Abstract
Objective
Pneumoconiosis, encompassing coal workers’ pneumoconiosis (CWP), silicosis and asbestosis, is one of the most common occupational diseases in China. Previous studies revealed significant associations between genetic variations and pneumoconiosis risk among individuals in different countries. With the known variability of genetic makeup between ethnicities, susceptibility to pneumoconiosis due to genetic differences is likely to be ethnicity-specific. The present review aimed at providing a comprehensive overview on the association between genetic polymorphisms and susceptibility of pneumoconiosis, specifically among people in China.
Methods
The literature search was performed in seven English and Chinese databases using keywords related to the review aim. An appraisal of the methodological quality of the included studies was conducted using the assessment tool derived from the Strengthening the Reporting of Genetic Association Studies (STREGA) statement.
Results
Forty-five studies were included in this review. Genotypes of specific genes which are associated with the risk of CWP, silicosis and asbestosis were reported. Our findings showed that genes encoding inflammatory cytokines have been examined extensively, and they demonstrated an association between these genes and pneumoconiosis risk. Gene–environment interactions in pneumoconiosis susceptibility were also reported by a number of studies.
Conclusions
This review summarised the evidence demonstrating the association between genetic polymorphisms and pneumoconiosis susceptibility among people in China, and that various genotypes could modify their risk to develop pneumoconiosis. The findings prompt that identification of individuals at high pneumoconiosis risk through genetic screening and strategies limiting their exposure to dust could be a potential strategy for the control of this occupational disease in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pneumoconiosis is a group of interstitial lung diseases caused by chronic inhalation of dust particles that induce inflammation of the alveoli and eventually result in irreversible lung damage and even death (Cullinan and Reid 2013). Pneumoconiosis appears in different forms depending on the type of dust inhaled, such as coal dust, silica and asbestos. China is among the countries that contribute to an alarming number of diagnosed pneumoconiosis cases around the world (Han et al. 2015a). In 2016, 27,992 new pneumoconiosis cases were reported in China, of which 16,658 (59.5%) had coal workers’ pneumoconiosis (CWP) and 10,072 (36%) had silicosis (Han et al. 2018). New cases of lung diseases caused by coal dust made up 44.83% of all occupational diseases (Han et al. 2018). By the end of 2018, the cumulative reports of occupational pneumoconiosis had been around 870,000 cases (Data available at http://www.gov.cn/zhengce/2019-05/14/content_5391271.htm), of which 193 cases (0.69%) had asbestosis (Chen et al. 2020). For the data of coal dust exposure, it was estimated that the maximum concentration of coal dust was between 198–3420 mg/m3, which was 49.5–855 times that of the standard of occupational exposure limit (Han et al. 2018).
In addition to its high prevalence, pneumoconiosis is also difficult to diagnose and treat. Difficult diagnosis is due to complicated assessment procedures and the need for highly-trained medical personnel. In general, patients are diagnosed with pneumoconiosis at later disease stages, when their conditions are often too serious for treatment to have any positive effect. There is no effective treatment that can reverse lung damage caused by coal dust, although certain interventions can help to slow down disease progression, relieve symptoms and improve patients’ quality of life (Ochmann et al. 2012).
Many studies had explored the contribution of genetic factors to the susceptibility to develop pneumoconiosis, and they demonstrated the combined influence of genotypes and genetic polymorphisms in risk alleles to such susceptibility. Nevertheless, with genetic makeup known to be different among individuals between ethnicities (Huang et al. 2015), it is likely that the genetic variations that contribute to susceptibility to pneumoconiosis among individuals would also likely be different between ethnicities. Also, given the different characteristics of the population and the environment of the coal mines alike in different countries, it is likely that the findings on the identified genetic variations associated with pneumoconiosis risk among individuals, reported by studies in different countries may not necessarily apply to China (Li et al. 2007), the country with the highest coal production, the highest number of coal miners and the highest prevalence of CWP in the world (Han et al. 2018). To date, many genetic association studies were conducted in China, uncovering the contribution of genetic background in susceptibility of pneumoconiosis in Chinese populations. Given the complex and ethnic-group-dependent interplay between genetic and environmental factors imposes a challenge for us to understand the aetiology of the complex diseases, a review on the identified association between genetic polymorphism and pneumoconiosis, specifically in China, is needed. This review aims to provide a systematic overview of evidence for the association between genetic polymorphisms and different types of pneumoconiosis in China.
Methods
Search strategy
We performed a literature search according to the criteria outlined in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline (Moher et al. 2009). No registration for the protocol was made. Literature search was conducted in December 2021 and repeated in May 2022, using seven databases including PubMed, Ovid Medline, Ovid Nursing, Embase and CINAHL, as well as Chinese databases of Wanfang and CNKI using the search strategy shown in Table 1. We also performed manual searches by screening the reference section of the included studies.
Eligibility criteria
Studies were eligible for inclusion if they were written in English or Chinese, reporting cohort or case–control studies comparing the genetic variations between control subjects and pneumoconiosis patients in China. Studies were excluded if they are reviews, study protocols, conference abstracts or original articles reporting animal- or cell-based studies. Studies with outcomes not measuring pneumoconiosis susceptibility or the study population not on Chinese were also excluded.
Data extraction
Using the Covidence software (available at www.covidence.org), duplicated articles retrieved during the literature search were first removed. The titles and abstracts of the retrieved articles were then screened, and articles reporting studies irrelevant to the review aim were removed. Examination of the full-text of the remaining articles was performed independently by two authors to confirm their eligibility for inclusion. For each included study, one author completed data extraction in full, and the second author verified all the data extracted to ensure their accuracy. Extracted data included study centre, study design, study population, cohort size, concerned gene polymorphism, statistical measures of outcome and confounders such as smoking status, age, dust exposure year. Conflicts were discussed between the two authors and a third author was consulted in case a consensus could not be reached. We anticipated a high degree of heterogeneity among the included studies in terms of the gene polymorphisms and the type of pneumoconiosis examined, resulting in an insufficient number of studies examining the association between a particular gene polymorphism and a particular type of pneumoconiosis for a meta-analysis. The conduction of a meta-analysis of the extracted data was therefore not planned. The findings of the included studies were presented in a narrative and tabular manner.
Quality assessment
All included studies were subjected to methodological quality assessment based on a 9-item quality checklist, with assessment items taken from the Strengthening the Reporting of Genetic Association Studies (STREGA) Statement that provides recommendations on the reporting of genetic association studies (Little et al. 2009). The quality assessment evaluates whether the included studies have provided an adequate description of the following: (1) The method and centre for genotyping; (2) Number of genotyped samples and successful rate; (3) Methods on determine genotypes, haplotypes and population stratification; (4) Statement on Hardy–Weinberg equilibrium and novelty of genetic association. A total quality score for each study was calculated by adding all the corresponding quality item scores (range: 0–9), with higher scores indicating higher overall quality. The quality item score was then expressed as a percentage, and studies were categorised as high quality, moderate quality or low quality based on this percentage score, as done previously (Andric et al. 2021). Studies achieving a percentage score of over 80% were considered high quality. Those with a percentage score of 50–80% were considered moderate quality, and those having a percentage score lower than 50% were of low quality. In addition to STREGA that evaluate the genotyping methods, we have considered other confounders such as smoking status and dust exposure time which have given importance of gene-environment interactions.
Results
Search results
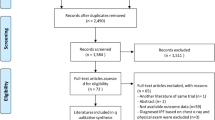
We retrieved 681 studies during the literature search. After excluding 77 duplicated articles and a further 518 studies owing to their inability to fit the eligibility criteria, we examined the full-text of the remaining 86 articles. Forty-four of these studies were excluded as they were either reporting irrelevant outcomes (not measuring susceptibility of pneumoconiosis, N = 40), irrelevant study design (not original research study, N = 3) or inappropriate study population (not Chinese population, N = 1). Three additional articles were sourced through reference list, resulting in the inclusion of 45 studies in this review. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram depicting the search results is shown in Fig. 1.
Quality of included studies
The quality score for each included study, as assessed using the 9-item checklist, is shown in Table 2. Among the included studies, their quality scores range between 22.2% and 66.7%. Nine studies scored as low quality and thirty-six studies as moderate quality. Most of the studies reported well on the genotyping methods (98%), number of genotypes samples (96%) and Hardy–Weinberg equilibrium consideration (89%). However, none of the studies provided a hint on whether the genotyping was done in one single batch or a few smaller batches. In addition, only one of the studies described the centre at which the genotyping was performed (2%) and few studies described any methods on determining genotypes or haplotypes (16%). About half of the studies (45%) indicated that the study was the first to show the association between gene polymorphism concerned and pneumoconiosis. Other confounding factors concerning gene-environment interactions was listed in the Supplementary Table.
Characteristics of included studies
The characteristics of the included studies are listed in the Supplementary Table. All included studies are case–control studies. These studies were published between 2001 and 2021 (Bian et al. 2014; Chen et al. 2014, 2015; Chu et al. 2014; Dang et al. 2012; Fan et al. 2021; Fang et al. 2011; Han et al. 2015b; Ji et al. 2012, 2014, 2015, 2017; Jin et al. 2012; Liu et al. 2017; Ni et al. 2007, 2009; Qian et al. 2010; Qu et al. 2007; Tang et al. 2020; Wang et al. 2011, 2012a, b, 2013, 2014, 2018a, b, c; Weng et al. 2015; Wu et al. 2008a, b, 2014, 2016; Xu et al. 2006, 2010; Yang et al. 2009, 2015, 2020; Yu et al. 2009; Yuan et al. 2017, 2018a, b, 2020; Zhai et al. 2001, 2002; Zhang et al. 2011). All investigated the association between genetic polymorphism and pneumoconiosis in the Chinese population. Among the included studies, 34 studies involved subjects with CWP (Bian et al. 2014; Chen et al. 2015; Chu et al. 2014; Dang et al. 2012; Han et al. 2015b; Ji et al. 2012, 2014, 2015, 2017; Jin et al. 2012; Liu et al. 2017; Ni et al. 2007, 2009; Qian et al. 2010; Tang et al. 2020; Wang et al. 2011, 2012b, 2013, 2014, 2018a; Wu et al. 2014, 2016; Xu et al. 2010; Yang et al. 2009, 2015, 2020; Yu et al. 2009; Yuan et al. 2017, 2018a, b, 2020; Zhai et al. 2001, 2002; Zhang et al. 2011) while ten other studies involved subjects with silicosis (Chen et al. 2014; Fan et al. 2021; Fang et al. 2011; Qu et al. 2007; Wang et al. 2012a; Wang et al. 2018b, c; Weng et al. 2015; Wu et al. 2008a, b). One additional study had subjects with asbestosis (Xu et al. 2006). Many of the included studies involved examination of genetic polymorphisms of cytokine genes including IL-1 (Dang et al. 2012; Ji et al. 2012; Weng et al. 2015; Wu et al. 2008b), IL-4 (Fang et al. 2011; Wang et al. 2011), IL-6 (Zhai et al. 2001), IL-10 (Wu et al. 2008a), IL-13 (Wang et al. 2011), IL-17 (Chen et al. 2014; Han et al. 2015b), IL-18 (Ji et al. 2012), TNF-α (Qu et al. 2007; Wang et al. 2012a; Wu et al. 2008a; Xu et al. 2006; Yang et al. 2009; Yu et al. 2009), IFN-γ (Wu et al. 2008a). The genes and genotypes associated with pneumoconiosis risk were summarized in Table 3.
Genetic susceptibility of pneumoconiosis in China
Association of cytokine genes with pneumoconiosis
Pneumoconiosis results from the accumulation of fine inhaled particles. As the inhaled particles enter the lung, alveolar macrophages take up the particles via phagocytosis and the resulting release of inflammatory cytokines is the first and most important step in pneumoconiosis. Therefore, the association between cytokine gene polymorphisms and pneumoconiosis was analysed.
In the included studies of this review, TNF-α genotype -308G/A was reported to be significantly associated with increased risk of CWP, silicosis and asbestosis (Wang et al. 2012a; Xu et al. 2006; Yang et al. 2009). The multivariate unconditional logistic regression model presented by Wang et al. (Wang et al. 2012a) showed that the association between 3 SNPs (TNF-α -308, TNF-α -238 and IL-1RA + 2018) and silicosis was statistically significant, where all three SNPs led to increased silicosis risk among the subjects. Consistent with this, Xu et al. (Xu et al. 2006) reported that TNF-α -308G/A genotype was associated with an increased risk of asbestosis (OR = 4.44, 95% CI 1.27–15.54, p = 0.014) (Xu et al. 2006), while Yang et al. (Yang et al. 2009) reported an increased risk of CWP among individuals carrying the minor allele of TNF-α -308G/A genotype (p < 0.05) (Yang et al. 2009). However, conflicting results were reported by Wu et al. (Wu et al. 2008a), where the authors showed no association between TNF-α -308 genotypes and the risk of silicosis (Wu et al. 2008a).
For IL-1β, four studies included in this review reported negative association of IL-1β genotype with the risk of CWP (Dang et al. 2012; Ji et al. 2012) and silicosis (Weng et al. 2015; Wu et al. 2008b). Nevertheless, Weng et al. (2015) reported no significant association between IL-1 genotype and silicosis risk when the data were stratified before analysis (Weng et al. 2015). Furthermore, Wu et al. (2008b) did not report any association between a number of cytokine genes (IL-1A, IL-1B and IL-1RN) and risk of silicosis (Wu et al. 2008b).
Two included studies demonstrated the protective effect of IL-4 genotypic variants on CWP and silicosis (Fang et al. 2011; Wang et al. 2011). Wang et al. (2011) reported that individuals bearing the minor C allele of IL-4 -590 showed were at lower risk of CWP when compared to those bearing the T allele (OR = 0.80, 95% CI 0.65–0.98, p = 0.034). Individuals with CT/CC genotype also had lower risk of CWP than those having the TT genotype (OR = 0.73, 95% CI .57–0.94, p = 0.016). In addition, combined polymorphism analysis of IL-4 and IL-13 demonstrated a dose-dependent increase in silicosis risk with increasing numbers of risk variant alleles (Ptrend = 0.023). A protective effect of IL-4 CT/CC genotype on silicosis was also reported by Fang et al. (p < 0.05) (Fang et al. 2011).
Zhai et al. (2001) investigated the prevalence of IL-6 -174G/C SNP in CWP patients and found that this IL-6 polymorphism at -174 is extremely rare in the study population and is unlikely to be associated with CWP susceptibility.
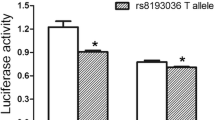
As shown by Han et al. (Han et al. 2015b), individuals bearing the AA genotype of the rs3748067 SNP of IL-17A were at significantly reduced risk of CWP compared to those having the GG genotype (OR: 0.43; 95% CI 0.23 – 0.83; p = 0.011), while those having the CC genotype of rs8193036 SNP of IL-17A were at significantly lower CWP risk when compared with those having the TT genotype (OR: 0.59; 95% CI 0.40 – 0.86; p = 0.006) (Han et al. 2015b). Further, Chen et al. reported the protective effect of genetic polymorphisms in another member of IL-17. They showed that GA genotype of IL17F of the 7488A/G SNP conferred a decreased risk of silicosis (OR: 0.374; 95% CI 0.198 – 0.705; p = 0.002).
No association was found between the examined SNPs of IL-10 (Wu et al. 2008a), IL-13 (Wang et al. 2011), IL-18 (Ji et al. 2012) and IFN-γ (Wu et al. 2008a) and the risk of pneumoconiosis.
Gene-environment interaction
Stratification analyses in some of the included studies revealed that the associations between gene polymorphisms and pneumoconiosis risk could be affected by environmental factors. For example, the association between SNPs of the potential pneumoconiosis susceptibility genes and CWP risk was demonstrated to be significantly modified under certain environmental conditions, including duration of dust exposure (Ji et al. 2012, 2015; Ni et al. 2007; Qian et al. 2010; Wang et al. 2012a, 2018a; Wu et al. 2016; Yang et al. 2020; Yuan et al. 2018a), smoking (Ji et al. 2012, 2014, 2015; Jin et al. 2012; Liu et al. 2017; Ni et al. 2007; Qu et al. 2007; Wang et al. 2014; Wu et al. 2016; Xu et al. 2006; Yang et al. 2020; Yuan et al. 2018a), age (Ji et al. 2012; Jin et al. 2012; Qian et al. 2010; Wang et al. 2012a, 2014; Yuan et al. 2018a) and pneumoconiosis stage (Ji et al. 2012, 2014; Jin et al. 2012; Qu et al. 2007; Wang et al. 2012a, 2018a; Weng et al. 2015; Wu et al. 2016; Xu et al. 2006; Yuan et al. 2018a). Nevertheless, findings on the effect of these environmental factors on such association appear inconsistent between studies. Association between genotypic variations and decreased risk of pneumoconiosis was reported in shorter dust exposure time (Liu et al. 2017; Yuan et al. 2017) and in longer dust exposure time (Han et al. 2015b; Wang et al. 2011), in smokers (Han et al. 2015b; Ji et al. 2017; Jin et al. 2012; Yuan et al. 2017) and non-smokers (Liu et al. 2017; Qian et al. 2010; Wu et al. 2016), in younger age (Han et al. 2015b; Ji et al. 2017; Wang et al. 2011; Yuan et al. 2017) and older age (Ji et al. 2017), as well as in different stages of CWP or silicosis (Stage I (Han et al. 2015b; Ji et al. 2017; Wang et al. 2011; Wu et al. 2016; Yuan et al. 2017), Stage II (Yuan et al. 2017). On the other hand, association between genotypic variations and increased risk of pneumoconiosis was reported in shorter dust exposure time (Ji et al. 2014; Yang et al. 2015) or in longer dust exposure time (Ni et al.2009; Wang et al.2014; Wu et al.2014; Yang et al.2015), in smoker (Ji et al. 2012, 2014) or non-smoker (Qian et al. 2010; Wang et al. 2013; Wu et al. 2014; Yang et al. 2015), in younger age (Ni et al. 2009) or older age (Wang et al. 2013), as well as in different stages of CWP or silicosis Stage I (Ji et al. 2012; Qian et al. 2010), Stage II/III (Qian et al. 2010; Wang et al. 2011, 2014, 2018c; Wu et al. 2014).
Three included studies reported the interaction of genotypes and different environmental factors using multifactor dimensionality reduction (MDR) analysis. Yang et al. (2020) and Tang et al. (2020) demonstrated that the strongest interaction in CWP was detected between genotypes and dust exposure time. Another study found that the number of cigarettes smoked was the strongest factor for predicting CWP risk (Wang et al. 2013).
Discussion
China, the United States and Australia were among the top three coal-producing countries in the world in 2017. However, the prevalence of CWP in China (6.02%) has been comparatively higher than that in the United States (1.88%) and Australia (< 0.5%) (Mo et al. 2014; Laney et al. 2010; Joy et al. 2012). The high prevalence of pneumoconiosis in China therefore raised a concern on the local population’s health. In the past decades, it is recognised that genetic variations may contribute greatly to the development of pneumoconiosis and such variations are influenced by environmental factors. In addition, genetic variations are also different among ethnic groups. For these reasons, we conducted a comprehensive review to summarize the findings in China exclusively. Our findings demonstrated that genes of cytokines and inflammasomes have strong association with the risk of pneumoconiosis. In addition, the interaction between gene polymorphisms and environmental factors is also critical on pneumoconiosis susceptibility.
Cytokines have been shown to play important roles in the pathogenesis of dust particle-induced pulmonary inflammatory reactions and chronic inflammatory diseases (Rimal et al. 2005; Wynn et al. 2004; Wilson et al. 2009). In the included studies, cytokines such as IL-1 (Dang et al. 2012; Ji et al. 2012; Weng et al. 2015; Wu et al. 2008b), IL-4 (Fang et al. 2011; Wang et al. 2011), IL-6 (Zhai et al. 2001), IL-17 (Chen et al. 2014; Han et al. 2015b), TNF-α (Qu et al. 2007; Wang et al. 2012a; Wu et al. 2008a; Xu et al. 2006; Yang et al. 2009; Yu et al. 2009) were reported to be associated with the risk of CWP and silicosis among people in China. Wang et al. (2012a) showed that the TNF-α (-308), TNF-α (-238) and IL-1RA (+ 2018) SNPs were significantly associated with silicosis. Similar findings were also reported by studies in other countries. For example, Yucesoy et al. (2001) examined the association between TNF-α and IL-1 polymorphisms and silicosis in a case–control study with 489 Caucasian in the United States (Yucesoy et al. 2001). Carriers of the minor variants of the TNF-α (-308) and IL-1RA (+ 2018) SNPs showed an increased risk for both moderate and severe disease groups. Carriers of the minor variant of the TNF-α (-238) SNP were also shown to be at markedly higher risk for severe silicosis (OR = 4.0), although they would be at 50% lower risk for moderate silicosis (OR = 0.52). No significant differences in the distribution of the IL-1α (+ 4845) or IL-1β (+ 3953) variants with respect to disease severity (Yucesoy et al. 2001).
Pneumoconiosis is a disease which is characterized by chronic inflammation and formation of fibrotic nodular lesions, leading to progressive fibrosis in the lungs (McCunney et al. 2009). Inflammasomes have been demonstrated to play a crucial role in the host response to silica and asbestos. Indeed, the Nalp3 inflammasome is an important innate immune receptor involved in the recognition of silica and asbestos by macrophages. Stimulation of macrophages with silica results in the activation of caspase-1 in a Nalp3-dependent manner (Cassel et al. 2008; Dostert et al. 2008). Macrophages deficient in components of the Nalp3 inflammasome were incapable of secreting the proinflammatory cytokines IL-1β in response to silica. Therefore, the impact of genetic polymorphisms of Nalp3, caspase-1 and IL-1β on pneumoconiosis may be crucial for pneumoconiosis susceptibility (Cassel et al. 2008; Dostert et al. 2008). One of the included studies in this review (Weng et al. 2015) reported the association between Nalp3, caspase-1 and IL-1β polymorphisms and the risk of silicosis among people in China. Subjects with the CT genotype of Ex4-849C > T in Nalp3 and the GA genotype of Ex2 + 37G > A in caspase-1 had increased risks of silicosis. Moreover, the association of genetic polymorphisms with silicosis risk may be affected by age, dust exposure or smoking status (Weng et al. 2015). The polymorphisms of another gene COX-2, which is also important for the synthesis of inflammatory mediator prostaglandin E2, was demonstrated to be associated with the risk of CWP in Chinese coal miners (Bian et al. 2014). Two COX-2 SNPs (rs689466 and rs20417) were found to be significant risk factors in decreasing the risk of CWP, providing further evidence for a role of inflammatory pathway in the pathogenesis of pneumoconiosis.
Reactive oxygen species (ROS) are key mediators of inflammation. Studies have suggested that exposure to mineral dust particles generate ROS, which are implicated directly in the pathogenesis of pneumoconiosis (Mastruzzo et al. 2002; Wallaert 1990; Schins et al. 1999). Therefore, the inhibitory effects of antioxidants on pneumoconiosis are of value to be further explored. Antioxidant gene polymorphisms were investigated in one of the included studies by Zhai et al. (2002). However, no association between MnSOD, GSTM1, GSTT1 or OGG1 genetic polymorphisms and CWP was found in Chinese coal miners. In the United States, a matched case–control study was conducted to determine the influence of the GSTP1, GSTT1 and MnSOD polymorphisms on the susceptibility to progressive massive fibrosis (PMF), a severe form of CWP (Yucesoy et al. 2005). Their findings were similar to those observed by Zhai et al. (2002). The allele frequencies for GSTP1, GSTT1 and MnSOD in the control population were similar to those determined in other studies involving Caucasian participants. No significant differences were found in the genotype frequencies or the allelic frequencies between PMF cases and controls for the GSTT1, GSTP1 or MnSOD. In addition, no significant gene–gene interactions were detected (Yucesoy et al. 2005). This may imply that polymorphisms of antioxidant genes are not associated with susceptibility of pneumoconiosis, and suggests that ROS may not be directly involved in the pathogenesis of pneumoconiosis.
The occurrence of pneumoconiosis is multifactorial. These factors include dust exposure time, smoking status, patients’ age and genetic makeup. Dust levels exposed to the patients are of most importance. Age and dust exposure time are directly accounted for the dust levels exposed, while smoking status and genetic makeup are modifiers of pneumoconiosis susceptibility. An early study in 1975 had demonstrated the effect of dust exposures time and smoking habits on the attack rate of pneumoconiosis among coal miners (Jacobsen et al. 1975). Few studies provided quantitative data to ascertain the relationships between occupational dust exposure, cigarette smoking, and emphysema severity (Ruckley et al. 1984; Leigh et al. 1994). Among the included studies of this review, 15 studies reported the effect of smoking status on the genotypic association with pneumoconiosis risk (Han et al. 2015b; Ji et al. 2012; Ji et al. 2015; Ji et al. 2017; Jin et al. 2012; Liu et al. 2017; Wang et al. 2013; Wang et al. 2014; Weng et al. 2015; Wu et al. 2014; Wu et al. 2016; Xu et al. 2006; Xu et al. 2010; Yang 2009; Yang et al. 2015) while nine studies reported the effect of dust exposure time on such association (Ji et al. 2014; Ni et al. 2009; Qu et al. 2007; Wang et al. 2011; Wang et al. 2012b, 2018b, c; Wu et al. 2016; Yang et al. 2015). Although smoking status and dust exposure time were found to be crucial factors in pneumoconiosis (Leung et al. 2012; Fan et al. 2020), the influence of these factors on genetic susceptibility in pneumoconiosis were not consistent among the included studies. Similarly, the effects of disease stage on risk of pneumoconiosis were inconsistently reported among the included studies and other studies conducted in other countries. However, in this review, more studies reported the association between genotypes and pneumoconiosis risk among individuals with stage I CWP/silicosis on such association (Han et al. 2015b; Ji et al. 2012, 2015, 2017; Liu et al. 2017; Ni et al. 2007, 2009; Qian et al. 2010; Wang et al. 2011; Wu et al. 2016) while fewer reported such association among individuals at stage II/III (Wang et al. 2013, 2018b, c; Wu et al. 2014). The results are more consistent among the studies reporting the effect of patients’ age of such association, where increased risk of CWP and silicosis with specific genotypes was more evident at higher age (Ji et al. 2017; Ni et al. 2009; Wang et al. 2011; Weng et al. 2015). Globally, the largest number of CWP cases was shifting to higher age groups, from aged 35–39 in 1990 to aged 50–54 in 2019 (Wang et al. 2021). However, statistical data are lacking in China to confirm if the increased pneumoconiosis risk is directly related to age.
The effect of environmental factors on genetic susceptibility of pneumoconiosis were found to be controversial. A possible reason for this may be the small sample sizes used in the studies, thereby generating a higher risk of bias in study findings that may contribute to inconsistencies of findings between studies. Moreover, the effect of the aforementioned environmental factors on the association of the genotype of a particular susceptibility gene and pneumoconiosis risk was understudied, with such effect reported by one single trial only. Therefore, more studies are needed to consolidate the findings related to the environmental effects on the association between genotypes and pneumoconiosis risks, which may shed light on the future planning of occupational disease control in China.
Limitations
There are some limitations of this review that need to be acknowledged as follows: (1) Due to the heterogeneity of various genetic polymorphisms examined in the studies, pooled effects using a meta-analysis was not feasible, limiting our ability to visualise the variations in the level of the association between the examined genotypes and pneumoconiosis risk; (2) Most of the genetic polymorphisms examined for a particular gene were only reported by one case–control study. More studies are required to consolidate the findings; (3) Given some studies of low quality rating, we should be cautious in interpreting the review findings; (4) The findings regarding the association between the reported genotypes and pneumoconiosis risks may not apply to populations in other countries because this review only include the studies conducted in China.
Conclusion
Identifying the factors affecting the susceptibility of pneumoconiosis may offer clues to the development of strategies to prevent or monitor the disease. As pneumoconiosis is a complicated and multifactorial disease without any effective treatment, and that the prevalence of this disease is high in China, it is of value to provide an overview on the potential factors affecting pneumoconiosis susceptibility in China. Genetic polymorphisms may explain the differences in outcomes of pneumoconiosis resulting from similar working conditions or exposure history. In this review, different genotypes were found to influence the pneumoconiosis susceptibility in patients in China, in particular the genes involved in the inflammatory pathway such as cytokines and inflammasomes. We also found that such associations were affected by various environmental factors, including duration of dust exposure, age, pneumoconiosis stage and smoking habits, although there were inconsistencies on such findings between studies. Given the effects of genetic variations on pneumoconiosis risks, genetic testing could potentially serve as a tool for the identification of individuals with increased genetic susceptibility to the disease especially in countries with high numbers of coal mine workers such as China if the conflicting results can be verified by more studies conducted on the issue.
References
Andric M, Jacimovic J, Jakovlijevic A, Nikolic N, Milasin J (2021) Gene polymorphisms in odontogenic keratocysts and ameloblastomas: a systematic review. Oral Dis. https://doi.org/10.1111/odi.13865
Bian LQ, Mao L, Shi J, Bi Y (2014) Polymorphisms in cyclooxygenase-2 gene and risk of developing coal workers’ pneumoconiosis: a case-control study. Am J Ind Med 57:866–871
Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA et al (2008) The Nalp3inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA 105:9035–9040
Chen Y, Fan XY, Jin YL, Yao SQ, Yun X, Hua ZB et al (2014) Association between polymorphisms of interleukin-17A and interleukin-17F genes and silicosis susceptibility in Chinese Han people. Asian Pac J Cancer Prev 15:8775–8778
Chen C, Wang L, Yang J, Wang T, Ji X, Wu B et al (2015) Gene variance in microsomal epoxide hydrolase and the susceptibility of coal workers’ pneumoconiosis. Chin J Ind Hyg Occup Dis 33:492–495 (in Chinese)
Chen M, Wang H, Zhang J, Yu C, Liu W, Xu Y (2020) Distribution of asbestos enterprises and asbestosis cases - China, 1997–2019. China CDC Wkly 2(18):305–309
Chu M, Ji X, Chen W, Zhang R, Sun C, Wang T et al (2014) A genome-wide association study identifies susceptibility loci of silica-related pneumoconiosis in Han Chinese. Hum Mol Genet 23:6385–6394
Cullinan P, Reid P (2013) Pneumoconiosis. Prim Care Respir J 22:249–252
Dang Z, Song Q, Yang W, Li W (2012) Relationship between interleukin-1β (511C/T) gene polymorphisms and coal worker’s pneumoconiosis in Ningxia. J Ningxia Med Uni 34:52–54 (in Chinese)
Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677
Fan Y, Xu W, Wang Y, Wang Y, Yu S, Ye Q (2020) Association of occupational dust exposure with combined chronic obstructive pulmonary disease and pneumoconiosis: a cross-sectional study in China. BMJ Open 10:e038874
Fan Y, Zheng C, Wu N, Li Y, Huang X, Ye Q (2021) Telomerase gene variants and telomere shortening in patients with silicosis or asbestosis. Occup Environ Med 78:342–348
Fang GF, Fan XY, Shen FH (2011) The relationship between polymorphisms of interleukin-4 gene and silicosis. Biomed Environ Sci 24:678–682
Han L, Han R, Ji X, Wang T, Yang J, Yuan J et al (2015a) Prevalence characteristics of coal workers’ pneumoconiosis (CWP) in a State-Owned Mine in Eastern China. Int J Environ Res Public Health 12:7856–7867
Han R, Ji X, Wu B, Wang T, Han L, Yang J et al (2015b) Polymorphisms in interleukin 17A gene and coal workers’ pneumoconiosis risk in a Chinese population. BMC Pulm Med 15:79
Han S, Chen H, Harvey M, Stemn E, Cliff D (2018) Focusing on coal workers’ lung diseases: a comparative analysis of China, Australia, and the United States. Intl J Environ Res Public Health 15:2565
Huang T, Shu Y, Cai Y (2015) Genetic differences among ethnic groups. BMC Genomics 16:1093
Jacobsen M, Burns J, Attfield MD (1975) Smoking and coal workers’ simple pneumoconiosis. Inhaled Part 4(Pt 2):759–772
Ji X, Hou Z, Wang T, Jin K, Fan J, Luo C et al (2012) Polymorphisms in inflammasome genes and risk of coal workers’ pneumoconiosis in a Chinese population. PLoS One 7:e47949
Ji X, Wu B, Jin K, Luo C, Han R, Chen M et al (2014) MUC5B promoter polymorphisms and risk of coal workers’ pneumoconiosis in a Chinese population. Mol Biol Rep 41:4171–4176
Ji X, Wang L, Wu B, Han R, Han L, Wang T et al (2015) Associations of MMP1, MMP2 and MMP3 genes polymorphism with coal workers’ pneumoconiosis in Chinese Han population. Int J Environ Res Public Health 12:13901–13912
Ji X, Wu B, Han R, Yang J, Ayaaba E, Wang T et al (2017) The association of LAMB1 polymorphism and expression changes with the risk of coal workers’ pneumoconiosis. Environ Toxicol 32:2182–2190
Jin K, Ji X, Wang S, Song Z, Hou Z, Wang T et al (2012) The MIF -173G/C polymorphism and risk of coal workers’ pneumoconiosis in a Chinese population. Int J Interferon, Cytokine Mediat Res 4:37–42
Joy GJ, Colinet JF, Landen D (2012) Coal workers’ pneumoconiosis prevalence disparity between Australia and the United States. Min Eng 64:65–71
Laney AS, Attfield MD (2010) Coal workers’ pneumoconiosis and progressive massive fibrosis are increasingly more prevalent among workers in small underground coal mines in the United States. Occup Environ Med 67:428–431
Leigh J, Driscoll TR, Cole BD, Beck RW, Hull BP, Yang J (1994) Quantitative relation between emphysema and lung mineral content in coal workers. Occup Environ Med 51:400–407
Leung CC, Yu IT, Chen W (2012) Silicosis. Lancet 379:2008–2018
Li C, Yu Q, Ye Z, Sun Y, He Q, Li X et al (2007) A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: potential like with severe adverse reactions to oseltamivir. Cell Res 17:357–362
Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E et al (2009) STrengthening the REporting of genetic association studies (STREGA) – an extension of the STROBE statement. Eur J Clin Invest 39:247–266
Liu Y, Yang J, Wu Q, Han R, Yan W, Yuan J et al (2017) LRBA gene polymorphisms and risk of coal workers’ pneumoconiosis: a case-control study from China. Int J Environ Res Public Health 14:1138
Mastruzzo C, Crimi N, Vancheri C (2002) Role of oxidative stress in pulmonary fibrosis. Monaldi Arch Chest Dis 57:173–176
McCunney RJ, Morfeld P, Payne S (2009) What component of coal causes coal workers’ pneumoconiosis? J Occup Environ Med 51:462–471
Mo J, Wang L, Au W, Su M (2014) Prevalence of coal workers’ pneumoconiosis in China: a systematic analysis of 2001–2011 studies. Int J Hyg Environ Health 217:46–51
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Ni C, Song Z, Jia X, Li A, Li C, Li S et al (2007) Relationship between FAS/FASL gene polymorphisms and susceptibility of coal worker’s pneumoconiosis. Chin J Ind Hyg Occup Dis 25:532–536 (in Chinese)
Ni C, Ye Y, Wang M, Qian H, Song Z, Jia X et al (2009) A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with risk of coal workers’ pneumoconiosis. J Toxicol Environ Health A 72:712–716
Ochmann U, Kotschy-Lang N, Raab W, Kellberger J, Nowak D, Jörres RA (2012) Long-term efficacy of pulmonary rehabilitation in patients with occupational respiratory diseases. Respiration 84:396–405
Qian H, Song Z, Wang M, Jia X, Li A, Yang Y et al (2010) Association of transforming growth factor-β1 gene variants with risk of coal workers’ pneumoconiosis. J Biomed Res 24:270–276
Qu Y, Tang Y, Cao D, Wu F, Liu J, Lu G et al (2007) Genetic polymorphisms in alveolar macrophage response-related genes, and risk of silicosis and pulmonary tuberculosis in Chinese iron miners. Int J Hyg Environ Health 210:679–689
Rimal B, Greenberg AK, Rom WN (2005) Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med 11:169–173
Ruckley VA, Gauld SJ, Chapman JS, Davis JMG, Douglas AN, Furney JM et al (1984) Emphysema and dust exposure in a group of coal workers. Am Rev Respir Dis 125:528–532
Schins RP, Borm PJ (1999) Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg 43:7–33
Tang Y, Duan J, Wang Y, Yuan L (2020) Associations of HMGB1 gene polymorphisms with risk of coal workers’ pneumoconiosis susceptibility in Chinese Han population. Inhal Toxicol 32:170–176
Wallaert B, Lassalle P, Fortin F, Aerts C, Bart F, Fournier E et al (1990) Superoxide anion generation by alveolar inflammatory cells in simple pneumoconiosis and in progressive massive fibrosis of nonsmoking coal workers. Am Rev Respir Dis 141:129–133
Wang M, Wang S, Song Z, Ji X, Zhang Z, Zhou J et al (2011) Associations of IL-4, IL-4R, and IL-13 gene polymorphisms in coal workers’ pneumoconiosis in China: a case-control study. PLoS One 6:e22624
Wang YW, Lan JY, Yang LY, Wang D, Kuang J (2012a) TNF-α and IL-1RA polymorphisms and silicosis susceptibility in Chinese workers exposed to silica particles: a case-control study. Biomed Environ Sci 25:517–525
Wang T, Wang S, Hou Z, Ji X, Song Z, Jia X et al (2012b) Annexin 5 gene polymorphism (-1C/T) and the susceptibility to pneumoconiosis in coal works. Chin J Ind Hyg Occup Dis 30:246–249 (in Chinese)
Wang T, Ji X, Luo C, Fan J, Hou Z, Chen M et al (2013) Polymorphisms in SELE gene and risk of coal workers’ pneumoconiosis in Chinese: a case-control study. PLoS One 8:e73254
Wang T, Yang J, Han R, Ji X, Wu B, Han L et al (2014) Polymorphisms in SPARC and coal workers’ pneumoconiosis risk in a Chinese population. PLoS One 9:e105226
Wang T, Li Y, Zhu M, Yao W, Wu H, Ji X et al (2018a) Association analysis identifies new risk loci for coal workers’ pneumoconiosis in Han Chinese Men. Toxicol Sci 163:206–213
Wang W, Yu Y, Wu S, Sang L, Wang X, Qiu A et al (2018b) The rs2609255 polymorphism in the FAM13A gene is reproducibly associated with silicosis susceptibility in a Chinese population. Gene 661:196–201
Wang W, Yu Y, Xiao J, Gao Y, Sang L, Lian Y et al (2018c) A novel variant of desmoplakin is potentially associated with silicosis risk. DNA Cell Biol 37:925–931
Weng S, Wang L, Rong Y, Liu Y, Wang X, Guan H et al (2015) Effects of the interactions between dust exposure and genetic polymorphisms in Nalp3, caspase-1, and IL-1β on the risk of silicosis: a case-control study. PLoS One 10:e0140952
Wilson MS, Wynn TA (2009) Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2:103–121
Wu F, Qu Y, Tang Y, Cao D, Sun P, Xia Z (2008a) Lack of association between cytokine gene polymorphisms and silicosis and pulmonary tuberculosis in Chinese iron miners. J Occup Health 50:445–454
Wu F, Xia Z, Qu Y, Tang Y, Cao D, Sun P et al (2008b) Genetic polymorphisms of IL-1A, IL-1B, IL-1RN, NFKB1, FAS, and FASL, and risk of silicosis in a Chinese occupational population. Am J Ind Med 51:843–851
Wu B, Ji X, Han R, Han L, Wang T, Yang J et al (2014) GITR promoter polymorphism contributes to risk of coal workers’ pneumoconiosis: a case-control study from China. Immunol Lett 162:210–216
Wu Q, Yan W, Han R, Yang J, Yuan J, Ji X et al (2016) Polymorphisms in long noncoding RNA H19 contribute to the protective effects of coal workers’ pneumoconiosis in a Chinese population. Int J Environ Res Public Health 13:903
Wynn TA (2004) Fibrotic disease and the Th1/Th2 paradigm. Nat Rev Immunol 4:583–594
Xu Z, Hou Q, Shi M, Xu M, Song Y, Pei X (2006) Study on the role of tumor necrosis factor-α and tumor necrosis factor receptor II gene polymorphisms in the pathogenesis of asbestosis. J Environ Occup Med 23:307–310 (in Chinese)
Xu J, Zhu M, Cai H, Zhang K, Duan W, Wang T et al (2010) SMAD4 gene polymorphisms and genetic susceptibility of coal work’s pneumoconiosis. Chin J Ind Hyg Occup Dis 28:766–771 (in Chinese)
Yang W, Wang F, Yang J, Song Q (2009) The relationship between gene polymorphism of TNF-α and coal worker pneumoconiosis in Ningxia. J Ningxia Med Uni 31:758–760 (in Chinese)
Yang J, Wang L, Wang T, Chen C, Han L, Ji X et al (2015) Associations of MMP-7 and OPN gene polymorphisms with risk of coal workers’ pneumoconiosis in a Chinese population: a case-control study. Inhal Toxicol 27:641–648
Yang X, Qin M, Cui S, Zhang Q (2020) Associations of VDR gene polymorphisms with risk of coal workers’ pneumoconiosis in Chinese Han population. Toxicol Res (camb) 9:399–405
Yu C, Li L, Qi F, Li D, Xu X (2009) Relationship between genetic polymorphisms of transforming growth factor-β and tumor necrosis factor and pneumoconiosis among coal workers. Chin J Ind Hyg Occup Dis 27:240–242 (in Chinese)
Yuan J, Han R, Esther A, Wu Q, Yang J, Yan W et al (2017) Polymorphisms in autophagy related genes and the coal workers’ pneumoconiosis in a Chinese population. Gene 632:36–42
Yuan B, Li B, Li C, Cui J (2018a) Correlation between single nucleotide polymorphisms of DPP9 gene and coal workers’ pneumoconiosis. Chi J Ind Med 31:87–89 (in Chinese)
Yuan B, Yuan W, Wen X, Li C, Gao L, Li B et al (2018b) Association of single nucleotide polymorphisms in the CYBA gene with coal workers’ pneumoconiosis in the Han Chinese population. Inhal Toxicol 30:492–497
Yuan B, Wen X, Li L, Li Y, Li C, Li B et al (2020) NAF1 rs4691896 is significantly associated with coal workers’ pneumoconiosis in a Chinese Han population: a case-control study. Med Sci Monit 26:e918709
Yucesoy B, Vallyathan V, Landsittel DP, Sharp DS, Weston A, Burleson GR et al (2001) Association of tumor necrosis factor-alpha and interleukin-1 gene polymorphisms with silicosis. Toxicol Appl Pharmacol 172:75–82
Yucesoy B, Johnson VJ, Kashon ML, Fluharty K, Vallyathan V, Luster MI (2005) Lack of association between antioxidant gene polymorphisms and progressive massive fibrosis in coal miners. Thorax 60:492–495
Zhai R, Liu G, Yang C, Huang C, Wu C, Christiani DC (2001) The G to C polymorphism at -174 of the interleukin-6 gene is rare in a Southern Chinese population. Pharmacogenetics 11:699–701
Zhai R, Liu G, Ge X, Yang C, Huang C, Wu C et al (2002) Genetic polymorphisms of MnSOD, GSTM1, GSTT1, and OGG1 in coal workers’ pneumoconiosis. J Occup Environ Med 44:372–377
Zhang H, Jin T, Zhang G, Chen L, Zou W, Li QQ (2011) Polymorphisms in heat-shock protein 70 genes are associated with coal workers’ pneumoconiosis in southwestern China. In Vivo 25:251–257
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chair, S.Y., Chan, J.Y.W., Law, B.M.H. et al. Genetic susceptibility in pneumoconiosis in China: a systematic review. Int Arch Occup Environ Health 96, 45–56 (2023). https://doi.org/10.1007/s00420-022-01893-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-022-01893-1