Abstract
Purpose
Accumulating evidence shows that effort–reward imbalance (ERI) at work can cause various health problems. However, few studies have investigated the biological pathways linking ERI and health outcomes, and their findings have been inconsistent. In this study, we investigated the associations between ERI, the hypothalamic–pituitary–adrenocortical axis, and inflammation in a sample of police officers.
Methods
One hundred forty-two male police officers that were engaged in a working system of 24-h shifts were followed up during the work shift as well as during the two subsequent work-free days. Throughout this period, the participants provided two saliva samples each day for the 3-day period, and we measured the concentrations of cortisol and C-reactive protein (CRP) in the saliva. The police officers also completed the Japanese short version of the Effort–Reward Imbalance Questionnaire.
Results
The results of linear mixed model analyses controlled for possible confounding variables indicated that higher effort scores (p = 0.031) as well as effort–reward ratio (p = 0.080) were associated with lower cortisol levels, and the effect of effort was strengthened in the younger police officers (p = 0.017). Furthermore, higher effort scores were associated with higher CRP levels in younger police officers (p = 0.037).
Conclusions
Our results indicate that effort, a component of ERI, has physiological effects in younger police officers, which possibly contribute to the development of stress-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effort–reward imbalance (ERI) model posits that work conditions of high-effort (e.g., work demands, obligation) coupled with low-reward (e.g., money, esteem, job security) are considered particularly stressful and could have adverse health effects (Siegrist 1996). Epidemiological studies have indicated that ERI in the work environment contributes to health problems such as coronary heart disease and depression (Kuper et al. 2002; Siegrist 1996; Siegrist et al. 1992; Tsutsumi et al. 2001a, b).
The physiological mechanism underlying the association between ERI and health problems remains unclear. A potential biological pathway linking ERI to health outcomes is the hypothalamic–pituitary–adrenocortical (HPA) axis. Acute psychosocial stress is known to activate the HPA axis, leading to elevated cortisol levels in the blood and saliva (Dickerson and Kemeny 2004). Moreover, HPA axis dysfunction is associated with various health problems such as depression and cardiovascular disease (McEwen 2000). Previous studies investigating the associations between ERI and cortisol levels (awakening and diurnal levels) yielded inconsistent results. Positive associations (Eller et al. 2006), inverse associations (Eller et al. 2012; Maina et al. 2009), and no association (Hanson et al. 2000; Harris et al. 2007; Irie et al. 2004; Marchand et al. 2016; Ota et al. 2014) between ERI and cortisol levels were reported. Characteristics of the participants (e.g., age, sex, and the type of occupation), sample size, and frequencies and timing of cortisol assessment differed between studies, which could affect their findings.
Another possible process linking ERI and health outcomes is inflammatory activity. It is well recognized that psychosocial stress could alter inflammatory activity (Segerstrom and Miller 2004). Further, inflammatory activity could contribute to the development of stress-related diseases, such as cardiovascular disease, by accelerating the process of arteriosclerosis (Steptoe and Brydon 2007). In previous studies, job stress was reported to be associated with higher levels of inflammatory markers including C-reactive protein (CRP), interleukin-6, and tumor necrosis factor (Nakata 2012). However, few studies have investigated the associations between ERI and inflammatory activity. Hamer et al. (2006) reported that higher ERI scores were associated with elevated CRP in response to acute experimental stress, and Almadi et al. (2012) reported a positive association between ERI and CRP in obese male workers. Finally, inflammatory activity is closely connected with HPA axis activity. Specifically, glucocorticoids inhibit the synthesis, release, and/or efficacy of cytokines and other mediators of immune and inflammatory responses (Sapolsky et al. 2000).
The present study focused on the job stress experienced by police officers working at police boxes in 24-h shifts. These officers are required to prepare for the possibility of accidents and emergencies by wearing stab jackets and carrying guns for a prolonged period, including nighttime, which could cause high stress and a chronic burden to their health. Previous studies indicate that the prevalence of cardiovascular diseases and cardiovascular risks in police offers is higher than that of the general population (Franke et al. 1998, 2002; Joseph et al. 2009). However, to our knowledge, no studies have investigated the biological consequences of job stress, such as that caused by ERI, in police officers.
In the present study, we investigated the associations between ERI, cortisol, and CRP in a sample of police officers during their 24-h work shift and the two subsequent work-free days. We took saliva samples and assessed their cortisol and CRP levels. Recent studies have demonstrated the biological validity of salivary CRP, including moderate correlations between circulating and salivary CRP (Ouellet-Morin et al. 2011; Punyadeera et al. 2011). We hypothesize that a stressful work environment, evaluated by ERI, alters the diurnal pattern or basal secretion level of cortisol, and contributes to higher CRP levels in police officers. In the sequence of statistical analyses, we investigated whether ERI components (effort and reward) altered the HPA and inflammatory activities, because previous studies reported biological correlates of only effort or reward (Eller et al. 2006; Maina et al. 2009). We also investigated the effects of ERI on biological parameters in younger and older police officers, considering a possible modification effect of age, because in the past the relationship between job stress and cardiovascular disease was stronger in younger workers (Chandola et al. 2008; Kuper and Marmot 2003; Theorell et al. 1998). Furthermore, correlations between cortisol and CRP levels were calculated considering the known anti-inflammatory effects of cortisol.
Methods
Participants
In this study, male police officers engaged in a working system of 24-h shifts were repeatedly measured for 3 days. The police officers were recruited from police offices located in Chiba Prefecture, Japan in August 2013. The sample initially consisted of 200 male police officers aged 31–59 years, randomly selected by their identification number. The sample size was determined by availability of financial resource. Fifty-eight subjects were excluded for various reasons: 21 for a history of possible inflammation- or adrenal-related diseases (cardiovascular diseases, liver disease, diabetes, or depression), 19 for current use of medications (steroidal, lipid-lowering, or anti-hypertensive medications, or analgesics), 15 for obesity (body mass index (BMI) ≥ 30), and 3 for missing data on the ERI questionnaire. Therefore, the final sample consisted of 142 male police officers. The participants’ mean (standard deviation [SD]) age was 43.0 (9.1) years. Their mean (SD) body mass index (BMI) was 24.3 (2.6) kg/m2, and 54 participants were current smokers. Written informed consent was obtained from participants, and the institute’s ethical committee approved the study (Kitasato University Medical Ethics Committee, B13-92).
Measurements
Saliva samples for cortisol and CRP measurements were obtained using a Salivette (Sarstedt Ltd.) with a polypropylene and polyethylene polymer swab. The participants were asked to place the swab under their tongue for 3 min to obtain the sample.
ERI was assessed using the 10-item Japanese short version of the Effort–Reward Imbalance Questionnaire (ERIQ), which includes three items for effort (e.g., “I have constant time pressure due to a heavy work load”) and seven items for reward assessment (e.g., “My job security is poor”). The 10-item Japanese short version of the ERIQ was reported to have acceptable internal consistency, reliability, and construct validity (Tsutsumi et al. 2002, 2008). In this sample, Cronbach’s alpha coefficients for effort and reward scales were 0.81 and 0.70, respectively. The effort–reward ratio was calculated by the formula effort/reward*c, where ‘c’ is a correction factor weighting the different numbers of items in numerator and denominator (3/7).
Procedure
The police officers engaged in 3-day rotating shifts with a 24-h work shift (0900–0900 h) followed by two work-free days. In this study, samples were collected during the 3 days, including the 24-h work shift and the 2 work-free days. Saliva samplings were conducted twice a day in the morning (0900 h) and evening (1900 h). Thus, the participants provided six samples in total: before the start of the 24-h shift (0900 h), during the shift (1900 h), after the end of the shift (0900 h), and 10-h (1900 h), 24-h (0900 h), and 34-h (1900 h) after the end of the shift. Previously, cortisol awakening response was frequently investigated in the context of work stress (Karlson et al. 2012). However, in this study, sleep patterns could be largely different between the participants after the 24-h work shift (e.g., time of sleep and awakening, deep sleep or short nap). Therefore, we assessed diurnal pattern of cortisol but not cortisol awakening response.
Before the study started, the participants were verbally instructed on saliva-sampling protocols and were requested to refrain from eating, drinking, smoking, heavy exercise, or brushing their teeth for 1 h before the saliva collections. Participants were also asked to record their bedtime and awakening time during the 3-day study period. Written instructions on the saliva collection protocol were also handed out. We further provided 1-h time windows for morning- and evening-saliva collections (0830–0930 h and 1830–1930 h, respectively) because a stricter schedule was expected to be too difficult for the police officers during their work. Saliva samples were frozen in the freezers of police offices or participants’ homes and transported frozen to the laboratory at the end of the experimental period.
Salivary assays
The samples were thawed and centrifuged at 3000 rpm for 15 min. The concentration of cortisol in saliva was determined by an enzyme-linked immunosorbent assay (ELISA) Kit (IBL International, Hamburg, Germany). The lower limit of detection was 0.14 nmol/L, and inter- and intra-assay variations were below 7.3 and 9.3 %, respectively. The concentration of CRP in saliva was determined using an ELISA kit (Salivary C-Reactive Protein ELISA Kit, Salimetrics LLC, PA, USA). The lower limit of detection was 0 pmol/L, and inter- and intra-assay variations were below 11.2 and 5.9 %, respectively.
Statistical analyses
Cortisol data were measured from all six saliva samples, and CRP data were measured from two saliva samples (the evening sample of the first day and the morning sample of the second day); salivary CRP levels were expected to be relatively stable over the course of 3 days (Izawa et al. 2013). Cortisol and CRP concentrations were logarithmically transformed (common logarithm) because the Kolmogorov–Smirnov test indicated a violation of the assumption of normality (skewed distribution).
The differences in the salivary cortisol levels were analyzed using linear mixed models with an unstructured error covariance matrix. We first examined variations in cortisol levels across 3 days, treating day (the first, second, and third days) and time (0900 and 1900 h) as fixed effects and individual intercept as a random effect. Possible confounding variables (age, BMI, and smoking status) were also included in the model as fixed effects, as these variables have previously been reported to be correlated with cortisol and CRP (Ouellet-Morin et al. 2011; Maina et al. 2012). Further, whether or not the sample was collected during the 1-h period after awakening was included in the model as a time-varying factor, because it is well known that cortisol levels rapidly increase during the first hour after awakening (Pruessner et al. 1997). Secondly, we examined the effects of effort, reward, and effort–reward ratio on the cortisol levels; effort score, reward score, or effort–reward ratio were additionally included in the model as fixed effects.
Differences in salivary CRP levels were analyzed by linear mixed models in the same manner as cortisol. We first examined variation in CRP levels during shifts, treating time (evening and morning) as fixed effects and individual intercept as a random effect. Possible confounding variables (age, BMI, and smoking status) were also included in the model as fixed effects. The effort score, reward score, or effort–reward ratio were additionally included in the model as fixed effects.
In preliminary analyses of cortisol and CRP, we found no significant interactions of ERI components with day and time, and thus we did not include these interaction terms in the models. We further examined the effects of ERI components on cortisol and CRP levels in younger (age <40, N = 72) and older (age ≥40, N = 70) participants, in order to consider the possible modification effect of age.
Pearson and Spearman correlation analyses and partial correlation analyses were conducted to investigate the association between cortisol and CRP. Statistical calculations were performed with the SPSS statistical package 18.0 for Windows (SPSS Software Inc., Tokyo, Japan).
Results
Characteristics of the participants are shown in Table 1. Younger participants exhibited higher reward scores (t [140] = 3.7, p < 0.001) and lower effort–reward ratios (t [140] = 2.1, p = 0.040) than older participants. BMI and smoking status did not differ between groups. Geometric means of cortisol levels (95 % CI) for morning and evening were 17.7 (16.6–18.9) nmol/L and 6.7 (6.0–7.4) nmol/L, respectively. Geometric means of CRP levels (95 % CI) for evening and morning were 126.7 (77.1–176.4) pmol/L and 125.9 (80.7–169.4) pmol/L, respectively. Logarithmically transformed cortisol levels across the 3 days are given in Table 2. In the first model, linear mixed model analyses detected significant differences in morning cortisol levels across the 3 days (β = 0.091, p = 0.014), and post hoc tests indicated that the cortisol levels on the morning of the third day were lower than those on the first and second days. Logarithmically transformed CRP levels during the shift are also shown in Table 2. Linear mixed model analysis of CRP detected no significant differences between the evening and morning levels.
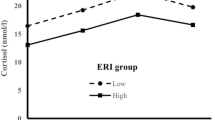
In the second model, linear mixed model analyses for cortisol detected significant effects of effort (β = −0.014, p = 0.031) as well as marginally significant effects of effort–reward ratio (β = −0.063, p = 0.080). The effects of reward were not significant. Further, the effects of effort on cortisol were strengthened in the younger group (β = −0.020, p = 0.017) but weakened in the older group (β = −0.009, p = 0.337, Fig. 1). The effects of the effort–reward ratio on cortisol were not significant in either group. Linear mixed model analyses for CRP detected marginally significant effects of effort (β = 0.026, p = 0.081) but not reward or effort–reward ratio. The effects of effort on CRP were significant in the younger group (β = 0.054, p = 0.037) but not in the older group (β = −0.008, p = 0.547, Fig. 1).
Pearson and Spearman correlation analyses revealed no significant associations between cortisol and CRP levels on either the first evening or the second morning (correlations ranged from −0.02 to 0.04). Partial correlation analyses controlling for age, BMI, smoking status, and timing of saliva collection (within 1 h of awakening or not) revealed no significant associations between cortisol and CRP levels. The correlation analyses were further divided into younger and older groups, but no significant associations were detected.
Discussion
In this study, we investigated the association between ERI and the HPA axis and inflammatory activities in a sample of police officers during a 24-h work shift and the 2 subsequent work-free days. We found that ERI, especially effort, had some physiological effects: higher effort scores were associated with lower salivary cortisol levels and higher salivary CRP levels in younger police officers. We did not find any association between cortisol and CRP levels. This study employed two measures, one endocrine and one inflammatory, to investigate the biological associations of ERI. We believe this to be valuable data because most previous studies employed only one biological parameter (e.g., cortisol or CRP). Further, to our knowledge, this is the first study to investigate the biological effects of ERI in a sample of police officers who are engaged in severe shiftwork (24-h shifts) with both a mentally and physically strenuous work load.
We found that police officers with higher ERI scores, especially effort scores, exhibited lower cortisol levels, consistent with previous findings. Maina et al. (2009) reported lower cortisol secretion after awakening and during the diurnal period in call-center workers with higher ERI or effort scores. Similarly, Eller et al. (2012) reported that higher ERI or effort scores were associated with lower cortisol levels after awakening in female participants. Furthermore, in the previous studies, high ERI or its component was associated with dysregulation of the HPA axis in that there was an attenuated cortisol response to acute psychosocial stress (Siegrist et al. 1997) and lower cortisol awakening response to the dexamethasone test, implying higher negative feedback sensitivity (Bellingrath et al. 2008). Taken together, it can be speculated that workers with high ERI are exposed to stress-induced cortisol for prolonged periods, which causes higher negative feedback sensitivity of the HPA axis, as well as lower cortisol levels or attenuated cortisol responses. This biological speculation was also supported by a recent study in burnout patients, which found that patients with severe burnout exhibited lower cortisol and ACTH response to acute psychosocial stress compared to patients with low burnout and healthy controls (Lennartsson et al. 2015).
Other studies failed to observe an association between ERI and cortisol (Hanson et al. 2000; Harris et al. 2007; Irie et al. 2004; Marchand et al. 2016; Ota et al. 2014). The reason for the discrepant findings remains unclear; however, characteristics of the participants, intensity of the stressor, sample size, and the frequency and timing of cortisol assessment could affect the results, as suggested in a recent review of work stress and cortisol (Karlson et al. 2012). Age could be an important factor, because we found pronounced effects of effort on cortisol in younger participants. No previous studies had investigated the modifying effect of age on the relationship between ERI and cortisol. Furthermore, our study assessed cortisol levels during the work shift as well as the work-free days and appropriately excluded any participants having factors that might affect HPA and inflammatory activities (e.g., history of diseases, medications, and obesity). This could help to increase the statistical power to detect the significance of the effect of ERI on cortisol levels.
The possible effects of a 24-h work shift including night shifts should also be mentioned. In previous studies, working night shifts could reduce cortisol secretion: Lower cortisol levels were observed in the morning after the night shift, compared with levels on a morning not following a night shift (Costa et al. 1994; Leese et al. 1996; Munakata et al. 2001). However, in this study, the cortisol levels after the 24-h shift were comparable to those before the shift (the morning of the first day). We speculate that this was because the police officers involved in this study were allowed to take a short nap during the shift. We found lower cortisol levels on the morning of the third day, compared with those of the first and second days. The previous studies reported that cortisol responses after awakening on the work-free day were lower than that during the work day (Kunz-Ebrecht et al. 2004; Schlotz et al. 2004), which was speculated to be linked to stress-related anticipation of the upcoming day (Fries et al. 2009). The lower morning cortisol levels on the third day might result from this phenomenon, and not from night shift working per se.
We also found that police officers with higher effort scores exhibited higher CRP levels during the shift. In the past, only two studies had investigated the association between ERI and CRP (Hamer et al. 2006; Almadi et al. 2012), and their findings were consistent with the finding of the current study. Further, we found pronounced effects of effort on CRP in younger participants, similar to the observed association between cortisol and effort. In a previous study on long-haul bus drivers (Tsai et al. 2014), a relationship between job stress (job demand) and CRP was observed only in younger participants, consistent with the finding of this study. In the past, it has been repeatedly reported that the relationship between job stress and incidence of coronary heart diseases was stronger among younger workers (Chandola et al. 2008; Kuper and Marmot 2003; Theorell et al. 1998). Biological factors associated with aging could contribute to the diminished effect of job stress on biological consequence. Furthermore, a survivor effect could be considered; Police officers who are highly vulnerable to the effect of job stress are less likely to work into old age, which could also diminish the biological effects.
In addition, we did not find negative correlations between cortisol and CRP, although we expected reduced cortisol secretion to contribute to increased inflammation. Previously, disturbances in the HPA axis were shown to be associated with higher inflammatory activity (e.g., Theorell et al. 2000). The reason for this finding remains unclear; however, a previous study (Bellingrath et al. 2013) demonstrated that high ERI is also associated with lower glucocorticoid sensitivity of interleukin-6 in vitro indicating the possibility that cortisol unsuccessfully regulated the inflammatory activity under the condition of high ERI. Therefore, a less effective anti-inflammatory regulation by cortisol, rather than reduced cortisol secretion, could largely contribute to increased inflammation in the high-ERI group.
This study has certain limitations that may affect the interpretation of the findings. Firstly, the study population was limited to male police officers because the majority of police officers in Japan are male. Secondly, our findings cannot be generalized to the general working population. The police officers involved in this study were in government employ, implying fairly provided chances of job promotion and salary and good employment security. Such an occupational status could contribute to the results we observed regarding the biological effect of effort, but not reward or effort–reward ratio. Therefore, caution should be exercised in over-generalizing the current findings to a wider population. Furthermore, we conducted many significance tests (effort/reward/ERI, young/old, cortisol, CRP), which should be exercised when interpreting the results.
In conclusion, we investigated the biological consequences of ERI using well-designed protocols. Randomly sampled police officers, selected using appropriate exclusion criteria, were investigated during their work shift as well as the following work-free days by employing endocrine and inflammatory measures. We provided evidence that younger police officers with higher effort scores exhibited lower salivary cortisol levels as well as higher salivary CRP levels. These biological features could possibly contribute to the future development of stress-related diseases such as cardiovascular diseases in the police officers.
References
Almadi T, Cathers I, Hamdan Mansour AM, Chow CM (2012) The association between work stress and inflammatory biomarkers in Jordanian male workers. Psychophysiology 49:172–177. doi:10.1111/j.1469-8986.2011.01296.x
Bellingrath S, Weigl T, Kudielka BM (2008) Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biol Psychol 78:104–113. doi:10.1016/j.biopsycho.2008.01.006
Bellingrath S, Rohleder N, Kudielka BM (2013) Effort-reward-imbalance in healthy teachers is associated with higher LPS-stimulated production and lower glucocorticoid sensitivity of interleukin-6 in vitro. Biol Psychol 92:403–409. doi:10.1016/j.biopsycho.2012.12.003
Chandola T, Britton A, Brunner E, Hemingway H, Malik M, Kumari M et al (2008) Work stress and coronary heart disease: what are the mechanism? Eur Heart J 29:640–648. doi:10.1093/eurheartj/ehm584
Costa G, Ghirlanda G, Tarondi G, Minors D, Waterhouse J (1994) Evaluation of a rapidly rotating shift system for tolerance of nurses to nightwork. Int Arch Occup Environ Health 65:305–311
Dickerson SS, Kemeny ME (2004) Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–391
Eller NH, Netterstrøm B, Hansen AM (2006) Psychosocial factors at home and at work and levels of salivary cortisol. Biol Psychol 73:280–287
Eller NH, Nielsen SF, Blønd M, Nielsen ML, Hansen ÅM, Netterstrøm B (2012) Effort reward imbalance, and salivary cortisol in the morning. Biol Psychol 89:342–348. doi:10.1016/j.biopsycho.2011.11.007
Franke WD, Collins SA, Hinz PN (1998) Cardiovascular disease morbidity in an Iowa law enforcement cohort, compared with the general Iowa population. J Occup Environ Med 40:441–444
Franke WD, Ramey SL, Shelley MC 2nd (2002) Relationship between cardiovascular disease morbidity, risk factors, and stress in a law enforcement cohort. J Occup Environ Med 44:1182–1189
Fries E, Dettenborn L, Kirschbaum C (2009) The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73. doi:10.1016/j.ijpsycho.2008.03.014
Hamer M, Williams E, Vuonovirta R, Giacobazzi P, Gibson EL, Steptoe A (2006) The effects of effort-reward imbalance on inflammatory and cardiovascular responses to mental stress. Psychosom Med 68:408–413
Hanson EK, Maas CJ, Meijman TF, Godaert GL (2000) Cortisol secretion throughout the day, perceptions of the work environment, and negative affect. Ann Behav Med 22:316–324
Harris A, Ursin H, Murison R, Eriksen HR (2007) Coffee, stress and cortisol in nursing staff. Psychoneuroendocrinology 32:322–330
Irie M, Tsutsumi A, Shioji I, Kobayashi F (2004) Effort-reward imbalance and physical health among Japanese workers in a recently downsized corporation. Int Arch Occup Environ Health 77:409–417
Izawa S, Miki K, Liu X, Ogawa N (2013) The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav Immun 27:38–41. doi:10.1016/j.bbi.2012.07.001
Joseph PN, Violanti JM, Donahue R, Andrew ME, Trevisan M, Burchfiel CM et al (2009) Police work and subclinical atherosclerosis. J Occup Environ Med 51:700–707
Karlson B, Lindfors P, Riva R, Mellner C, Theorell T (2012) Psychosocial work stressors and salivary cortisol. In: Kristenson M, Garvin P, Lundberg U (eds) The role of saliva cortisol measurement in health and disease. Bentham Science Publishers, Bussum, pp 43–66
Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A (2004) Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology 29:516–528
Kuper H, Marmot M (2003) Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health 57:147–153
Kuper H, Singh-Manoux A, Siegrist J, Marmot M (2002) When reciprocity fails: effort-reward imbalance in relation to coronary heart disease and health functioning within the Whitehall II study. Occup Environ Med 59:777–784
Leese G, Chattington P, Fraser W, Vora J, Edwards R, Williams G (1996) Short-term night-shift working mimics the pituitary-adrenocortical dysfunction in chronic fatigue syndrome. J Clin Endocrinol Metab 81:1867–1870
Lennartsson AK, Sjörs A, Währborg P, Ljung T, Jonsdottir IH (2015) Burnout and hypocortisolism—a matter of severity? A study on ACTH and cortisol responses to acute psychosocial stress. Front Psychiatry 6:8. doi:10.3389/fpsyt.2015.00008
Maina G, Bovenzi M, Palmas A, Larese Filon F (2009) Associations between two job stress models and measures of salivary cortisol. Int Arch Occup Environ Health 82:1141–1150. doi:10.1007/s00420-009-0439-0
Maina G, Bovenzi M, Palmas A, Rossi F, Filon FL (2012) Psychosocial environment and health: methodological variability of the salivary cortisol measurements. Toxicol Lett 213:21–26. doi:10.1016/j.toxlet.2011.08.019
Marchand A, Juster RP, Durand P, Lupien SJ (2016) Work stress models and diurnal cortisol variations: the SALVEO study. J Occup Health Psychol 21:182–193. doi:10.1037/a0039674
McEwen BS (2000) Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22:108–124
Munakata M, Ichi S, Nunokawa T, Saito Y, Ito N, Fukudo S et al (2001) Influence of night shift work on psychologic state and cardiovascular and neuroendocrine responses in healthy nurses. Hypertens Res 24:25–31
Nakata A (2012) Psychosocial job stress and immunity: a systematic review. Methods Mol Biol 934:39–75. doi:10.1007/978-1-62703-071-7_3
Ota A, Mase J, Howteerakul N, Rajatanun T, Suwannapong N, Yatsuya H et al (2014) The effort-reward imbalance work-stress model and daytime salivary cortisol and dehydroepiandrosterone (DHEA) among Japanese women. Sci Rep 4:6402. doi:10.1038/srep06402
Ouellet-Morin I, Danese A, Williams B, Arseneault L (2011) Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav Immun 25:640–646. doi:10.1016/j.bbi.2010.12.020
Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S et al (1997) Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sc 61:2539–2549
Punyadeera C, Dimeski G, Kostner K, Beyerlein P, Cooper-White J (2011) One-step homogeneous C-reactive protein assay for saliva. J Immunol Methods 373:19–25. doi:10.1016/j.jim.2011.07.013
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Schlotz W, Hellhammer J, Schulz P, Stone AA (2004) Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med 66:207–214
Segerstrom SC, Miller GE (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130:601–630
Siegrist J (1996) Adverse health effects of high-effort/low-reward conditions. J Occup Health Psychol 1:27–41
Siegrist J, Peter R, Motz W, Strauer BE (1992) The role of hypertension, left ventricular hypertrophy and psychosocial risks in cardiovascular disease: prospective evidence from blue-collar men. Eur Heart J 13:89–95
Siegrist J, Klein D, Voigt KH (1997) Linking sociological with physiological data: the model of effort-reward imbalance at work. Acta Physiol Scand Suppl 640:112–116
Steptoe A, Brydon L (2007) Psychosocial factors and coronary heart disease: the role of psychoneuroimmunological processes. In: Ader R (ed) Psychoneuroimmunology, 4th edn. Elsevier Academic Press, MA, pp 945–974
Theorell T, Tsutsumi A, Hallquist J, Reuterwall C, Hogstedt C, Fredlund P et al (1998) Decision latitude, job strain, and myocardial infarction: a study of working men in Stockholm. The SHEEP Study Group. Stockholm Heart epidemiology Program. Am J Public Health 88:328–382
Theorell T, Hasselhorn HM, Vingård E, Andersson B, the MUSIC-Norrtälje Study Group (2000) Interleukin 6 and cortisol in acute musculoskeletal disorders: results from a case-referent study in Sweden. Stress Med 16:27–35
Tsai SS, Lai CH, Shih TS, Lin MH, Liou SH (2014) High job strain is associated with inflammatory markers of disease in young long-haul bus drivers. J Occup Health Psychol 19:336–347. doi:10.1037/a0036600
Tsutsumi A, Ishitake T, Peter R, Siegrist J, Matoba T (2001a) The Japanese version of the Effort-Reward Imbalance Questionnaire: a study in dental technicians. Work Stress 15:86–96
Tsutsumi A, Kayaba K, Theorell T, Siegrist J (2001b) Association between job stress and depression among Japanese employees threatened by job loss in a comparison between two complementary job-stress models. Scand J Work Environ Health 27:146–153
Tsutsumi A, Nagami M, Morimoto K, Matoba T (2002) Responsiveness of measures in the effort-reward imbalance questionnaire to organizational changes: a validation study. J Psychosom Res 52:249–256
Tsutsumi A, Iwata N, Wakita T, Kumagai R, Noguchi H, Kawakami N (2008) Improving the measurement accuracy of the effort-reward imbalance scales. Int J Behav Med 15:109–119. doi:10.1080/10705500801929718
Acknowledgments
This study was supported by a research grant from the Japan Police Meaningful Life Promotion Foundation.
Funding
This study was supported by a research grant from the Japan Police Meaningful Life Promotion Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Rights and permissions
About this article
Cite this article
Izawa, S., Tsutsumi, A. & Ogawa, N. Effort–reward imbalance, cortisol secretion, and inflammatory activity in police officers with 24-h work shifts. Int Arch Occup Environ Health 89, 1147–1154 (2016). https://doi.org/10.1007/s00420-016-1154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-016-1154-2