Abstract
Purpose
Shift work, short sleep duration, employment as a flight attendant, and exposure to light at night, all potential causes of circadian disruption, have been inconsistently associated with breast cancer (BrCA) risk. The aim of this meta-analysis is to quantitatively evaluate the combined and independent effects of exposure to different sources of circadian disruption on BrCA risk in women.
Methods
Relevant studies published through January 2014 were identified by searching the PubMed database. The pooled relative risks (RRs) and corresponding 95 % confidence intervals (CIs) were estimated using fixed- or random effects models as indicated by heterogeneity tests. Generalized least squares trend test was used to assess dose–response relationships.
Results
A total of 28 studies, 15 on shift work, 7 on short sleep duration, 3 on flight attendants, and 6 on light at night were included in the analysis. The combined analysis suggested a significantly positive association between circadian disruption and BrCA risk (RR = 1.14; 95 % CI 1.08–1.21). Separate analyses showed that the RR for BrCA was 1.19 (95 % CI 1.08–1.32) for shift work, 1.120 (95 % CI 1.119–1.121) for exposure to light at night, 1.56 (95 % CI 1.10–2.21) for employment as a flight attendant, and 0.96 (95 % CI 0.86–1.06) for short sleep duration. A dose–response analysis showed that each 10-year increment of shift work was associated with 16 % higher risk of BrCA (95 % CI 1.06–1.27) based on selected case–control studies. No significant dose–response effects of exposure to light at night and sleep deficiency were found on BrCA risk.
Conclusions
Our meta-analysis demonstrates that circadian disruption is associated with an increased BrCA risk in women. This association varied by specific sources of circadian disrupting exposures, and a dose–response relationship remains uncertain. Therefore, future rigorous prospective studies are needed to confirm these relationships.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is an important public health problem and a major cause of morbidity and mortality in women internationally (Ferlay et al. 2010; Jemal et al. 2011). The development of breast cancer has been attributed to multiple risk factors including the disruption of circadian rhythms due to shift work, short sleep duration, exposure to light at night, and specific occupations that may alter circadian rhythms, such as employment as a flight attendant.
The association between shift work and breast cancer risk in women has been extensively studied since the beginning of the twenty-first century. The potential mechanisms of night shift work and breast cancer include increased likelihood of light exposure at night and decreased length of sleep, both of which influence the circadian rhythm (Haus and Smolensky 2013). Previous studies on the association of shift work with breast cancer were primarily based on the assumption that shift work could lead to the suppression of melatonin, a hormone capable of regulating the initiation, promotion and progression of cancer (Blask 2009; Blask et al. 2005). These hypotheses have been supported by laboratory animal models demonstrating an increased breast cancer risk resulting from simulated rotating shift work (Filipski et al. 2004, 2005), and by human studies indicating an inverse association of breast cancer risk with urinary 6-sulfatoxy melatonin levels (Schernhammer et al. 2008, 2010; Schernhammer and Hankinson 2009).
Sleep deficiency, which may be a surrogate for exposure to light at night, is common among shift workers, especially in night workers. According to a meta-analysis of 36 studies, permanent night workers slept an average 6.6 h compared to 7.0 h by day-shift workers (Pilcher et al. 2000). The popularity of round-the-clock service in modern society places an increasing number of persons at risk of being exposed to artificial light at night. Night shift work, short sleep duration, and exposure to light at night, all may reduce melatonin secretion by the pineal gland, thereby affecting the circadian rhythm. Despite this, a limited number of studies thus far have been conducted to examine the impact of either short sleep duration or exposure to light at night on the risk of breast cancer. In addition, no study has assessed the combined effect of exposure to shift work, sleep deficiency, and light at night on breast cancer risk in women.
Employment as a flight attendant is considered a common occupation that may require night work. A meta-analysis by Buja et al. found an elevated breast cancer risk among female flight attendants (Buja et al. 2006). Some authors tend to include individuals who are employed in these types of occupations in their estimates of the effect of night shift work on breast cancer (Megdal et al. 2005). However, there is evidence suggesting that airline cabin crew members are occupationally exposed to twice the amount of ionizing radiation as compared to the general population (Bartlett 2004; Pukkala et al. 2012). Ionizing radiation is a potential risk factor for breast cancer, given its ability to damage DNA in cells (Haldorsen et al. 2001). As a consequence, the inclusion of this subgroup of employees may cause an overestimation of the association between shift work and the risk of breast cancer. Therefore, isolating the effect of employment as a flight attendant is important in obtaining an accurate assessment of the influence of shift work on breast cancer incidence.
A dose–response relationship between the duration of shift work and breast cancer risk has been considered in the literature, but has shown conflicting results. For example, according to Wang et al., a five-year increase in exposure to night shift work increased the risk of female breast cancer by 3 % (Wang et al. 2013). However, Ijaz et al. (2013) failed to identify a significant dose–response effect of night shift work on breast cancer risk among cohort studies (RR = 1.01, 95 % CI 0.97–1.05). In addition, no studies have investigated the dose–response effect of exposure to light at night or sleep deficiency on breast cancer risk among women.
In consideration of the potential connections among shift work, short sleep duration, employment as a flight attendant, and exposure to light at night, coupled with the limitations of previous meta-analyses, we performed a series of meta-analyses to quantitatively assess (1) the combined effect of exposure to different sources of circadian disruption on breast cancer risk in women and (2) the independent effect of exposure to shift work, employment as a flight attendant, light at night or sleep deficiency on breast cancer risk in women. Finally, we investigated the dose–response relationship between exposure to shift work, light at night and sleep deficiency, and female breast cancer risk.
Materials and methods
The meta-analysis was conducted in accordance with the checklist of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (Stroup et al. 2000). The MOOSE checklist was developed by a group of experts in clinical practice, trials, statistics, epidemiology, social sciences, and biomedical editing. It contains specifications on how to report background, search strategy, methods, results, discussion, and conclusion for the meta-analysis of observational studies in epidemiology (Appendix I).
Search strategy
A PubMed search of the literature was conducted to identify manuscripts published from inception to January 2014. A comprehensive search strategy was used to retrieve relevant publications. Search terms included [shift work OR night work OR rotating shift OR shiftwork OR evening shift OR night time shift OR night shift work] OR [light at night OR light exposure OR artificial light OR electric light] OR [flight attendant OR cabin crew OR airline crew] OR [sleep deficiency OR sleep duration OR sleep disturbance] OR [circadian disrupt OR circadian disruption OR circadian rhythm OR chronobiology disruption OR chronobiology disrupt] AND [breast cancer OR breast neoplasm OR endometrial cancer OR mammary cancer]. Relevant articles were also selected based on the reference lists of publications identified in the original PubMed search.
Study selection
All references obtained from PubMed were imported into EndNote c version X6 reference database. Duplicate articles were automatically identified and excluded by EndNote. At the first stage of analysis, article titles were screened for relevance (e.g., observational studies). Abstracts of relevant articles were then reviewed to identify eligible papers (e.g., quantified analyses between exposure to shift work, light at night, short sleep duration, employment as a flight attendant, and risk of breast cancer). Thereafter, full-text versions of potentially eligible articles were obtained and assessed.
Studies had to fulfil the following criteria for inclusion in the meta-analysis: (1) were observational studies (e.g., cohort study or case–control study); (2) evaluated the association of breast cancer risk among women exposed to any type of circadian disruption (e.g., shift work and/or exposure to light at night and/or sleep disruption and/or employment as a flight attendant); and (3) presented hazard ratio (HR), odds ratio (OR), relative risk (RR), or standardized incidence ratio (SIR) estimates with 95 % confidence intervals (CI). For studies with more than one article based on the same study population, inclusion was limited to the one with (1) the most recent publication date; (2) the biggest sample size; and/or (3) adjustment for potential confounders.
Quality assessment
The quality of the eligible studies was assessed using the Newcastle-Ottawa Scale (NOS) (Wells et al.). A star system (maximum of nine stars) is employed by the NOS scale to assess observational studies on three domains: participant selection (maximum of four stars), comparability of study groups (maximum of two stars), and ascertainment of exposures or assessment of outcome (maximum of three stars).
For participant selection, we focused on the representativeness of the study cases and cohorts and the selection of controls. One star was awarded to studies that utilized a random-digit-method to recruit free-of-cancer controls. For comparability of study groups, we examined the following six confounding variables: age, age at menarche, body mass index (BMI), family history of breast cancer, parity (e.g., number of children and age at the first birth), and alcohol consumption. One star was assigned to studies that adjusted for at least four out of these six variables in the risk estimate. An extra star was awarded to studies that adjusted for additional variables, such as hormone therapy and smoking. Regarding assessment of exposures, one star was assigned to studies with either quantitative classification of exposures (e.g., studies containing stratified analyses according to the duration of exposure) or objective assessment of exposures (e.g., exposure status was ascertained through shift schedules). Moreover, we evaluated the follow-up/response rate in the selected studies. Studies with ≤80 % follow-up/response rate received no star. Finally, cohort studies with the maximum follow-up time of greater than 8 years received one star.
Data extraction
A data extraction form was developed, piloted, and assessed by CH, SWR, and STA. Information including the first author’s last name, year of publication, geographic location, study design, number of cases, total sample size, follow-up/response rate, covariates, exposure assessment, exposure categories, quality score, measure of association, and corresponding 95 % CI were extracted. All data were extracted by one investigator (CH) and validated by the second investigator (STA) for key study characteristics such as study design, study setting, and measures of association. Consensus was reached by discussions or resolutions by the third investigator (SWR).
Statistical analysis
Standard errors (SEs) for ORs, RRs, or HRs were derived from the formula SE = (log(upper limit of 95 % confidence interval (CI)/lower limit of 95 % CI))/(2 × 1.96), while standard errors for SIRs were calculated as \(SE = \sqrt {O/E^{2} }\) (O denotes the observed number of cases and E denotes the expected number of cases). RR was used to summarize the measure of association between circadian disruption and breast cancer risk among women. Distinctions between various measures of association (e.g., OR, RR, HR, and SIR) were ignored based on the assumption that breast cancer is a rare disease, which indicates that the RR and OR are approximately equivalent (Greenland and Thomas 1982).
The heterogeneity of RRs across studies was tested using Cochran’s Q statistic and I 2 statistic (Higgins et al. 2003). A P value less than 0.1 from the Q statistic was considered to be indicative of statistically significant heterogeneity. The I 2 statistic was calculated to express the fraction of variation between studies that was due to heterogeneity (Higgins et al. 2003). The pooled RR was estimated based on the fixed effects model when no significant heterogeneity was detected. Otherwise, a random effects model was used (DerSimonian and Laird 1986). To explore heterogeneity among various circadian disrupting exposures, separate analyses were carried out based on studies concerning shift work, employment as a flight attendant, exposure to light at night, or short sleep duration. To explore heterogeneity among selected studies, we conducted subgroup analyses over a number of key study characteristics including study design (cohort or case–control design), measures of association (OR, RR, HR, or SIR), geographic location (Europe, USA, or other countries), study quality (quality score ≤7 or quality score >7), and follow-up/response rate (follow-up/response rate <80 % or follow-up/response rate ≥80 %). Moreover, subgroup analyses were carried out on the basis of cross-classification (i.e., double-stratification according to two factors) of follow-up/response rate and quality score, and of follow-up/response rate and study design. Heterogeneity between subgroups was tested with meta-regression. Finally, we performed sensitivity analyses omitting one study at a time to determine whether the pooled effect size was unduly influenced by a specific study.
For the dose–response meta-analysis, we used the generalized least squares for trend (GLST) method to compute study-specific slopes (linear trends) and 95 % CIs from the natural logs of the RRs and CIs across categories of various sources of circadian disruption, as described by Greenland (Greenland and Longnecker 1992) and Orsini (Orsini and Greenland 2006). This method requires information regarding the number of cases, person-time, or non-cases for at least three quantitative exposure categories in order to derive the dose–response trend. For studies that reported the circadian disruption by ranges, we used the midpoint of the lower and upper bound in each category as the average dose of exposure. The width of the open-ended interval was assumed to be the same as that of the adjacent interval if the highest category did not have an upper bound. The lower bound was set to be zero if the lowest category did not have a lower bound. The pooled RRs for dose–response effects were obtained based on three factors: (1) increments of 10 years of shift work (reference group: never exposed to shift work); (2) increments of 8 times/month an individual turned on a light while sleeping (reference group: no light turned on while sleeping); or (3) decrement of 1 h of sleep per night (reference group: slept 7–8 h per night). For comparability, conversion was carried out based on the assumption that there are 28 days in a month (i.e., 2 times/week = 8 times/month).
Finally, potential publication bias was examined using a funnel plot and Egger’s test (Egger et al. 1997). All analyses were performed in either STATA 12 (StataCorp, College Station, TX, USA) or SAS 9.3 for Windows (SAS Institute, Inc., Cary, NC). P values <0.05 were considered statistically significant for all analyses except heterogeneity tests.
Results
Search results
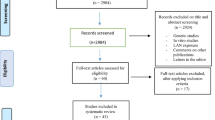
A total of 2,112 citations were retrieved from the PubMed database. Of these, 598 were initially discarded by EndNote reference database due to duplications. From the remaining citations, the majority were excluded based on titles or abstracts, primarily because they were molecular studies, clinical trials, or lacked exposure or outcome information. Sixty-three articles were eligible for full-text review. After close examination, 35 publications were excluded because they used the same study population source, were literature reviews, or had no clear definition of exposures. Ultimately, a total of 28 studies met our inclusion criteria and were included in the meta-analysis (Bauer et al. 2013; Davis et al. 2001; Fritschi et al. 2013; Girschik et al. 2013; Grundy et al. 2013; Hansen 2001; Hansen and Lassen 2012; Hansen and Stevens 2012; Kakizaki et al. 2008; Kloog et al. 2011; Knutsson et al. 2013; Kojo et al. 2005; Li et al. 2010; Lie et al. 2011; McElroy et al. 2006; Menegaux et al. 2012; O’Leary et al. 2006; Pesch et al. 2010; Pinheiro et al. 2006; Pronk et al. 2010; Pukkala et al. 2012; Rafnsson et al. 2003; Reynolds et al. 2002; Schernhammer et al. 2001, 2006; Schwartzbaum et al. 2007; Verkasalo et al. 2005; Wu et al. 2008) (Fig. 1).
Characteristics of studies
Descriptive characteristics of the 28 studies, comprising 1,728,237 participants that were selected for analysis are presented in Table 1. Overall, the meta-analysis included 18 case–control and 10 cohort studies. Nine out of 10 (90 %) cohort studies reached a follow-up rate of 80 % or higher. The response rate for more than half of the case–control studies (61 %) was less than 80 %. The association between breast cancer risk and circadian disruption was primarily measured using ORs (n = 17) and HRs (n = 5). The relevant studies were conducted in several regions: twelve in Europe, nine in the USA, and seven in other countries such as Australia, Canada, and China. The median quality score of selected studies was 7, with a range of 5–9. Eleven studies had a quality score >7.
Three studies evaluated the association between breast cancer and circadian disruption with more than one source of exposure. For example, the effects of both night shift work and exposure to light at night on breast cancer risk were assessed in Davis et al.’s, Fritschi et al.’s and O’Leary et al.’s studies (Davis et al. 2001; Fritschi et al. 2013; O’Leary et al. 2006). Fritschi et al.’s study also reported the risk estimate of sleep disruption. However, since the study population from Fritschi et al.’s (2013) and Girschik et al.’s (2013) studies (;) were the same and the latter study had a more detailed evaluation of sleep duration on breast cancer risk, the risk estimate from the latter study was the only one included in the analysis. In total, the relationship between shift work and breast cancer was investigated in 15 studies (Davis et al. 2001; Fritschi et al. 2013; Grundy et al. 2013; Hansen 2001; Hansen and Lassen 2012; Hansen and Stevens 2012; Knutsson et al. 2013; Lie et al. 2011; Menegaux et al. 2012; O’Leary et al. 2006; Pesch et al. 2010; Pronk et al. 2010; Schernhammer et al. 2001, 2006; Schwartzbaum et al. 2007). Breast cancer risk estimates were obtained in six studies concerning exposure to light at night (Bauer et al. 2013; Davis et al. 2001; Fritschi et al. 2013; Kloog et al. 2011; Li et al. 2010; O’Leary et al. 2006), seven studies regarding sleep deficiency (Girschik et al. 2013; Kakizaki et al. 2008; Kojo et al. 2005; McElroy et al. 2006; Pinheiro et al. 2006; Verkasalo et al. 2005; Wu et al. 2008), and three involving employment as a flight attendant (Pukkala et al. 2012; Rafnsson et al. 2003; Reynolds et al. 2002).
Overall meta-analysis
Our meta-analysis was first performed over all selected studies regardless of the source of exposure, the type of study design, or any other potential confounders. Of note, only one measure of association (either the original estimate or a pooled estimate generated from the fixed effects model) was obtained from each study for the overall meta-analysis: for studies presenting breast cancer RRs stratified according to source of exposure, only the risk estimates pertaining to shift work was included in the overall meta-analysis; for studies presenting several breast cancer risk estimates in terms of factors such as duration or frequency of exposure, a fixed effects model was carried out to obtain a pooled RR estimate. A sensitivity analysis was carried out by omitting study’s one at a time and assessing the impact on the pooled estimate of the effect of circadian disruption on risk of breast cancer, but no substantial difference was identified (data not shown). Visual examination of Fig. 2 indicates an approximately symmetric funnel plot across all included studies. The Egger’s test performed over all studies was not statistically significant (P = 0.548) indicating no publication bias. Significant heterogeneity was identified across all studies (P < 0.001, I 2 = 77.5 %), and thus a random effects model was performed. Overall, we found a significant positive relationship between circadian disruption and breast cancer risk in women (RR = 1.14; 95 % CI 1.08–1.21) (Fig. 3).
Subgroup analyses
A positive association between breast cancer risk and circadian disruption was consistently found in almost all of the stratified analyses (Table 2). When stratifying studies by source of exposure, shift work (RR = 1.19, 95 % CI 1.08–1.32) and exposure to light at night (RR = 1.120, 95 % CI 1.119–1.121) appeared to have similar RR estimates. No substantial association was found between sleep deficiency and breast cancer risk (RR = 0.96; 95 % CI 0.86–1.06). The highest RR estimate was obtained among studies concerning employment as a flight attendant and breast cancer risk (RR = 1.56; 95 % CI 1.10–2.21). An additional subgroup analysis, which included all studies except those focusing on flight attendants, yielded a slightly smaller RR estimate as compared to the overall meta-analysis result (RR = 1.12, 95 % CI 1.05–1.19 vs. RR = 1.14, 95 % CI 1.08–1.21, respectively). Moreover, the magnitude of the effect of shift work on breast cancer risk increased slightly when considering studies including either shift work or flight attendants (compared to those studies looking at shift work only) (i.e., RR = 1.19, 95 % CI 1.08–1.32 for studies concerning shift work only; RR = 1.23, 95 % CI 1.11–1.36 for studies concerning either shift work or employment as a flight attendant).
When the analyses were stratified by study design, the pooled RR was 1.21 (95 % CI 1.11–1.32) for case–control studies, and 1.04 (95 % CI 0.95–1.3) for cohort studies. When using 80 % follow-up/response rate as a cutoff point, we found a lower RR estimate in studies with a follow-up/response rate ≥80 % (RR = 1.16; 95 % CI 1.08–1.25). When subgroup analyses were stratified by quality score (quality score ≤7 or quality score >7), we found that studies with a lower quality score tended to have higher breast cancer risk estimates (RR = 1.16; 95 % CI 1.08–1.25) as compared to studies with a higher quality score (RR = 1.14; 95 % CI 1.00–1.31). Cross-classification of follow-up/response rate and quality score revealed the strongest RR estimate among studies with a follow-up/response ≥80 % and a quality score >7 (RR = 1.20; 95 % CI 1.08–1.32). Cross-classification of follow-up/response rate and study design identified the lowest RR estimate across cohort studies with follow-up/response rate ≥80 % (RR = 1.03; 95 % CI 0.99–1.09). The lowest pooled RR estimate was obtained among studies measured with HRs (RR = 1.05, 95 % CI 0.88–1.26) while stratifying in terms of measure of association. When analyses were stratified according to geographic location, the highest pooled RR estimate was obtained among studies conducted in Europe (RR = 1.32; 95 % CI 1.12–1.56).
Meta-regression analysis was used to evaluate the influence of type of exposure, study design, geographic location, follow-up/response rate, study quality, or measure of association on the heterogeneity across studies (Table 2). We failed to identify any substantial cause of heterogeneity in this study (P > 0.05). Based on Egger’s test, a significant publication bias was found among studies with less than 80 % follow-up/response rate (P = 0.009), among studies with lower follow-up/response rate and higher study quality (P = 0.029), and among case–control studies with higher a follow-up/response rate (P = 0.016).
Dose–response analyses
Dose–response analyses were performed separately to analyze the pooled effect of shift work exposure on the risk of breast cancer in cohort studies, case–control studies, and an overall analysis. Nine case–control (Davis et al. 2001; Fritschi et al. 2013; Grundy et al. 2013; Hansen and Lassen 2012; Hansen and Stevens 2012; Lie et al. 2011; Menegaux et al. 2012; O’Leary et al. 2006; Pesch et al. 2010) and three cohort studies (Pronk et al. 2010; Schernhammer et al. 2006; Schernhammer et al. 2001) were included in the dose–response analysis for shift work. An increment of 10 years of shift work exposure was significantly associated with a 16 % increased risk of breast cancer (RR = 1.16; 95 % CI 1.06–1.27) based on case–control studies (Fig. 4a). However, no significant dose–response relationship between shift work and breast cancer risk was found in either cohort studies (Fig. 4b: RR = 1.03; 95 % CI 0.95–1.11) or in the overall analysis (Fig. 4c: RR = 1.06; 95 % CI 0.98–1.15). Two studies (Davis et al. 2001; O’Leary et al. 2006) presented breast cancer risk estimates according to the number of times/month a light was turned on during sleep, but no significant increase in risk was found (Fig. 4d). Similarly, no significant dose–response relationship was identified between sleep deficiency and breast cancer risk based on four selected studies (Fig. 4e) (Girschik et al. 2013; McElroy et al. 2006; Pinheiro et al. 2006; Verkasalo et al. 2005).
The dose–response relationship between breast cancer risk and a duration of shift work among case–control studies; b duration of shift work among cohort studies; c duration of shift work among all selected studies; d number of times/month a light was turned on while sleeping; e duration of sleep deficiency (reference group: slept 7–8 h per night)
Discussion
This meta-analysis included 28 observational studies and provided a comprehensive assessment of the associations of breast cancer risk in women with various sources of circadian disrupting exposures including shift work, short sleep duration, exposure to light at night, and employment as a flight attendant. We found a significant increase in the risk of breast cancer in the combined meta-analysis, which aggregated all studies regardless of the source of circadian disruption (RR = 1.14; 95 % CI 1.08–1.21). Neither visual funnel plot nor Egger’s test showed evidence of publication bias across all studies.
Melatonin, a hormone produced by the pineal gland, regulates the circadian rhythm. Night work can disrupt the normal circadian rhythm through exposure to light at night and reduced sleep length. It is hypothesized that female shift workers have a higher risk of breast cancer. Some studies indicated a positive association between shift work and the risk of breast cancer in women (Davis et al. 2001; Hansen 2001; Hansen and Stevens 2012; Knutsson et al. 2013; Schernhammer et al. 2001). Others, however, found a reverse association (Pronk et al. 2010; Schwartzbaum et al. 2007) or failed to observe any significant relationship between shift work and breast cancer risk (Lie et al. 2011; Menegaux et al. 2012; Pesch et al. 2010). Our meta-analysis, which incorporated 15 articles on shift work only, found a substantially increased breast cancer risk among women (RR = 1.19; 95 % CI 1.08–1.32). This finding is consistent with previous meta-analyses on the topic (Ijaz et al. 2013; Jia et al. 2013). In addition, we found an average of a 16 % increased risk per 10 years of shift work exposure based on case–control studies (RR = 1.16; 95 % CI 1.06–1.27), but no significant increase in risk was found among cohort studies or in the overall analysis.
Some occupational exposure studies demonstrated an excess in breast cancer incidence among female flight attendants (Linnersjo et al. 2003; Lynge 1996; Pukkala et al. 2012; Reynolds et al. 2002). The underlying mechanism is related to increased exposure to ionizing radiation and the hormonal alteration resulting from the circadian disturbance (Kojo et al. 2005). The separate meta-analysis based on three articles regarding employment as a flight attendant suggested an elevated breast cancer risk (RR = 1.56; 95 % CI 1.10–2.21). This is slightly higher than previous findings by Megdal et al. in 2005 (RR = 1.44; 95 % CI 1.26 1.65) (Megdal et al. 2005) and by Buja et al. in 2006 (RR = 1.40; 95 % CI 1.19–1.65) (Buja et al. 2006). Differences in the findings may be attributed to the distinctions in the inclusion criteria for data analysis. Two studies reported by Lynge et al. and Wartenberg et al. were excluded from the present analysis because of a lack of a clear description of the study population source (Lynge 1996; Wartenberg and Stapleton 1998). Data from Linnersjö et al.’s study (Linnersjo et al. 2003) were also removed from the present analysis because it was conducted in the same population as Pukkala et al.’s study (Pukkala et al. 2012).
Sleep duration may be a surrogate of an individuals’ exposure to light at night and the reduction in the number of hours of sleeping could therefore suppress the production of melatonin via circadian disruption. This hypothesis has been examined by a number of studies. There is evidence demonstrating a positive association between sleep duration at night and nocturnal blood melatonin levels (Akerstedt et al. 1979; Wehr et al. 2001). However, the effect of sleep deficiency on the risk of breast cancer remains controversial. Similar to the recent meta-analysis by Qin et al. (Qin et al. 2014), the present study failed to identify a statistically significant association between short sleep duration and breast cancer in women (RR = 0.96; 95 % CI 0.86–1.06). In addition, we did not find a substantial dose–response relationship between breast cancer risk and sleep deficiency.
A potential mechanism for the elevated risk of breast cancer among female shift workers involves the increased likelihood of exposure to light at night (Haus and Smolensky 2013). Exposure to light during sleeping can reset the circadian phase and reduce the secretion of melatonin by the pineal gland (Boivin et al. 1996; Haus and Smolensky 2013). A large number of studies have investigated the relationship between night shift work and the risk of breast cancer in women. However, the effect of light at night on breast cancer risk is not frequently investigated. Our study is the first to summarize the risk estimate for exposure to light at night. Consistent with the hypothesis, we found an increased breast cancer risk among women who were ‘ever’ exposed to light during sleep (RR = 1.120; 95 % CI 1.119–1.121). Nonetheless, no significant dose–response effect of light exposure at night was found on breast cancer.
As expected, flight attendants had a 37 % higher breast cancer risk than the average shift worker (RR = 1.56 vs. RR = 1.19, respectively). The indiscriminate combination of shift work and employment as a flight attendant tends to overestimate the effect of shift work on breast cancer risk. Consequently, apart from exposure to shift work, other factors such as ionizing radiation may also be related to the elevated breast cancer risk among female flight attendants.
Our study has several strengths over previous reviews on the topic. To the best of our knowledge, this is the first comprehensive and quantitative assessment of the association between circadian disruption and breast cancer risk. As mentioned previously, shift work, exposure to light at night, employment as a flight attendant, and short sleep duration, are all potential causes of circadian dysregulation and are highly interrelated. The combination of these exposures provides general evidence on the effect of circadian disruption on breast cancer risk in women. It is difficult to identify the potential differences in various sources of circadian disrupting exposures; therefore, separate meta-analyses were carried out to assess the specific role of these exposures on the risk of breast cancer. This study found a higher breast cancer risk among female flight attendants than among other types of shift workers, which suggests that other mechanisms apart from circadian disruption may contribute to the development of breast cancer among this occupational subset. Moreover, stratified analyses based on other key variables, such as study design and geographic location, were performed to investigate the potential sources of heterogeneity across studies. An additional strength is that, we excluded studies without a clear description of study participant selection or ascertainment of exposures to reduce bias among included studies. Finally, dose–response relationships were explored according to the duration of night shift work, frequency of exposure to light at night during sleep, and duration of sleep deficiency. However, we were not able to perform a dose–response analysis for employment as a flight attendant because of lack of quantitative evidence.
Our study has several limitations. First, significant heterogeneity existed among studies, which may result from inconsistent definitions of exposure across studies. For example, night shift work was defined as working the fulltime period between 12:00 a.m. and 5:00 a.m. according to Pesch et al. (2010), as any work spanning the time period 11:00 p.m.–5:00 a.m. according to Menegaux et al. (2012), as a shift that lasted from at least 12 p.m. until 6 a.m. according to Lie et al. (2011), and as a shift between 22:00 p.m. and 6:00 a.m. according to Knutsson et al. (2013). Also, the substantial heterogeneity we observed over studies may have been caused by differences in study population, or in the selection of covariates, among other differences. Random effects models may not have eliminated all sources of heterogeneity, therefore, the pooled risk estimate should be interpreted with caution.
Secondly, the present meta-analysis was performed over observational studies, which are vulnerable to a number of biases. Most of the included studies (64 %) were designed as case–control studies and were therefore particularly susceptible to recall bias. In addition, most of the studies did not employ standardized questionnaires to assess the exposure, which may have resulted in information bias. It should also be noted that it is not possible to identify the exact mechanisms and/or establish the cause-effect relationship between circadian disruption and breast cancer risk in women based on evidence aggregated from observational studies.
Finally, we did not differentiate the risk estimates for night shift work from those for shift work not including night. All of the selected studies on shift work (15 out of 15) used ‘ever work at night shift’ as the reference group to some extent, which inhibited our analysis from making this type of differentiation. In addition, the effect of night shift works on breast cancer risk has been recently assessed by Jia et al. (2013). Their results suggested an elevated breast cancer risk among women who ‘ever worked at night’ (RR = 1.16, 95 % CI 1.06–1.27).
In conclusion, this comprehensive meta-analysis provides evidence to support a positive association between circadian disruption and breast cancer risk in women. However, the aggregate risk of circadian disruption can be either under- or overestimated due to distinctions between various sources of exposures. Shift work, exposure to light at night and employment as a flight attendant were consistently associated with elevated breast cancer risk. However, the effect of sleep deficiency on breast cancer remains uncertain. Furthermore, this study provides insufficient evidence to support a dose–response relationship between breast cancer risk and shift work, exposure to light at night, or sleep deficiency. The temporal relationship between circadian disruption and risk of breast cancer is crucial. Future rigorous prospective studies with relatively long follow-up periods, objective assessment of exposures, and extensive adjustment for confounding variables, should be conducted to confirm or refute the association between circadian disruption and breast cancer in women.
References
Akerstedt T, Froberg JE, Friberg Y, Wetterberg L (1979) Melatonin excretion, body temperature and subjective arousal during 64 hours of sleep deprivation. Psychoneuroendocrinology 4(3):219–225
Bartlett DT (2004) Radiation protection aspects of the cosmic radiation exposure of aircraft crew. Radiat Prot Dosimetry 109(4):349–355. doi:10.1093/rpd/nch311
Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE (2013) A case-referent study: light at night and breast cancer risk in Georgia. Int J Health Geogr 12(1):23. doi:10.1186/1476-072X-12-23
Blask DE (2009) Melatonin, sleep disturbance and cancer risk. Sleep Med Rev 13(4):257–264. doi:10.1016/j.smrv.2008.07.007
Blask DE et al (2005) Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65(23):11174–11184. doi:10.1158/0008-5472.CAN-05-1945
Boivin DB, Duffy JF, Kronauer RE, Czeisler CA (1996) Dose-response relationships for resetting of human circadian clock by light. Nature 379(6565):540–542. doi:10.1038/379540a0
Buja A et al (2006) Cancer incidence among female flight attendants: a meta-analysis of published data. J Women Health 15(1):98–105. doi:10.1089/jwh.2006.15.98
Davis S, Mirick DK, Stevens RG (2001) Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93(20):1557–1562
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917. doi:10.1002/ijc.25516
Filipski E et al (2004) Effects of chronic jet lag on tumor progression in mice. Cancer Res 64(21):7879–7885. doi:10.1158/0008-5472.CAN-04-0674
Filipski E et al (2005) Effects of light and food schedules on liver and tumor molecular clocks in mice. J Natl Cancer Inst 97(7):507–517. doi:10.1093/jnci/dji083
Fritschi L et al (2013) The association between different night shiftwork factors and breast cancer: a case-control study. Br J Cancer. doi:10.1038/bjc.2013.544
Girschik J, Heyworth J, Fritschi L (2013) Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case–control study. Am J Epidemiol 177(4):316–327. doi:10.1093/aje/kws422
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135(11):1301–1309
Greenland S, Thomas DC (1982) On the need for the rare disease assumption in case-control studies. Am J Epidemiol 116(3):547–553
Grundy A et al (2013) Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. doi:10.1136/oemed-2013-101482
Haldorsen T, Reitan JB, Tveten U (2001) Cancer incidence among Norwegian airline cabin attendants. Int J Epidemiol 30(4):825–830
Hansen J (2001) Increased breast cancer risk among women who work predominantly at night. Epidemiology 12(1):74–77
Hansen J, Lassen CF (2012) Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med 69(8):551–556. doi:10.1136/oemed-2011-100240
Hansen J, Stevens RG (2012) Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer 48(11):1722–1729. doi:10.1016/j.ejca.2011.07.005
Haus EL, Smolensky MH (2013) Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17(4):273–284. doi:10.1016/j.smrv.2012.08.003
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. doi:10.1136/bmj.327.7414.557
Ijaz S et al (2013) Night-shift work and breast cancer—a systematic review and meta-analysis. Scand J Work Environ Health 39(5):431–447. doi:10.5271/sjweh.3371
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Jia Y et al (2013) Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer epidemiology 37(3):197–206. doi:10.1016/j.canep.2013.01.005
Kakizaki M et al (2008) Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer 99(9):1502–1505. doi:10.1038/sj.bjc.6604684
Kloog I, Portnov BA, Rennert HS, Haim A (2011) Does the modern urbanized sleeping habitat pose a breast cancer risk? Chronobiol Int 28(1):76–80. doi:10.3109/07420528.2010.531490
Knutsson A et al (2013) Breast cancer among shift workers: results of the WOLF longitudinal cohort study. Scand J Work Environ Health 39(2):170–177. doi:10.5271/Sjweh.3323
Kojo K, Pukkala E, Auvinen A (2005) Breast cancer risk among Finnish cabin attendants: a nested case-control study. Occup Environ Med 62(7):488–493. doi:10.1136/oem.2004.014738
Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M (2010) Light at night and breast cancer risk: results from a population-based case-control study in Connecticut, USA. Cancer Causes Control 21(12):2281–2285. doi:10.1007/s10552-010-9653-z
Lie JA, Kjuus H, Zienolddiny S, Haugen A, Stevens RG, Kjaerheim K (2011) Night work and breast cancer risk among Norwegian nurses: assessment by different exposure metrics. Am J Epidemiol 173(11):1272–1279. doi:10.1093/aje/kwr014
Linnersjo A, Hammar N, Dammstrom BG, Johansson M, Eliasch H (2003) Cancer incidence in airline cabin crew: experience from Sweden. Occup Environ Med 60(11):810–814
Lynge E (1996) Risk of breast cancer is also increased among Danish female airline cabin attendants. BMJ 312(7025):253
McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM (2006) Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res 15(3):241–249. doi:10.1111/j.1365-2869.2006.00523.x
Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES (2005) Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer 41(13):2023–2032. doi:10.1016/j.ejca.2005.05.010
Menegaux F et al (2012) Night work and breast cancer: a population-based case–control study in France (the CECILE study). Int J Cancer. doi:10.1002/ijc.27669
O’Leary ES et al (2006) Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol 164(4):358–366. doi:10.1093/aje/kwj211
Orsini NBR, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6:40–70
Pesch B et al (2010) Night work and breast cancer—results from the German GENICA study. Scand J Work Environ Health 36(2):134–141
Pilcher JJ, Lambert BJ, Huffcutt AI (2000) Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep 23(2):155–163
Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB (2006) A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res 66(10):5521–5525. doi:10.1158/0008-5472.can-05-4652
Pronk A et al (2010) Night-shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol 171(9):953–959. doi:10.1093/aje/kwq029
Pukkala E et al (2012) Cancer incidence among Nordic airline cabin crew. Int J Cancer 131(12):2886–2897. doi:10.1002/Ijc.27551
Qin Y, Zhou Y, Zhang X, Wei X, He J (2014) Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer 134(5):1166–1173. doi:10.1002/ijc.28452
Rafnsson V, Sulem P, Tulinius H, Hrafnkelsson J (2003) Breast cancer risk in airline cabin attendants: a nested case-control study in Iceland. Occup Environ Med 60(11):807–809
Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S (2002) Cancer incidence in California flight attendants (United States). Cancer Causes Control 13(4):317–324
Schernhammer ES, Hankinson SE (2009) Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses’ Health Study cohort. Cancer Epidemiol Biomark Prevent 18(1):74–79. doi:10.1158/1055-9965.EPI-08-0637
Schernhammer ES et al (2001) Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 93(20):1563–1568
Schernhammer ES, Kroenke CH, Laden F, Hankinson SE (2006) Night work and risk of breast cancer. Epidemiology 17(1):108–111
Schernhammer ES et al (2008) Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 100(12):898–905. doi:10.1093/jnci/djn171
Schernhammer ES et al (2010) Urinary 6-Sulphatoxymelatonin levels and risk of breast cancer in premenopausal women: the ORDET cohort. Cancer Epidemiol Biomark Prevent 19(3):729–737. doi:10.1158/1055-9965.EPI-09-1229
Schwartzbaum J, Ahlbom A, Feychting M (2007) Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health 33(5):336–343
Stroup DF et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Verkasalo PK et al (2005) Sleep duration and breast cancer: a prospective cohort study. Cancer Res 65(20):9595–9600. doi:10.1158/0008-5472.can-05-2138
Wang F et al (2013) A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann Oncol 24(11):2724–2732. doi:10.1093/annonc/mdt283
Wartenberg D, Stapleton CP (1998) Risk of breast cancer is also increased among retired US female airline cabin attendants. BMJ 316(7):1902
Wehr TA, Aeschbach D, Duncan WC Jr (2001) Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol 535(Pt 3):937–951
Wells GA et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC (2008) Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis 29(6):1244–1248. doi:10.1093/carcin/bgn100
Acknowledgments
This work was funded, in part, by the Georgia Cancer Coalition (Proposal 038505) to the Cancer Epidemiology, Prevention and Control Program (CEPC) at the Georgia Cancer Center.
Conflict of interest
The authors have declared no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, C., Anand, S.T., Ebell, M.H. et al. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health 88, 533–547 (2015). https://doi.org/10.1007/s00420-014-0986-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-014-0986-x