Abstract
Purpose
The purpose of this review is to summarize the current knowledge from field studies on how many consecutive night shifts are required for adaptation of diurnal rhythms in cortisol, melatonin and heart rate variability (HRV) to night work.
Methods
A systematic search of the databases PubMed and Web of Science resulted in 18 studies selected for review.
Results
Cortisol was measured in five studies, melatonin in 11 studies and HRV in four studies. Diurnal rhythms were assessed by use of several different measures based on three to eight samples per day for cortisol and melatonin and 24-h recordings for HRV. Most of the studies in the review were small studies with less than 30 participants, and most studies evaluated diurnal rhythms after only two consecutive night shifts whereas only six studies used seven or more consecutive night shifts. The majority of studies found that adaptation to night work had not occurred after two consecutive night shifts, whereas a small number found evidence for full adaptation after seven consecutive night shifts based on diurnal rhythms in cortisol and melatonin.
Conclusion
There are methodological differences in the field studies analyzing diurnal rhythms and large diversity in the occupational fields studied. Nevertheless, we conclude that diurnal rhythms in cortisol, melatonin and HRV are not adapted to night work after 1–3 consecutive night shifts. Studies are needed to establish how many consecutive night shifts are needed for full adaptation of diurnal rhythms to night work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Night work is a necessity in many occupations. It is estimated that 15–20 % of the working population in Europe are involved in night work. Night work is associated with both acute and reversible effects such as poor sleep (Sallinen and Kecklund 2010), decreased cognitive function (Dula et al. 2001; Griffiths et al. 2006) and gastro-intestinal problems (Knutsson and Bøggild 2010). Also chronic disorders, e.g., peptic ulcer disease (Knutsson and Bøggild 2010), cardiovascular disease (Bøggild and Knutsson 1999; Frost et al. 2009) and breast cancer (Costa et al. 2010) are reported in association with night work. In 2007, the Agency for Research on Cancer classified ‘shift work that involves circadian disruption’ as a probable human carcinogen based on sufficient evidence from animal studies and limited evidence from epidemiological studies (Stevens et al. 2011).
Although unequivocal evidence of a causal effect of night work on, e.g., breast cancer and cardiovascular disease is lacking, several mechanisms of disease onset and progression have been suggested (Bonde et al. 2007; Puttonen et al. 2010). One proposed mechanism is related to disruption of circadian rhythms of physiological systems (Costa et al. 2010; Puttonen et al. 2010). Circadian rhythms of physiological systems include sleep/wake cycles, and fluctuations in body temperature, blood pressure, hormone secretion, digestion, metabolism, and cell turnover. They are pivotal for survival and are driven and maintained in a hierarchical manner by a central pacemaker (the biologic master clock) located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Buijs et al. 2003). The SCN receives signals from external zeitgebers such as light and darkness, social rhythms, timing of meals, and activities such as work and physical activity (Grandin et al. 2006; Zelinski et al. 2014).
Since humans generally are day-oriented, working at night requires a phase change in the sleep/wake cycle and circadian rhythms of other physiological systems. This change occurs gradually with increasing number of consecutive night shifts (Haus and Smolensky 2006), but it is not clear how many consecutive night shifts are needed for full adaptation to night work in terms of being synchronized relative to the new work schedule and sleep times (and therefore disrupted in relation to the ambient dark/light cycle). Rhythms may be characterized by amplitude, phase and frequency (Hofstra and De Weerd 2008). Preferably, the adaptation of circadian rhythms is assessed by measurements at several time points in order to assess all the characteristics. However, lack of adaptation may also be approximated by just a few measurements taken at comparable times during night work and day work schedules.
Circadian rhythms and circadian disruption have been investigated in several different ways in laboratory studies, e.g., via dim light melatonin onset (DLMO), body temperature and sleep–wake logbooks (Figueiro et al. 2014; Mirick and Davis 2008;Sargent et al. 2012). Laboratory studies have the advantage of a high level of control of the environment, but lack the inherent exposures of the real-life setting. Laboratory studies have shown that when working nights in a real-life setting, adaptation of the circadian rhythm is complicated by the fact that a night worker continues to be exposed to external zeitgebers like sunlight and social factors that promote day orientation. The influence of the external environment and social factors is not necessarily captured in a laboratory setting with simulated night work. Therefore, laboratory studies must be supplemented with field studies to evaluate how the human body is affected by night work in real life (Kantermann et al. 2012).
Of the many possible physiological markers of diurnal rhythms that have been studied, we chose to focus on two central indicators of physiological adaptation to the environment, that is, cortisol as a measure of the hypothalamus–pituitary–adrenal (HPA) axis (Tsigos and Chrousos 2002) and heart rate variability (HRV) as a proxy for autonomic regulation (Pagani et al. 1986). Cortisol has a pronounced effect on the immune system and is a vital part of the body’s stress response, thus changes in the rhythmicity of cortisol release may have effects on overall health (McEwen and Karatsoreos 2015). The autonomic nervous system (ANS) is involved in many physiological process and is of vital importance of health (Pagani et al. 1986). Moreover, we included melatonin to capture information on the central circadian pacemaker to the external environment (Haus and Smolensky 2006). Melatonin suppression has also been linked to shift worker health as a possible mechanism leading from shift work to cancer (Stevens and Rea 2001).
Cortisol is produced in the adrenal gland and is the principal marker of the activation of the hypothalamus–pituitary–adrenal (HPA) axis (Tsigos and Chrousos 2002). The HPA axis plays a central role in homeostatic processes, and it is commonly thought to reflect attempts to adjust to daily pressures and joys (Rosmond and Björntorp 2000). Cortisol has a pronounced diurnal rhythm and is viewed as a good and robust marker of the overall circadian rhythm (Hofstra and De Weerd 2008). The concentration of cortisol can be influenced by awakening time, physical activity and stress (Garde et al. 2008) Cortisol can be measured in blood, urine and saliva (Gatti et al. 2009; Jensen et al. 2011), and there is a good correlation between serum and saliva levels of cortisol (Cadore et al. 2008).
Melatonin is synthesized and secreted primarily by the pineal gland (Arendt 1995); this molecule contributes to synchronizing the internal hormonal environment to the light–dark cycle of the external environment (Haus and Smolensky 2006). Melatonin can be measured in blood or saliva and its metabolite 6-sulfatoxymelatonin can be measured in urine. Circulating melatonin level and 6-sulfatoxymelatonin have been shown to be good biomarkers of the circadian rhythm (Arendt 1986; Jensen et al. 2011). Melatonin production is affected by light intensity. Illumination of sufficient intensity can completely suppress melatonin production. This means that the production of melatonin in night workers can be suppressed by the light in the environment (Mirick and Davis 2008).
HRV is controlled by the ANS, which oscillates over the 24 h (Pagani et al. 1986; Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology 1996). HRV is an indicator of the ANS’s regulation of the cardiac rhythm, which encompasses the influences from the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS) activity. The SNS are associated with energy mobilization, and the PNS with vegetative and restorative functions. These two branches of the ANS are in constant dynamic balance (Thayer et al. 2010). Imbalance in autonomic regulation, characterized by a relative dominant SNS hyperactivity over PNS hypoactivity, is a reliable predictor of morbidity and mortality (Dekker et al. 2000; Tsuji et al. 1996). The relative activity in the SNS and PNS can be disentangled by analyzing HRV (Kleiger et al. 2005). High frequency (HF) variation (normally calculated for the frequency range 0.15–0.4 Hz) reflects PNS modulation of cardiac rhythm, while low frequency (LF) variation in (0.04–0.15 Hz) reflects SNS modulation with a significant contribution from PNS HRV (Malliani et al. 1998; Montano et al. 1994; Pagani et al. 1986; Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology 1996). In addition, the ratio between LF and HF is interpreted as the balance between the sympathetic and parasympathetic modulation of cardiac rhythm (sympathovagal balance) (Malliani et al. 1998; Montano et al. 1994; Pagani et al. 1986). Most measures of HRV show a pronounced circadian profile that is influenced by the sleep–wake cycle (Bonnemeier et al. 2003; Vandewalle et al. 2007) and work versus leisure time and physical activity (Collins et al. 2005; Vrijkotte et al. 2000).
Aim
We aimed at evaluating to what extent night workers adapt to night shifts and how many consecutive shifts are required in real-life settings. This was done by reviewing field studies explicitly investigating the effect of the number of consecutive night shifts on diurnal rhythms of cortisol, melatonin and HRV.
Materials and methods
Search strategy
The literature search (Fig. 1) was performed during august 2014. We searched the database ‘PubMed’ and ‘Web of Science.’ The search strategy was developed by the first author and a search specialist at (workplace is to be revealed after blinded peer-review). The search resulted in total of 900 articles. All titles and abstracts were read by the first author, and 65 papers were scrutinized further. Of these 65 papers, 15 papers fulfilled the inclusion criteria given below.
The criteria for inclusion in the present review were:
-
Original research written in English and published in a peer-reviewed journal
-
Data from humans in field studies
-
Studies on one or more night shifts, i.e., primary working hours between 23:00 and 07:00
-
Results on cortisol, melatonin/6-sulfatoxymelatonin and/or HRV
-
At least three measurements on the same day to ensure that there was an indication of circadian rhythm
-
A comparison of night work with day work or days off
Exclusion criteria were:
-
Combined field and laboratory studies
-
Intervention studies, e.g., of the effect of light therapy and melatonin treatment
In addition to the literature search, we scanned the reference lists of the selected papers for additional studies of relevance revealing two relevant papers that were included in the study, giving a total of 18 papers for the present review.
Evaluation of the degree of adaptation to night work
We classified the degree of adaptation to night work for cortisol, melatonin and HRV in the studies as indicating no degree of adaptation, some signs of adaptation or a high degree of adaptation based on how much the circadian rhythm on night shifts deviated from the circadian rhythm during the day shift in relation to working hours. If there were permanent night workers as part of the study population, days off were used for comparison. No adaptation was characterized by no change in the timing of the phase or amplitude of the circadian rhythm compared to a day shift. Some signs of adaptation were characterized by changes in the timing of the phase or amplitude of the circadian rhythm, but not a full phase shift. A high degree of adaption was characterized by a rhythm that followed the same pattern as on a day shift only shifted to match a night shift schedule.
Results
In total, 18 papers were included in the present review. Cortisol was measured in five studies (of which one also included melatonin and one HRV, Table 1). Melatonin was measured in 11 studies (of which one also measured cortisol, Table 2) and HRV was measured in four studies (of which one also measured cortisol, Table 3). The 18 studies cover several different occupational fields. Five studies had participants from the offshore oil industry (Barnes et al. 1998a, b; Gibbs et al. 2007; Hansen et al. 2010; Harris et al. 2010). Six studies had nurses as participants (Anjum et al. 2011; Borugian et al. 2005; Costa et al. 1994; Grundy et al. 2011; Hansen et al. 2006; Kobayashi et al. 1997), and one study had paramedics as participants (Wong et al. 2012). One study had workers from a power station (Vangelova and Dalbokova 1998). Three studies had participants from other industries such as mining, the glass industry and electronic manufacturing (Choosong et al. 2006; Ferguson et al. 2012; Kudielka et al. 2007). The largest study in the present review consisted of 170 participants (Hansen et al. 2006), and only two additional studies had more than 100 participants: Grundy et al. (2011) with 123 participants and Kudielka et al. (2007) with 102 participants. The rest of the studies had small sample sizes of around 10–30 participants (Anjum et al. 2011; Barnes et al. 1998a, b; Borugian et al. 2005; Choosong et al. 2006; Ferguson et al. 2012; Furlan et al. 2000; Gibbs et al. 2007; Hansen et al. 2010; Harris et al. 2010; Kobayashi et al. 1997; Rauchenzauner et al. 2009; Vangelova and Dalbokova 1998; Wong et al. 2012). The number of consecutive night shifts ranged from a single night shift (Rauchenzauner et al. 2009) to 14 consecutive night shifts (Harris et al. 2010). The frequency of saliva, urine and blood sampling ranged from hourly saliva samples over 24 h (Ferguson et al. 2012) to blood samples taken at the start, middle and end of each shift (Costa et al. 1994).
Cortisol
We found five relevant papers with cortisol as a circadian marker (Table 1). Four studies used cortisol in saliva and one study used cortisol in blood as the circadian marker.
Four studies used saliva samples. Harris et al. (2010) used saliva taken five times a day to evaluate the level of cortisol decrease during the day and used it as an indicator of the diurnal rhythm. Kudielka et al. (2007) used saliva samples at +4, +8, +12 and +16 h after awakening to assess the cortisol level during the day. Wong et al. used saliva samples taken 30 min after awakening and samples taken +1, +6 and +12 h and at bedtime on rest days and at the beginning of the shift, mid-shift, end of shift and at bed time on work days. They used this to calculate the slope of the cortisol curve. They also looked at the overall daily production of cortisol (Wong et al. 2012). Anjum et al. (2011) compared the concentration of cortisol in saliva in evening, night and morning samples during a night shift and during a day shift.
One study used blood samples: Costa et al. (1994) used three blood samples collected at the start, middle and end of a shift on days with morning, afternoon and night shifts.
Table 4 shows an overview of the degree of adaptation of the diurnal rhythm of cortisol in relation to the number of consecutive night shifts.
In the study by Harris et al. (2010), offshore workers tested two types of shifts: fixed shift (14 day shifts or 14 night shifts) and swing shift (seven night shifts followed by 7 day shifts). The offshore workers adapted to night work within seven consecutive night shifts. This is in contrast to the findings by Anjum et al. (2011) that did not find statistically significant results to indicate adaptation to night work in diurnal rhythms of cortisol after nine night shifts in a study with nurses, but the authors did see a tendency toward an alteration in cortisol patterns after two night shifts. Wong et al. (2012) claimed a tendency toward a flattened diurnal slope for cortisol, but found no statistically significant differences between night and day work after two shifts. Two studies found no changes in plasma cortisol patterns after two consecutive night shifts (Costa et al. 1994; Kobayashi et al. 1997).
Overall, no or little adaptation after two consecutive night shifts was found. There are very few studies that investigate the effects of more than two consecutive night shifts, although the study by Harris et al. indicate that full adaptation may occur after seven consecutive night shifts in offshore workers.
Melatonin
Of the 11 papers on melatonin as a diurnal marker (Table 2), seven used 6-sulfatoxymelatonin measured in urine as a marker of diurnal rhythm (Barnes et al. 1998a, b; Costa et al. 1994; Gibbs et al. 2007; Hansen et al. 2006, 2010; Vangelova and Dalbokova 1998), three studies used salivary melatonin (Borugian et al. 2005; Choosong et al. 2006; Ferguson et al. 2012) and one study used both 6-sulfatoxymelatonin in urine and melatonin in saliva (Grundy et al. 2011). Urine and saliva collection were conducted in slightly different ways. Five studies compared fixed time points (Borugian et al. 2005; Choosong et al. 2006; Costa et al. 1994; Grundy et al. 2011; Vangelova and Dalbokova 1998), five studies used 1- to 4-h intervals between samples (Barnes et al. 1998a, b; Ferguson et al. 2012; Gibbs et al. 2007; Hansen et al. 2010) and one study used all urine voids during 24 h (Hansen et al. 2006).
Table 4 shows an overview of the degree of adaptation of the diurnal rhythm of melatonin in relation to the number of consecutive night shifts.
After two consecutive night shifts, Grundy et al. (2011) also did not find any difference in the pattern of melatonin in saliva in a study of rotating shift workers. In a study of 10 female shift workers on a glass manufacturing factory, Choosong et al. (2006) found that the older group (age 35–40 years, n = 5) adapted to night work after two consecutive night shifts while the younger group (age 20–25) did not. Costa et al found the excretion of 6-sulfatoxymelatonin showed a normal diurnal pattern with higher levels at night during two consecutive night shifts compared to day shifts, indicating that the diurnal rhythm did not adapt to night work. The authors found no difference between the first and second night in a row when looking at the excretion of 6-sulfatoxymelatonin (Costa et al. 1994). In a study of both male and female office workers and nurses working either rotating shift or day shifts, Borugian et al. (2005) found that rotating shift workers had abnormally high melatonin levels at arising and during the work day and abnormally low melatonin levels during sleep indicating a disrupted diurnal rhythm after two consecutive night shifts.
In the two large studies of female nurses (n = 170 and n = 123), little evidence for adaptation of the diurnal rhythm of 6-sulfatoxymelatonin after two or more consecutive night shifts was found (Grundy et al. 2011; Hansen et al. 2006). Hansen et al. (2006) did, however, show that nurses working fixed nights had a lower excretion of 6-sulfatoxymelatonin compared to day shift nurses on both a workday and a day off. Vangelova and Dalbokova (1998) also found no adaptation in the rhythm of 6-sulfatoxymelatonin to night work after two consecutive night shift, but significantly lower overall levels of 6-sulfatoxymelatonin on the second night shift compared with the first night shift.
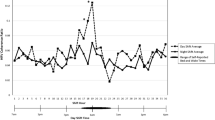
Gibbs et al. (2007) found that 19 of the 23 male offshore oil workers adapted to night shifts after seven consecutive night shifts by a delay in 6-sulfatoxymelatonin rhythm. Hansen et al. (2010) also studied offshore workers and found that the rhythm of 6-sulfatoxymelatonin shifted from day 1 to 7 with a rate of adaptation of 0.84 h per day. In another study of offshore workers, Barnes et al. (1998a) showed significant phase delay in 6-sulfatoxymelatonin rhythm in workers working 2 weeks of night shifts. Ferguson et al. used saliva sampling and showed only small changes in the diurnal rhythm of melatonin and little adaptation to night work after seven days of night shifts in people working in a mining operation (Ferguson et al. 2012).
The findings in the included studies suggest little or no adaptation (a high level of circadian disruption) after two consecutive night shifts in diurnal rhythms of melatonin, but also indicate a tendency to increased level of adaptation of diurnal rhythm of melatonin after seven consecutive night shifts.
HRV
We found four relevant papers with HRV as a diurnal marker. The results are shown in Table 3.
Fulan et al. (2000) and Rauchenzauner et al. (2009) used normalized markers of cardiac sympathetic (LFnu) and vagal (HFnu) modulation of cardiac rhythm and of the sympathovagal balance (LF/HF) for one-hour periods for a 24-h period. Kobayashi et al. (1997) used LF and LF/HF for 1-h periods for 24 h. Wong et al. (2012) used the square root of the mean of the squares of successive NN interval differences (RMSSD), pNN50 (the percentage of intervals >50 ms different from the preceding interval) and HF for 12 h as indicators of parasympathetic activity.
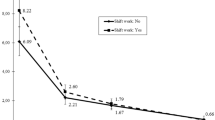
Table 4 shows an overview of the degree of adaptation of the diurnal rhythm of HRV in relation to the number of consecutive night shifts. Furlan et al. (2000) found that the autonomic balance was shifted in direction of lower sympathetic and higher parasympathetic activity during night work compared to day work after an adaptation period of 2 days. In contrast, Kobayashi et al. (1997) found a tendency that the autonomic balance was shifted in direction of higher sympathetic and lower parasympathetic activity during night shifts following a half day on day shift compared to a night shift that followed a full or two full day shifts, although the difference between the two conditions was not significant. Wong et al. (2012) did not find statistically significant differences, but did report a tendency toward lower parasympathetic cardiac modulation in shift workers compared to day workers after two work days. Rauchenzauner et al. (2009) found higher sympathovagal balance (LFnu and a tendency to higher LF/HF) on a 24-h on-call shift compared to a normal day. In summary, one to three consecutive night shifts is not enough for adaptation to work at night, but there is a lack of studies of the effects of more consecutive nights on diurnal rhythms in HRV.
Discussion
We aimed at evaluating to what extent night workers adapt to night shifts and how many consecutive shifts are required in real-life settings. This was done by reviewing field studies explicitly investigating the effect of the number of consecutive night shifts on diurnal rhythm of cortisol, melatonin and HRV. The main result is that night work with one to three consecutive night shifts does not result in adaptation to night work by a full shift in diurnal rhythms in salivary melatonin, cortisol and HRV. In total, 18 studies were included in the present review: three measured cortisol, 10 studies measured melatonin, three measured HRV, one studies measured both cortisol and HRV and one study measured both cortisol and melatonin. There were large methodological differences in the field studies. Since we wanted to look at the effects of night shifts in a real-life setting, we excluded laboratory studies and combined laboratory and field studies as the environmental exposures in these settings (e.g., in terms of light, noise level and meals) are highly controlled. We argue that this is a very different exposure compared to sleeping at home where the participants are in their natural environment which may include high levels of light, noise etc. all of which may contribute to the circadian disruption or degree of adaptation that occur during spells of night work. We also excluded interventions such as light therapy, melatonin supplementation or wearing tinted glasses to limit sun light since we also viewed this as a different exposure than night work in a natural setting.
There was a high degree of diversity with respect to the use of the diurnal markers in the 18 studies in this review. Four studies used cortisol in saliva and two used cortisol in blood. For cortisol, four studies used the CAR and others the cortisol decrease during the day as an indicator of circadian disruption. For the studies using melatonin, four studies looked at melatonin in saliva and eight at 6-sulfatoxymelatonin in urine. The HRV studies mostly focused on the balance between sympathetic and parasympathetic activity by analyzing LF/HF, LFnu and HFnu. There are also differences in the number of points and timing of measurement and some studies use only very few. The lack of adequate sampling is a major limitation of most of the studies in this review making it difficult to assess what characteristic of the diurnal rhythm, e.g., amplitude or phase-shift is adapted. Several measurements taken throughout the day would allow for a more precise description of rhythm characteristics and therefore which characteristic of the diurnal rhythm is adapted. In contrast, lack of adaptation may also be approximated by just a few measurements taken at comparable times during night work and day work schedules. Another limitation is that it was not always possible to verify if the day shifts analyzed were unaffected by prior night shifts and thus that the timing of the rhythms was ‘normal.’
The degree of adaptation to night work varied between the included studies and was studied in different ways and with diverging results. Two of the studies using melatonin that looked at two consecutive nights shift and did not find a significant change in diurnal rhythm (Costa et al. 1994; Grundy et al. 2011) indicate that two consecutive night shifts will not cause a shift in the diurnal rhythm at least not in melatonin excretion, whereas two studies found signs of adaptation in the diurnal rhythm of melatonin (Borugian et al. 2005; Choosong et al. 2006). Also the studies using HRV as a diurnal marker found some contradicting results. Furlan et al. (2000) found a lowered sympathovagal balance, while Wong et al. (2012) and Rauchenzauner et al. (2009) found higher sympathovagal balance in response to night work. A review from Folkard (2008) concluded that even permanent night shift workers rarely fully adapt to their night shift in terms of the diurnal rhythm of melatonin with only one in four permanent night workers adjusting enough to benefit from the adjustment.
Factors influencing the degree of adaptation to night work are mostly related to specific shift characteristics like timing, number of consecutive night shifts, duration and direction of shift rotation as well as factors related to the actual light exposure (intensity, wavelength and timing) (Haus and Smolensky 2006). In addition, individual factors (e.g., diurnal preference and sleep pattern) and other environmental factors (e.g., eating and lighting when not at work) which have not been specifically assessed in the studies reviewed here may critically influence the circadian adjustment (Haus and Smolensky 2013). This means that it is difficult to give a specific answer to how many night shifts are required for adaptation in field studies because this to a very high degree depends on both the individual and environmental factors. The behavioral and environmental influences on the evaluated biological markers can also mask some of the circadian effects making it difficult to evaluate whether or not a rhythm has adapted. Only few studies found a high degree of adaptation in diurnal rhythm of melatonin and cortisol to night work after seven consecutive nights (Gibbs et al. 2007; Harris et al. 2010), and the study by Anjum et al. (2011) indicated that adaptation in diurnal rhythm of cortisol may not be achieved after nine consecutive night shifts. The speed of adaptation may differ between different diurnal rhythms, as different physiological systems may adapt to night work in different tempos (Haus and Smolensky 2006; Turek 2008).
There may be many reasons for lack of adaptation. Circadian type may be relevant because being predisposed to an earlier circadian phase (‘morningness’) decrease the speed of adaptation to night work (Mirick and Davis 2008). Further, ‘eveningness’, i.e. being predispose to a later circadian phase, compared to morningness seems to better suited to permanent night work, but not to rotating shift work. (Bonde et al. 2012). Accordingly, future studies should take these factors into account.
The studies of offshore workers generally showed a high degree of adaptation to night work. Thus, Harris et al. (2010) demonstrated a very fast adaptation to night work with the workers’ cortisol rhythms begin fully adapted to night work within 7 days. In offshore work, the workers are typically on remote locations, isolated from the rest of society and free from commitments to daily family activities. Their meal times and exposure to light are also much more controlled than a typical shift worker onshore. Offshore night workers thereby lack input from conflicting zeitgebers which prevent adaptation in onshore workers. This might be the reason why the studies using offshore shift workers typically show more and faster adaptation to night work (Bjorvatn et al. 2006). Also Studies of night shifts workers in remote polar regions have also found evidence for a similar fast adaption to night shifts indicating that adaptation to night shifts might occur faster in these remote locations (Midwinter and Arendt 1991; Ross et al. 1995).
Our aim was to identify and summarize the effects of consecutive night shifts in field studies and we thus excluded both laboratory studies and combined field and laboratory studies. However, combined field and laboratory studies can add some important knowledge to the effects of night work in the field, and a laboratory circadian assessment following a period of night or day work is likely to provide an accurate picture of circadian status. A combined field study compared the diurnal rhythm of cortisol and melatonin in permanent night workers (normally 5–6 consecutive night shifts) and controls on a day off in the laboratory. They found that the permanent night workers did not have a permanent shift in their diurnal rhythm of cortisol and melatonin (Roden et al. 1993). This evidence again suggests that even permanent night workers have a normal diurnal rhythm on their days off. Boivin et al. (2012) conducted a combined field and laboratory study with police officers on seven consecutive night shifts in the field and circadian assessment in the laboratory and found a phase delay of approximately 6 h in the rhythm of melatonin. This evidence of a significant, but not complete adaptation to night work after seven consecutive night shifts are comparable with the results found in this review.
Even if it is established how many consecutive night shifts are required to adapt diurnal rhythms to work at night, it is still questionable what is the optimal way to organize night work. Thus, it is debated whether it is pivotal for health to adapt diurnal rhythms to work at night (by having many consecutive night shifts) or to minimize phase shifts of diurnal rhythms (by having few consecutive night shifts). Complete adaptation of diurnal rhythms may also not be desired, given that individuals would experience circadian misalignment when returning to a day-active schedule. In a study by Merkus et al. (2015), it was shown that following a 2-week 12-h night shift periods offshore, recovery was not fully complete up to day 11. In a consensus report published in 2012, Bonde et al. (2012) suggest to minimize the number of consecutive night shifts (i.e. one to two consecutive nights), since disruption of the diurnal melatonin secretion pattern can be diminished by restricting the number of consecutive night shifts. However, a consequence is that the sleep–wake cycle is affected, because it is out of phase with circadian rhythms of other physiological systems (as well as the light–dark cycle). For this reason, it may be argued that diurnal rhythms should be adapted to the sleep–wake cycle rather than the light–dark cycle by having more consecutive night shifts. This may be particularly relevant when focus is on safety rather than health. Studies have shown that both number of errors, reaction time and concentration are most affected during the first, second and third night shifts, but less so in the following shifts (Lamond et al. 2003;2004).
Most of the studies in the present review are small in size and some of the smaller studies did not find statistically significant differences (Anjum et al. 2011; Wong et al. 2012) which could be due to low statistical power. Field studies can be both expensive and time-consuming. They also require a company that will allow for its workers to participate in research projects during working hours and that there are participants who will volunteer for the study. However, field studies do provide important knowledge on how people are affected by night work in a real-life setting and they can provide insights regarding why and how diseases develop in night workers.
Conclusion
There are methodological differences in the field studies analyzing diurnal rhythms, and many use only few points of measurement. The lack of measuring points and proper timing of the measurements makes it difficult to assess which characteristics of the diurnal rhythm are adapted. For melatonin, cortisol and HRV, the results of the studies indicate that night work with one to three consecutive night shifts does not result in adaptation to night work by a full shift in diurnal rhythms. However, there is a need for studies investigating more than three consecutive night shifts to make conclusions on how many number on consecutive night shifts are required for adaptation in diurnal rhythms of cortisol, melatonin and HRV. There is also a need for larger field studies in order to secure statistically significant results.
References
Anjum B, Verma NS, Tiwari S, Singh R, Mahdi AA, Singh RB, Singh RK (2011) Association of salivary cortisol with chronomics of 24 h ambulatory blood pressure/heart rate among night shift workers. Biosci Trends 5:182–188
Arendt J (1986) Assay of melatonin and its metabolites: results in normal and unusual environments. J Neural Transm Suppl 21:11–33
Arendt J (1995) Melatonin and the mammalian pineal gland. Chapman & Hall, Cambridge, pp 1–331
Barnes RG, Deacon SJ, Forbes MJ, Arendt J (1998a) Adaptation of the 6-sulphatoxymelatonin rhythm in shiftworkers on offshore oil installations during a 2-week 12-h night shift. Neurosci Lett 241:9–12
Barnes RG, Forbes MJ, Arendt J (1998b) Shift type and season affect adaptation of the 6-sulphatoxymelatonin rhythm in offshore oil rig workers. Neurosci Lett 252:179–182
Bjorvatn B, Stangenes K, Oyane N, Forberg K, Lowden A, Holsten F, Akerstedt T (2006) Subjective and objective measures of adaptation and readaptation to night work on an oil rig in the North Sea. Sleep 29:821–829
Bøggild H, Knutsson A (1999) Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health 25:85–99
Boivin DB, Boudreau P, Tremblay GM (2012) Phototherapy and orange-tinted goggles for night-shift adaptation of police officers on patrol. Chronobiol Int 29:629–640
Bonde JPE, Andersen JH, Frost P, Kærgaard A, Kolstad HA, Thulstrup AM (2007) Helbredsundersøgelser ved natarbejde. Ugeskr Laeger 169:2005–2007
Bonde JP, Hansen J, Kolstad HA, Mikkelsen S, Olsen JH, Blask DE, Harma M, Kjuus H, de Koning HJ, Olsen J, Moller M, Schernhammer ES, Stevens RG, Akerstedt T (2012) Work at night and breast cancer-report on evidence-based options for preventive actions. Scand J Work Environ Health 38:380–390
Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, Katus HA (2003) Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol 14:791–799
Borugian MJ, Gallagher RP, Friesen MC, Switzer TF, Aronson KJ (2005) Twenty-four-hour light exposure and melatonin levels among shift workers. J Occup Environ Med 47:1268–1275
Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A (2003) The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol 177:17–26
Cadore E, Lhullier F, Brentano M, Silva E, Ambrosini M, Spinelli R, Silva R, Kruel L (2008) Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J Sports Sci 26:1067–1072
Choosong T, Arporn S, Chaikittiporn C (2006) A study of melatonin levels and stress in female shift workers. Southeast Asian J Trop Med Public Health 37:1048–1053
Collins SM, Karasek RA, Costas K (2005) Job strain and autonomic indices of cardiovascular disease risk. Am J Ind Med 48:182–193
Costa G, Ghirlanda G, Tarondi G, Minors D, Waterhouse J (1994) Evaluation of a rapidly rotating shift system for tolerance of nurses to nightwork. Int Arch Occup Environ Health 65:305–311
Costa G, Haus E, Stevens R (2010) Shift work and cancer—considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health 36:163–179
Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG (2000) Low heart rate variability in a 2-min rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis risk in communities. Circulation 102:1239–1244
Dula DJ, Dula NL, Hamrick C, Wood GC (2001) The effect of working serial night shifts on the cognitive functioning of emergency physicians. Ann Emerg Med 38:152–155
Ferguson SA, Kennaway DJ, Baker A, Lamond N, Dawson D (2012) Sleep and circadian rhythms in mining operators: limited evidence of adaptation to night shifts. Appl Ergon 43:695–701
Figueiro MG, Plitnick B, Rea MS (2014) The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Med
Folkard S (2008) Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int 25:215–224
Frost P, Kolstad HA, Bonde JP (2009) Shift work and the risk of ischemic heart disease—a systematic review of the epidemiologic evidence. Scand J Work Environ Health 35:163–179
Furlan R, Barbic F, Piazza S, Tinelli M, Seghizzi P, Malliani A (2000) Modifications of cardiac autonomic profile associated with a shift work schedule of work. Circulation 102:1912–1916
Garde AH, Persson R, Hansen AM, Österberg K, Ørbæk P, Eek FC, Karlson B (2008) Effects of lifestyle factors on concentrations of salivary cortisol in healthy individuals. Scand J Clin Lab Invest 49:242–250
Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF (2009) Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clin Biochem 42:1205–1217
Gibbs M, Hampton S, Morgan L, Arendt J (2002) Adaptation of the circadian rhythm of 6-sulphatoxymelatonin to a shift schedule of seven nights followed by seven days in offshore oil installation workers. Neurosci Lett 325:91–94
Gibbs M, Hampton S, Morgan L, Arendt J (2007) Predicting circadian response to abrupt phase shift: 6-sulphatoxymelatonin rhythms in rotating shift workers offshore. J Biol Rhythms 22:368–370
Grandin LD, Alloy LB, Abramson LY (2006) The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev 26:679–694
Griffiths JD, McCutcheont C, Silbert BS, Maruff P (2006) A prospective observational study of the effect of night duty on the cognitive function of anaesthetic registrars. Anaesth Intensive Care 34:621–628
Grundy A, Tranmer J, Richardson H, Graham CH, Aronson KJ (2011) The influence of light at night exposure on melatonin levels among Canadian rotating shift nurses: cancer epidemiology. Biomark Prev 20:2404–2412
Hansen ÅM, Garde AH, Hansen J (2006) Diurnal urinary 6-sulfatoxymelatonin levels among healthy danish nurses during work and leisure time. Chronobiol Int 23:1203–1215
Hansen JH, Geving IH, Reinertsen RE (2010) Adaptation rate of 6-sulfatoxymelatonin and cognitive performance in offshore fleet shift workers: a field study. Int Arch Occup Environ Health 83:607–615
Harris A, Waage S, Ursin H, Hansen ÅM, Bjorvatn B, Eriksen HR (2010) Cortisol, reaction time test and health among offshore shift workers. Psychoneuroendocrinology 35:1339–1347
Haus E, Smolensky M (2006) Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 17:489–500
Haus EL, Smolensky MH (2013) Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17:273–284
Hofstra WA, De Weerd AW (2008) How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav 13:438–444
Jensen MA, Hansen AM, Abrahamsson P, Norgaard AW (2011) Development and evaluation of a liquid chromatography tandem mass spectrometry method for simultaneous determination of salivary melatonin, cortisol and testosterone. J Chromatogr B Analyt Technol Biomed Life Sci. 879:2527
Kantermann T, Wehrens SM, Ulhoa MA, Moreno C, Skene DJ (2012) Noisy and individual, but doable: shift-work research in humans. Prog Brain Res 199:399–411
Kleiger RE, Stein PK, Bigger JT Jr (2005) Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 10:88–101
Knutsson A, Bøggild H (2010) Gastrointestinal disorders among shift workers. Scand J Work Environ Health 36:85–95
Kobayashi F, Furui H, Akamatsu Y, Watanabe T, Horibe H (1997) Changes in psychophysiological functions during night shift in nurses. Influence of changing from a full-day to a half-day work shift before night duty. Int Arch Occup Environ Health 69:83–90
Kudielka BM, Buchtal J, Uhde A, Wüst S (2007) Circadian cortisol profiles and psychological self-reports in shift workers with and without recent change in the shift rotation system. Biol Psychol 74:92–103
Lamond N, Dorrian J, Roach GD, McCulloch K, Holmes AL, Burgess HJ, Fletcher A, Dawson D (2003) The impact of a week of simulated night work on sleep, circadian phase, and performance. Occup Environ Med 60
Lamond N, Dorrian J, Burgess HJ, Holmes AL, Roach GD, McCulloch K, Fletcher A, Dawson D (2004) Adaptation of performance during a week of simulated night work. Ergonomics 47:154–165
Malliani A, Pagani M, Montano N, Mela GS (1998) Sympathovagal balance: a reappraisal. Circulation 98:2640–2643
McEwen BS, Karatsoreos IN (2015) Sleep deprivation and circadian disruption: stress, allostasis, and allostatic load. Sleep Med Clin 10:1–10
Merkus SL, Holte KA, Huysmans MA, Hansen AM, van de Ven PM, van MW, van der Beek AJ (2015) Neuroendocrine recovery after 2-week 12-h day and night shifts: an 11-day follow-up. Int Arch Occup Environ Health 88:247–257
Midwinter MJ, Arendt J (1991) Adaptation of the melatonin rhythm in human subjects following night-shift work in Antarctica. Neurosci Lett 122:195–198
Mirick DK, Davis S (2008) Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomark Prev 17:3306–3313
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A (1994) Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90:1826–1831
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelly P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59:178–193
Puttonen S, Härmä M, Hublin C (2010) Shift work and cardiovascular disease—pathways from circadian stress to morbidity. Scand J Work Environ Health 36:96–108
Rauchenzauner M, Ernst F, Hintringer F, Ulmer H, Ebenbichler CF, Kasseroler MT, Joannidis M (2009) Arrhythmias and increased neuro-endocrine stress response during physicians’ night shifts: a randomized cross-over trial. Eur Heart J 30:2606–2613
Roden M, Koller M, Pirich K, Vierhapper H, Waldhauser F (1993) The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol 265:R261–R267
Rosmond R, Björntorp P (2000) Occupational status, cortisol secretory pattern, and visceral obesity in middle-aged men. Obes Res 8:445–450
Ross JK, Arendt J, Horne J, Haston W (1995) Night-shift work in Antarctica: sleep characteristics and bright light treatment. Physiol Behav 57:1169–1174
Sallinen M, Kecklund G (2010) Shift work, sleep and sleepiness—differences between shift schedules and systems. Scand J Work Environ Health 36:121–133
Sargent C, Darwent D, Ferguson SA, Kennaway DJ, Roach GD (2012) Sleep restriction masks the influence of the circadian process on sleep propensity. Chronobiol Int 29:565–571
Stevens RG, Rea MS (2001) Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control 12:279–287
Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castaño-Vinyals G, Davis S, Frings-Dresen MHW, Fritschi L, Kogevinas M, Kogi K, Lie JA, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V, Straif K (2011) Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med 68:154–162
Task Force of the European Society of Cardiology, The North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065
Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141:122–131
Tsigos C, Chrousos GP (2002) Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53:865–871
Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D (1996) Impact of reduced heart rate variability on risk for cardiac events: the Framingham Heart Study. Circulation 94:2850–2855
Turek FW (2008) Staying off the dance floor: when no rhythm is better than bad rhythm. Am J Physiol Regul Integr Comp Physiol 294:R1672–R1674
Vandewalle G, Middleton B, Rajaratnam SMW, Stone BM, Thorleifsdottir B, Arendt J, Dijk D-J (2007) Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res 16:148–155
Vangelova KK, Dalbokova DL (1998) Variations in 6-sulphatoxymelatonin excretion and oral temperature under a 12-h shiftwork environment. Rev Environ Health 13:221–226
Vrijkotte TG, van Doornen LJ, de Geus EJ (2000) Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension 35:880–886
Wong IS, Ostry AS, Demers PA, Davies HW (2012) Job strain and shift work influences on biomarkers and subclinical heart disease indicators: a pilot study. J Occup Environ Hyg 9:467–477
Zelinski EL, Deibel SH, McDonald RJ (2014) The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev 40:80–101
Acknowledgments
Elizabeth Bengtsen is acknowledged for her help in constructing the search strategy. The study was funded by The Danish Working Environment Research Fund (10-2011-09) and a Ph.D. scholarship from the University of Copenhagen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jensen, M.A., Garde, A.H., Kristiansen, J. et al. The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): a systematic review of field studies. Int Arch Occup Environ Health 89, 531–545 (2016). https://doi.org/10.1007/s00420-015-1093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-015-1093-3