameter circular double-turn surface coil was attached to a cradle located at the mid-forearm, centered mainly over the flexor digitorium superficialis muscle; it was tuned to both the 31P and 1H frequencies (34.6 MHz and 85.4 MHz, respectively). The homogeneity of the magnetic field was adjusted to <0.28 parts per million on the proton signal from water. 31P spectra were obtained using a 60-µs pulse width to optimize signal intensity. The pulse repetition time was 2 s. Five pulses were averaged during to obtain a free induction decay (FID), so each spectrum was obtained every 10 s.
The FIDs were obtained and Fourier transformed into spectra; the peak areas were fitted by a non-linear, curve-fitting method to calculate the areas under the β-ATP, PCr, and Pi peaks in the frequency domain that minimizes the sum of squares between the spectral resonances. To acquire the saturation factors for each phosphate compound, a ratio was calculated between each area obtained at 20-s repetition time (fully relaxed spectra) and those obtained at 2-s pulse intervals. Then, PCr and Pi peak areas were quantified fom the ratio of PCr and Pi areas to β-ATP area, with corrections made for the saturation factor. Because there were no data available for a direct chemical analysis of the forearm muscles, the ATP concentration reported from the muscle biopsies of the human vastus lateralis ([ATP]=8.2 mM) was used for PCr and Pi quantification (Harris et al. 1974). The intracellular pH was calculated from the median chemical shift between the Pi and PCr peaks (Kushmerick and Meyer 1985).
Data analysis
The area of the PCr peaks during recovery was integrated and fitted to a single-exponential curve, and the time constant for PCr resynthesis (PCrTc) was determined using the following equation:
where PCr is the PCr concentration at time t during recovery, PCr0 is the PCr concentration at the end of exercise, ΔPCr is the difference between final and initial PCr, t is the time, k is the rate constant of the monoexponential curve, and 1×k−1is the time constant (Tc). In fact, the PCr resynthesis rate is independent of exercise intensity, as long as muscle pH is close to 7.0 (Yoshida and Watari 1993; Hamaoka et al. 2000c). Therefore, this PCrTc is used as the indicator of muscle oxidative capacity (Arnold et al. 1984; McCully et al. 1993; McCully et al. 1994).
The post-exercise PCr resynthesis rate measured by 31P-MRS has been recognized as one of the most reliable parameters for quantifying the rate of oxidative ATP production (Chance et al. 1985; Mahler 1985; Kemp et al. 1993; McCully et al. 1993) because PCr cannot be resynthesized without mitochondrial oxidative phosphorylation (Quistorff et al. 1992). The muscle V̇O2 at the end of exercise (muscle V̇O2EX) was calculated using the equation described below:
While the FBF index was only evaluated as relative to the resting value, resting muscle V̇O2 (muscle V̇O2REST) could be obtained from the PCr breakdown rate during 15 min arterial occlusion (Blei et al. 1993; Hamaoka et al. 1996; Sako et al. 2001). Hence, the ratio of muscle V̇O2EX/muscle V̇O2REST can be determined for each subject as a muscle V̇O2 index. Both muscle V̇O2EX and muscle V̇O2REST were expressed as millimoles ATP per second. The absolute values of muscle V̇O2 (in μM O2 s−1) were also calculated using P-to-O2 ratio, which is 6 for in vivo skeletal muscle (Blei et al. 1993; Hamaoka et al. 1996; Sako et al. 2001).
Statistics
Using a paired t-test, we conducted statistical analyses of the differences between the muscle V ̇O2 and FBF indexes. Comparisons of PCrTc, muscle V̇O2EX, the FBF index, and the Reoxy-rate were made using linear correlation. Values are presented as means (SD). Results were considered statistically different at P<0.05.
Results
Reoxygenation rate after exercise
Figure 2A shows a typical example of changes in Oxy-Hb/Mb and Hbtot during and after a maximal isometric handgrip exercise. At the beginning of exercise, Hbtot showed a biphasic response that decreased steeply and remained stable thereafter. On the other hand, Oxy-Hb/Mb dropped linearly until the termination of exercise. After exercise, Oxy-Hb/Mb and Hbtot rose exponentially. All the subjects but one showed similar kinetics as shown in Fig. 2A. Figure 2B displays the changes in Oxy-Hb/Mb and Hbtot of the highest oxidative capacity muscle (subject A). Similar to the previous example, Oxy-Hb/Mb dropped rapidly at the beginning of exercise. However, immediately after exercise, although the Hbtot rose rapidly back to the resting level, Oxy-Hb/Mb continued to deoxygenate until approximately 15 s after exercise and then proceeded to gradually rise. Therefore, this subject's Reoxy-rate indicates a negative value (Table 1). The Reoxy-rate ranged from 0.53% s−1 to 12.47% s−1 [7.30(4.7)% s−1; Table 1]. The Reoxy-rate in relation to the PCrTc is shown in Fig. 3. There was a significant positive correlation between the Reoxy-rate and PCrTc (r 2=0.939, P<0.001).
Measurement of O2 dynamics
The average values of muscle V̇O2REST, and muscle V̇O2EX were 0.0098 mM ATP s−1 and 0.273 mM ATP s−1, respectively (Table 1). These values were also equivalent to 1.65 μM O2 s−1 and 45.5 μM O2 s−1, respectively, which were calculated using P-to-O2 ratio of 6. At the end of exercise, the muscle V̇O2 index increased on average by 25-fold compared to the resting value. On the other hand, the FBF index at the end of exercise increased by 3-fold compared to the average resting value. The muscle V̇O2 index was significantly higher than the FBF index immediately after exercise (P<0.001).
Muscle V̇O2EX and the FBF index in relation to the Reoxy-rate are shown in Fig. 4. There was a significant correlation between muscle V̇O2EX and the Reoxy-rate (P<0.05). No correlation, however, was found between the FBF index at the end of exercise and the Reoxy-rate. The O2 balance, estimated from the separate measurements of the muscle V̇O2 index and the FBF index (muscle V̇O2 index/FBF index), for each subject are shown in Table 1. The estimated O2 balance (muscle V̇O2 index/FBF index) ranged from 5.62 to 16.08 [9.50 (3.63)]. The relationship between the estimated O2 balance and NIRcws O2 balance (Reoxy-rate) are shown in Fig. 5. The estimated muscle O2 balance closely correlated with the Reoxy-rate (r 2=0.820, P<0.001). A significant correlation was also found between the muscle V̇O2 index/FBF index and PCrTc (r 2=0.799, P<0.001)
Discussion
We found a significant relationship between the Reoxy-rate and the PCrTc as shown in Fig. 3. Specifically, we found that higher oxidative capacity muscle exhibited slower reoxygenation after exercise. At the end of the exercise, the muscle V̇O2 exceeded the resting level by approximately 25-fold, while the FBF index exceeded the resting level by only 3-fold on average. These results support our hypothesis that after maximal short-term isometric exercise, when O2 demand is high and O2 supply is not fully increased, higher oxidative capacity muscle exhibits slower muscle reoxygenation. Some studies have reported that higher oxidative capacity muscle exhibits a faster reoxygenation rate after exercise (Chance et al. 1992; McCully et al. 1994). This is the first report indicating that higher oxidative capacity muscle exhibits a slower reoxygenation rate after exercise.
Also in this study, the estimated O2 balance from the separate measurement of muscle V̇O2 and O2 supply closely correlated with the Reoxy-rate. This suggests that the Reoxy-rate is determined by the balance between muscle V̇O2 and O2 supply. Furthermore, the Reoxy-rate was not closely correlated with the FBF index, but instead with muscle V̇O2EX as shown in Fig. 4. These results imply that the Reoxy-rate after this type of exercise may be influenced more by muscle V̇O2 than by O2 supply.
Reliability of this study
In this study, the average value of muscle V̇O2REST was 0.0098 mM ATP s−1, which is similar to 0.008 mM ATP s−1 (Blei et al. 1993), 0.0073 mM ATP s−1 (Hamaoka et al. 1996), and 0.0076 mM ATP s−1 (Sako et al. 2001) as previously measured by 31P-MRS. The muscle V̇O2REST (0.0098 mM ATP s−1) was also equivalent to 1.6 μM O2 s−1, This value is very close to the value of 1.4 μM O2 s−1 from Harris et al. (1975). The average value of muscle V̇O2EX in this study was 0.273 mM ATP s−1. This result is similar to 0.28 mM ATP s−1 obtained from average muscle V̇O2 following fully excited muscle contraction (Blei et al. 1993). These results demonstrate that our study's muscle V̇O2 results support the validity of quantitative values. These results also indicate that the finger flexor muscles, that served as agonist muscles in this study, were maximally activated.
According to several studies, a severe decrease in intracellular pH and a restriction of mitochondrial oxidative phosphorylation causes a delay in the PCr resynthesis rate (Arnold et al. 1984; Iotti et al. 1993; Takahashi et al. 1995). Following a non-steady-state exercise, a delayed pHmin response may be observed (Vestergaard-Poulsen et al. 1995). This phenomenon is also supported by the creatine kinase reaction with a rapid PCr resynthesis. In this exercise protocol, however, all of the pHmin values were above 6.82 (Table 1) and therefore did not restrict mitochondrial oxidative phosphorylation (Takahashi et al. 1995). Some studies have demonstrated a linear relationship between the PCr resynthesis rate and mitochondrial enzyme activities in human skeletal muscle (McCully et al. 1993; Mizuno et al. 1999). Therefore, the time constant for PCr recovery in the experiment should be considered as an indicator of skeletal muscle oxidative capacity.
O2 balance estimated from separate measurements of muscle O2 consumption and O2 supply
A significant linear relationship between the estimated O2 balance and NIRcws O2 balance (Reoxy-rate) was found as shown in Fig. 5. This correlation demonstrates that the estimated O2 balance from the separate measures of O2 supply and demand corresponds with the NIR method of O2 balance. These separate measurements can provide important insight into the factors determining O2 balance in muscle, which the Reoxy-rate alone cannot achieve.
As mentioned earlier, according to our hypothesis, the O2 balance after maximal short-term isometric contraction is much more influenced by muscle V̇O2 than O2 supply. This study found that the ratio of muscle V̇O2 index/FBF index of all subjects was above 1.0 as shown in Table 1. Van Beekvelt at al. (2001) also reported a greater increase in muscle V̇O2 than O2 supply after isometric handgrip contraction. Moreover, the Reoxy-rate closely correlated with muscle V̇O2EX, but not with the FBF index (Fig. 4). These results suggest that the Reoxy-rate after this type of exercise may be influenced more by muscle V̇O2 than by O2 supply. This is because increased O2 demand promotes greater O2 extraction from the capillaries.
It is possible that the muscle V̇O2 index/FBF index is equal to the relative change of the arteriovenous (a–v) O2 difference. The muscle V̇O2 index/FBF index at the end of exercise ranged between 5.6 and 16.1 compared to the resting value. These values are higher than the reported (a–v) O2 difference during maximal exercise (2.7- to 8.8-fold of the resting value) (Hartling et al. 1989, Saltin et al. 1998, Bangsbo et al. 2000). One possible explanation for this is the different type of exercise used in this study. Almost all of these studies that measured (a–v) O2 difference used dynamic exercise for more than a few minutes to fully activate blood flow. In this study, short-term isometric exercise was used to prevent an increase in blood flow after exercise. As a result, FBF index increased by only 3-fold of the resting value after exercise. Lower BF induces longer red blood cell transit time. This may cause greater O2 extraction.
Another possible reason for the high muscle V̇O2 index/FBF index value is that the venous effluent at the in vivo sampling sites was a mixture of blood from exercising and non-exercising tissue. Compared to the blood sampling method, 31P-MRS and NIRS can specifically measure only dominant active muscle information.
Factors affecting the reoxygenation rate
O2 inflow to the exercising muscle may be completely interrupted during isometric exercise by increased intramuscular pressure (Saltin et al. 1998; Hamaoka et al. 2000b). Stable Hbtot during isometric exercise also supports blood flow interruption during the exercise (Fig. 2). These reports suggest that the availability of O2, but not of convective O2 transport, may limit muscle V̇O2 during isometric exercise.
Several studies have reported that the difference in (a–v) O2 content does not increase at the onset of exercise (Hughson et al. 1996). However, Bangsbo et al. (2000) found that O2 extraction increased after a few seconds of exercise by obtaining blood transit time from the capillaries to the collection point of the venous blood sample. Recent studies have demonstrated that the ability to detect human skeletal muscle Mb deoxygenation using 1H-MRS makes it possible to estimate intracellular PO2 (Richardson et al. 1995; Tran et al. 1999). Richardson et al. (1995) demonstrated that partial Mb deoxygenation occurs even at 50% of maximum O2 consumption within 20 s of exercise onset (the time resolution of the MRS system). These findings suggest that the intracellular PO2 may decrease within a few seconds, which may induce a greater O2 extraction at the onset of blood reperfusion.
A large number of studies have proposed that HOCmuscle is characterized by high mitochondrial oxidative enzyme activity, high mitochondrial density, and high capillary density (Esbjornsson et al. 1993; McCully et al. 1993; Mizuno et al. 1999). Consequently, at the onset of exercise HOCmuscle may produce a more rapid adjustment in muscle V̇O2 (Phillips et al. 1995; Hamaoka et al. 1998). In addition, considering the high O2 utilization at the end of exercise in HOCmuscle, intracellular PO2 in HOCmuscle may be lower than that of LOCmuscle. Therefore, the O2 gradient from capillary blood to myocyte at the onset of blood reperfusion may be higher in HOCmuscle than in LOCmuscle. The combination of previous studies with our own present findings suggests that the speed and magnitude of Mb deoxygenation during exercise may be one of the most important factors influencing the Reoxy-rate.
Summary
This study produced three important findings:
-
1.
Significantly positive correlation between the Reoxy-rate and PCrTc suggests that HOCmuscle shows slow reoxygenation after maximal isometric exercise.
-
2.
The Reoxy-rate was not closely correlated with the FBF index, but instead with muscle V̇O2EX. This result implies that the Reoxy-rate after 10 s of maximal isometric exercise may be influenced greater by muscle V̇O2 than by O2 supply.
-
3.
The linear correlation between estimated O2 balance (muscle V̇O2 index/FBF index) and NIRcws O2 balance (Reoxy-rate) suggests that the Reoxy-rate determines the balance between muscle V̇O2 and O2 supply.
The present study's results may depend heavily on the exercise protocol. The inverse proportional relationship between the Reoxy-rate and muscle oxidative capacity should not be applied for other types of exercise protocol (i.e., dynamic or whole body exercise). Compared to other exercises, short-term isometric contraction is easily performed. Also, NIRcws is more portable and inexpensive than the MRS device. Therefore, the Reoxy-rate after short-term isometric exercise, as determined by NIRcws, allows us to easily measure the muscle oxidative capacity.
References
Arnold DL, Matthews PM, Radda GK (1984) Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med 1:307–315
Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. (2000) Muscle oxygen kinetics at onset of intense dynamic exercise in human. Am J Physiol 279:R899–R906
Blei M, Conley K, Kushmerick M (1993) Separate measurement of ATP utilization and recovery in human skeletal muscle. J Physiol (Lond) 465:203–222
Chance B, Leigh JS, Clark BJ, Maris JM, Kent J, Nioka S (1985) Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A 82:8384–8388
Chance B, Leigh JS, Kent J, McCully K, Nioka S, Clark BJ, Maris JM, Graham T (1986) Multiple controls of oxidative metabolism of living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci U S A 83:9458–9462
Chance B, Dait TM, Chang C, Hamaoka T, Hagerman F (1992) Recovery from exercise induced desaturation in the quadriceps muscle of elite competitive rowers. Am J Physiol 262:C766–C775
Chilibeck PD, McCreary CR, Marsh GD, Paterson DH, Noble EG, Taylor AW, Thompson RT (1998) Evaluation of muscle oxidative potential by 31P-MRS during incremental exercise on old and young humans. Eur J Appl Physiol 78:460–465
Esbjornsson M, Jansson E, Sundberg CJ, Sylven C, Eiken O, Nygren A, Kaijser L (1993) Muscle fiber types and enzyme activities after training with local leg ischaemia in man. Acta Physiol Scand 148:231–241
Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B (1996) Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol 81:1410–1417
Hamaoka T, Mizuno M, Katsumura T, Osada T, Shimomitsu T, Quistorff B (1998) Correlation between indicators determined by near infrared spectroscopy and muscle fiber types in humans. Jpn J Appl Physiol 28:243–248
Hamaoka T, Katsumura T, Murase N, Nishio S, Osada T, Sako T, Higuchi H, Kurosawa Y, Shimomitsu T, Miwa M, Chance B (2000a) Quantification of ischemic muscle deoxygenation by near infrared time-resolved spectroscopy. J Biomed Opt 5:102–105
Hamaoka T, Katsumura T, Murase N, Osada T, Sako T, Murakami M, Esaki K, Kime R, Homma T, Sugeta A, Kurosawa Y, Shimomitsu T (2000b) Changes in phosphocreatine, muscle oxygen consumption and forearm blood flow at onset of intermittent isometric exercise. Med Sci Sports Exerc 32:S320
Hamaoka T, McCully KK, Katsumura T, Shimomitsu T, Chance B (2000c) Noninvasive measurements of muscle metabolism. In: Sen CK, Packer L, Hanninen O (eds) Handbook of Oxidants and Antioxidants in Exercise. Elsevier Science, Amsterdam, pp 485–509
Harris R, Hultman E, Nordesjo L-O (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33:109–120
Harris R, Hultman E, Kaijser L, Nordesjo L-O (1975) The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand J Clin Lab Invest 35:87–95
Hartling O, Kelbaek H, Gjorup T, Schibye B, Klausen K, Trap-Jensen J (1989) Forearm oxygen uptake during maximal forearm dynamic exercise. Eur J Appl Physiol 58:466–470
Higuchi H, Hamaoka T, Sako T, Nishio S, Kime R, Murakami M, Katsumura T (2002) Oxygenation in vastus lateralis and lateral head of gastrocnemius during treadmill walking and running in humans. Eur J Appl Physiol 87:343–349
Homma S, Eda H, Ogasawara S, Kagaya A (1996a) Near-infrared estimation of O2 supply and consumption in forearm muscles working at varying intensity. J Appl Physiol 80: 1279–1284
Homma S, Fukunaga T, Kagaya A (1996b) Influence of adipose tissue thickness on near infrared spectroscopic signals in the measurement of human muscle. J Biomed Opt 1:418–424
Hughson RL, Shoemaker JK, Tschakovsky ME, Kowalchuk JM (1996) Dependence of muscle V̇O2 on blood flow dynamics at onset of forearm exercise. J Appl Physiol 81:1619–1626
Huonker M, Halle M, Keul J (1996) Structural and functional adaptations of the cardiovascular system by training. Int J Sports Med 17: S164-S172
Iotti S, Lodi R, Frassineti C, Zaniol P, Barbiroli B (1993) In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. NMR Biomed 6:248–253
Kemp GJ, Taylor DJ, Radda GK (1993) Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed 6:66–72
Kushmerick MJ, Meyer RA (1985) Chemical changes in rat leg muscle by phosphorus nuclear magnetic resonance. Am J Physiol 248:542-549
Mahler M (1985) First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implication for the control of respiration. J Gen Physiol 77:135–165
Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR (1994) Validation of near-infrared spectroscopy in human. J Appl Physiol 77:2740–2747
McCully KK, Boden BP, Tuchler M, Fountain MR, Chance B (1989) Wrist flexor muscles of elite rowers measured with magnetic resonance spectroscopy. J Appl Physiol 67:926–932
McCully KK, Fielding RA, Evans WJ, Leigh JS, Posner JD (1993) Relationship between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 77:2740–2747
McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh JS, Chance B (1994) Simultaneous in vivo measurement of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol 77:5–10
Mizuno M, Hamaoka T, Osada T, Shimomitsu T, Katsumura T, Quistorff B (1999) Correlation between mitochondrial enzyme activities and the rate of hemoglobin deoxygenation at onset of exercise in human gastrocnemius muscles. Med Sci Sports Exerc 31:S275
Patterson MS, Chance B, Wilson BC (1989) Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. J Appl Opt 28:2331–2336
Phillips SM, Green HJ, MacDonald MJ, Hughson RL (1995) Progressive effect of endurance training on V̇O2 kinetics at the onset of submaximal exercise. J Appl Physiol 79:1914–1920
Quaresima V, Homma S, Azuma K, Shimizu S, Chiarotti F, Ferrari M, Kagaya A (2001) Calf and shin muscle oxygenation patterns and femoral artery blood flow during dynamic plantar flexion exercise in humans. Eur J Appl Physiol 84:387–394
Quaresima V, Ferrari M (2002) Quantitation of muscle oxygenation by near-infrared spectroscopy methods. Eur J Appl Physiol 86:283–284
Quistorff B, Johansen L, Sahlin K (1992) Absence of phosphocreatine resynthesis in human calf muscle during ischemic recovery. Biochem J 291:681–686
Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD (1995) Myoglobin O2 desaturation during exercise. Exercise of limited O2 transport. J Clin Invest 96:1916–1926
Sako T, Hamaoka T, Higuchi H, Kurosawa Y, Katsumura T (2001) Validity of NIR spectroscopy for quantitatively measuring muscle oxidative metabolic rate in exercise. J Appl Physiol 90:338–344
Saltin B, Radegran G, Koskolou MD, Roach RC (1998) Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162:421–436
Shiga T, Yamamoto K, Tanabe K, Nakase Y, Chance B (1997) Study of an algorithm based on model experiments and diffusion theory for a portable tissue oximeter. J Biomed Opt 2: 152–161
Takahashi H, Inaki M, Fujimoto K, Katsuta S, Anno I, Niitsu M, Itai Y (1995) Control of the rate of phosphocreatine resynthesis after exercise in trained and untrained human quadriceps muscles. Eur J Appl Physiol 71:396–404
Tran T-K, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole P, Kuno S, Jue T (1999) Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am J Physiol 276:R1682–R1690
Van Beekvelt MCP, Colier WNJM, Wevers RA, Van Engelen BGM (2001) Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol 90:511–519
Vestergaard-Poulsen P, Thomsen C, Sinkjaer T, Henriksen O (1995) Simultaneous 31P-NMR spectroscopy and EMG in exercising and recovering human skeletal muscle: a correlation study. J Appl Physiol 79:1469–1478
Yoshida T, Watari H (1993) 31P-Nuclear magnetic resonance spectroscopy study of the time course of energy metabolism during exercise and recovery. Eur J Appl Physiol 66:494-499
Acknowledgements
The authors would like to thank the subjects who participated in the study, Dr Sang Yong Bae for his assistance in completing this project, and Toshio Kimura and Gregg Goldstein for all of their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kime, R., Hamaoka, T., Sako, T. et al. Delayed reoxygenation after maximal isometric handgrip exercise in high oxidative capacity muscle. Eur J Appl Physiol 89, 34–41 (2003). https://doi.org/10.1007/s00421-002-0757-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-002-0757-3