Abstract
Object: The purpose of this study was to compare the stress levels of Japanese ambulance men between on-duty and off-duty days, by using the physiological indices of heart rate variability (HRV) and cortisol in urine, measured over each 24-h period. Methods: Measurements were made during one on-duty and one off-duty day for each subject. The participants were monitored for 24 h with a Holter recording system and a parameter reflecting overall stress levels was obtained by measuring the cortisol level in urine collected over 24 h. Results: The circadian variation of cardiac autonomic nervous system activity was affected when the subjects were on duty. The low-frequency/high-frequency power ratio (=low-frequency power/high-frequency power: LF/HF), which is a useful parameter that reflects the balance of cardiac autonomic nervous activity, differed significantly between the waking and sleeping times on the off-duty day (P=0.03), while it did not differ between these two states on the on-duty day (P=0.56). Similarly, the normalized high-frequency power [=high-frequency/(high-frequency+low-frequency) power: HF/(HF+LF)] ratio, which is a useful measure of the activity of the parasympathetic nervous system, differed significantly between these two states on the off-duty day (P=0.04), while there was no significant difference in the ratio between the two states on the on-duty day (P=0.13). Conclusion: These results show that the diurnal balance of the cardiac autonomic nervous system is affected on the on-duty day, even though it is possible for ambulance men to sleep regular hours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investigations of cardiovascular reactivity have traditionally used heart rate and blood pressure to track responses. Power spectral analysis of heart rate variability (HRV) has been used as an alternative, sensitive index of cardiac autonomic nervous activity (Sayers 1973; Pomeranz et al. 1985; Pagani et al. 1986; Fujita et al. 2000). In humans, power spectral analysis of R–R interval variability has revealed that there are two major spectral components: the high-frequency (HF) component (0.15–0.40 Hz), which varies with the respiratory frequency, and the low-frequency (LF) component (0.04–0.15 Hz). The LF/HF power ratio (=low-frequency power/high-frequency power: LF/HF) is a useful parameter that reflects the balance of cardiac autonomic nervous activity (Baselli et al. 1986; Lombardi et al. 1987; Rimoldi et al. 1990), and the normalized HF power [=high-frequency power/(high-frequency+low-frequency) power: HF/(HF+LF)] ratio is a useful measure of the activity of the parasympathetic nervous system (Koizumi et al. 1985; Furlan et al. 1998; Montano et al. 1998; Petelenz et al. 2004).
During nighttime sleep, the activity of the sympathetic nervous system declines and that of the parasympathetic nervous system increases so that the LF/HF ratio decreases and the HF/(HF+LF) ratio increases, reflecting normal circadian changes in the sympathovagal balance (Parati et al. 1990; Furlan et al. 1990; Molgaard et al. 1991). Similarly, these indices have proven useful in investigating the responses of the autonomic nervous system to mental arithmetic stress tasks, emotional stress, job stress, physical loads and posttraumatic stress disorder (Cohen et al. 1997, 1998; Dishman et al. 2000; Matthews et al. 2001). Recent research on the relationship between the HRV and shift work has revealed that nighttime work reduced cardiac sympathetic modulation, compared with the morning or evening work periods (Kobayashi et al. 1997; Furlan et al. 2000).
On the other hand, serum cortisol levels are controlled by adrenocorticotrophic hormone (ACTH), a hypophysiotropic hormone, and are affected by stress (Aguilera 1994). Free cortisol is an appropriate index of stress. This is metabolized in the liver and 0.5% of free cortisol in serum is directly eliminated in urine. Free cortisol (cortisol in urine) follows a circadian pattern similar to that of ACTH, which is low at night and higher during the day (Yehuda et al. 2003). Reliable data, reflecting overall stress levels, are obtained by measuring cortisol in urine collected over 24 h.
The purpose of this study was to compare the stress levels of Japanese ambulance men between the on-duty and off-duty days by using the physiological indices of HRV and cortisol levels in urine, measured over each 24-h period.
Methods
Subjects
The subjects were nine Japanese men, 28–52-year old (39.7±9.3), who had been employed with the ambulance service for 1–25 years (17.8±9.5), and had worked 24-h shifts on alternate days. All were ambulance men and licensed paramedics. Of the nine subjects, eight were married, and seven reported some use of alcohol. The total time of sleep averaged 5.5±1.4 h on the on-duty days, and 6.5±2.2 h on the off-duty days (t=1.15, P=0.28).
In Japan, fire service workers, including ambulance men, firefighters, rescue staff and chiefs, customarily work 24-h shifts that begin at 9:00 a.m. They are permitted to take a nap during the night if they are not called out. At 9:00 a.m. the following morning, their duties are completed and they remain off-duty for the next day. Their working hours are about 40 h per week, essentially on an on-off-off shift.
On the off-duty day, none of the ambulance men had a duty call during the present study period.
The main inclusion criteria were having no insurmountable objections to shift work such as heart disease, hypertension, diabetes, severe physical disorder, no current medications, and no previous hospitalization for cardiovascular diseases. All subjects gave written informed consent before being included. This study was conducted with the approval of the Ethics Committee of the Kyoto University Graduate School.
Data collection
Measurements were made during one on-duty and one off-duty day for each subject. To minimize the possible effects accompanied by the installation of a Holter recorder, the order of the test was randomized. Therefore, in order to avoid a time sequence effect, half of the subjects started the recording on the on-duty day, and the other half started on the off-duty day.
Twenty-four-hour Holter recordings
The participants were monitored for 24 h with a Holter recording system, starting at 9:00 a.m. All subjects were instructed to keep a diary of all activities, noting the times when sleep, work, meals, leisure, physical activities and other possibly relevant events or activities began and ended. The sleeping period was defined as the time between 30 min after going to sleep and 30 min before getting up, as recorded in the diary.
Cortisol in urine
In this study, 24-h urine was collected using URIN-MATE (MD-63350, Sumitomo Chemical Co. Ltd., Japan), which could accumulate 1/50th of whole urine, for each 24-h monitoring period. The free cortisol level was measured with a commercially available radioimmunoassay kit (Mitsubishi Kagaku Bio-Clinical Laboratories Inc., Japan).
HRV assessment
The 24-h Holter recordings were analyzed with a Marquette Series 800 Holter Analyzer (Tokyo, Japan) by an experienced Holter analyst. The complete recording was split into 5-min segments. We selected ECG recordings consisting predominantly of sinus rhythm. Recordings with frequent supraventricular or ventricular ectopic rhythms or atrial fibrillation were excluded from subsequent analysis. Heart rate and HRV parameters were computed for each segment. Spectral analysis was conducted by computing the following frequency domain variables for HRV by means of fast Fourier transformation. The direct current component was excluded in the calculation of the power spectrum to remove the nonharmonic components in the very LF region (<0.04 Hz). Spectral analysis was used to compute the following frequency domain variables for HRV by means of fast Fourier transformation: low-frequency (LF, 0.04–0.15 Hz) power in ms2, high-frequency (HF, 0.15–0.40 Hz) power in ms2 ; the LF/HF ratio and the HF/(HF+LF) ratio were subsequently calculated for each 5-min segment.
Data analysis
Since all nine subjects were awake from 10:00 to 12:00 a.m., and asleep from 3:00 to 5:00 a.m. on both the on-duty and off-duty days, HR and parameters of HRV were averaged during these time periods.
Demographic and physiological data are presented as the mean ± SD. The differences between the time periods were analyzed with a paired t test, using SPSS for Windows Version 10.0.
Results
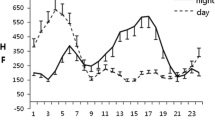
A representative pattern of the disturbed cardiac autonomic nervous activity is shown in Fig. 1. The circadian rhythm of HR was unaffected on both the on-duty and off-duty days (Fig. 1a). The HR was high in the waking time and low in the sleeping time on both days. The circadian rhythm of the LF/HF ratio was disturbed on the on-duty day, that is, low in the waking time and high in the sleeping time, while the circadian rhythm was well preserved on the off-duty day (Fig. 1b). Similarly, the circadian rhythm of HF/(HF+LF) was affected on the on-duty day, that is, high in the waking time and low in the sleeping time, while it was well preserved on the off-duty day (Fig. 1c).
Table 1 shows the comparison of HR, LF/HF and HF/(HF+LF) between the waking (10:00–12:00 a.m.) and sleeping (3:00–5:00 a.m.) times on both the on-duty and off-duty days. There were significant differences in HR between the waking and sleeping times on both the on-duty and off-duty days. The mean value of the LF/HF ratio, which reflects the sympathovagal interaction, differed significantly between the waking and sleeping times on the off-duty day. In contrast, it did not differ between these two conditions on the on-duty day, indicating that the activity of the sympathetic nervous system remained elevated even during sleeping on the on-duty day. Similarly, the mean value of the HF/(HF+LF) ratio, which is an index of parasympathetic nervous system activity, differed significantly between the waking and sleeping times on the off-duty day; however, there was no significant difference between these two conditions on the on-duty day.
Cortisol concentration in urine was 47.3±19.9 μg/day on the off-duty day and 51.0±23.3 μg/day on the on-duty day, but the difference did not reach statistical significance (t=0.64, P=0.54).
Discussion
We found a significant diurnal change in the activity of the cardiac sympathetic and parasympathetic nervous systems between waking and sleeping conditions on the off-duty day, but not on the on-duty day. These results show that the circadian variation of the cardiac autonomic nervous system is disturbed on the on-duty day, even though it is possible for Japanese ambulance men to sleep for similar durations on the on-duty day by napping.
Circadian variation of cardiac autonomic nervous activity
The natural circadian rhythm of the cardiac autonomic nervous system causes a nocturnal decrease in the total power of HRV and the LF component, and an increase in the HF component. The afternoon peak and the midnight trough of LF/HF (Fig. 1b) and the afternoon trough and midnight peak of HF/(HF+LF) (Fig. 1c) on the off-duty day all disappeared on the on-duty day.
Shift workers display a significantly decreased standard deviation of the R–R interval during sleep, compared with daytime workers, reflecting decreased activity of the parasympathetic nervous system (van Amelsvoort et al. 2000). These reports demonstrate that shift work is accompanied by disturbances of the sympathovagal balance. In the present study, the circadian rhythm of HR was well preserved on both the on-duty and off-duty days, however, the circadian rhythm of cardiac autonomic nervous activity obtained from the analysis of HRV was affected on the on-duty day. These findings are in agreement with the aforementioned reports (Furlan et al. 2000; van Amelsvoort et al. 2000).
Difference between the on-duty and off-duty days
The HRV analysis indicated that the circadian variation of sympathetic and parasympathetic nervous system activities is disturbed on the on-duty day and recovered on the off-duty day. However, the HR measurements could not detect the disturbance of the circadian variation. In the present study, HR differed significantly between the waking and sleeping times on both the on-duty and off-duty days. However, HR during waking and sleeping times was comparable between the on-duty and off-duty days. A similar study revealed higher HRs on the off-duty day, during both the waking and sleeping times, which may be explained by more time spent standing on nonworkdays (Goldstein et al. 1992).
Additionally, concentration differences of 24-h cortisol in urine between on-duty and off-duty days did not reach statistical significance, however, levels on the off-duty day were lower than on the on-duty day. These conflicting results reflect, at least in part, the fact that HR and cortisol in urine are greatly influenced by physical state and activity, and therefore may not be useful indices for the assessment of job-related stress.
Clinical implications
Some studies have reported that important cardiovascular risk factors (hypertension, low-HDL cholesterol and high triglyceride levels) are higher for shift workers than for day workers (Peter et al. 1999; Karlsson et al. 2003). Additionally, in many clinical studies, the effects of pharmacological or other treatments on autonomic nervous function in chronic heart failure (CHF) are assessed with indices of HRV. Because abnormal HRV is related to higher risk of subsequent death in patients with CHF (Eckberg et al. 1971; Janszky et al. 2004) and diabetes (Lio et al. 2002), it is important to assess HRV in our subjects.
Our data on cardiac autonomic nervous activity suggest that subjects could not sleep well on the on-duty day. Indeed, it has been reported that delta waves in electroencephalograms disappear in association with the increased sympathetic tone during sleep (Gronfier et al. 1999). Thus, further studies are necessary to elucidate the precise pathophysiology of sleep on the on-duty day. It is important to point out that normal cardiovascular neural regulation is essential to maintain a 24-h circadian periodicity. In fact, patients with diabetes mellitus (Bernardi et al. 1992), coronary heart disease (Lombardi et al. 1992), and hypertension (Guzzetti et al. 1991) were found to be associated with blunted 24-h fluctuations of power spectral markers of cardiac autonomic control. On the other hand, there is a report that circadian variation of cardiac autonomic activity is well preserved in patients with mild to moderate CHF (Miyamoto et al. 2004).
In conclusion, the results of the present study may indicate that the affected balance of cardiac autonomic nervous systems of the participating shift workers may also be a potential risk factor for cardiovascular diseases.
References
Aguilera G (1994) Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 15:321–350
van Amelsvoort LG, Schouten EG, Maan AC, Swenne CA, Kok FJ (2000) Occupational determinants of heart rate variability. Int Arch Occup Environ Health 73:255–262
Baselli G, Cerutti S, Civardi S, Liberati D, Lombardi F, Malliani A, Pagani M (1986) Spectral and cross-spectral analysis of heart rate and arterial blood pressure variability signals. Comput Biomed Res 19:520–534
Bernardi L, Ricordi L, Lazzari P, Solda P, Calciati A, Ferrari MR, Vandea I, Finardi G, Fratino P (1992) Impaired circadian modulation of sympathovagal activity in diabetes. Circulation 86:1443–1452
Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y (1997) Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry 41:627–629
Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, Cassuto Y (1998) Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry 44:1054–1059
Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN (2000) Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol 37:121–133
Eckberg DL, Drabinsky M, Braunwald E (1971) Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 285:877–883
Fujita M, Miyamoto S, Sekiguchi H, Eiho S, Sasayama S (2000) Effects of posture on sympathetic nervous modulation in patients with chronic heart failure. Lancet 356:1822–1823
Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A (1990) Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and R-R variabilities in ambulant subjects. Circulation 81:537–547
Furlan R, Piazza S, Dell’Orto S, Barbic F, Bianchi A, Mainardi L, Cerutti S, Pagani M, Malliani A (1998) Cardiac autonomic patterns preceding occasional vasogal reactions in healthy humans. Circulation 98:1756–1761
Furlan R, Barbic F, Piazza S, Tinelli M, Seghizzi P, Malliani A (2000) Modifications of cardiac autonomic profile associated with a shift schedule of work. Circulation 102:1912–1916
Goldstein IB, Jamner LD, Shapiro D (1992) Ambulatory blood pressure and heart rate in health male paramedics during a workday and a nonworkday. Health Psychol 11:48–54
Gronfier C, Simon C, Piquard F, Ehrhart J, Brandenberger G (1999) Neuroendocrine processes underlying sleep regulation in man. J Clin Endocrinol Metab 84:2686–2690
Guzzetti S, Dassi S, Pecis M, Casati R, Masu AM, Longoni P, Tinelli M, Cerutti S, Pagani M, Malliani A (1991) Altered pattern of circadian neural control of heart period in mild hypertension. J Hypertens 9:831–838
Janszky I, Ericson M, Mittleman MA, Wamala S, Al-Khalili F, Schenck-Gustafsson K, Orth-Gomer K (2004) Heart rate variability in long-term risk assessment in middle-aged women with coronary heart disease: The Stockholm female coronary risk study. J Intern Med 255:13–21
Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS (2003) Metabolic disturbance in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health 76:424–430
Kobayashi F, Furui H, Akamatsu Y, Watanabe T, Horibe H (1997) Changes in psychophysiological functions during night shift in nurses: Influence of changing from a full-day to a half-day work shift before night duty. Int Arch Occup Environ Health 69:83–90
Koizumi K, Terui N, Kollai M (1985) Effect of cardiac vagal and sympathetic nerve activity on heart rhythmic fluctuations. J Auton Nerv Syst 12:251–259
Lio D, Carnethon M, Evans GW, Cascio WE, Heiss G (2002) Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes 51:3524–3531
Lombardi F, Sandrone G, Pernpruner S, Sala R, Garimoldi M, Cerutti S, Baselli G, Pagani M, Malliani A (1987) Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction. Am J Cardiol 60:1239–1245
Lombardi F, Sandrone G, Mortara A, La Rovere MT, Colombo E, Guzzetti S, Malliani A (1992) Circadian variation of spectral indices of heart rate variability after myocardial infarction. Am Heart J 123:1521–1529
Matthews KA, Gump BB, Owens JF (2001) Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Physiol 20:403–410
Miyamoto S, Fujita M, Tambara K, Sekiguchi H, Eiho S, Hasegawa K, Tamaki S (2004) Circadian variation of cardiac autonomic nervous activity is well preserved in patients with mild to moderate chronic heart failure: effect of patient position. Int J Cardiol 93:247–252
Molgaard H, Sorensen KE, Bjerregaard P (1991) Circadian variation and influence of risk factors on heart rate variability in healthy subjects. Am J Cardiol 68:777–784
Montano N, Cogliati C, Porta A, Pagani M, Malliani A, Narkiewicz K, Abboud FM, Birkett C, Somers VK (1998) Central vagotonic effects of atropine modulate spectral oscillations of sympathetic nerve activity. Circulation 98:1394–1399
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59:178–193
Parati G, Castiglioni P, Di Rienzo M, Omboni S, Pedotti A, Mancia G (1990) Sequential spectral analysis of 24-hour blood pressure and pulse interval in humans. Hypertension 16:414–421
Petelenz M, Gonciarz M, Macfarlane P, Rudner R, Kawecki P, Musialik J, Jalowiecki P, Gonciarz Z (2004) Sympathovagal balance fluctuates during colonoscopy. Endoscopy 36:508–514
Peter R, Alfredsson L, Siegrist J, Westerholm P (1999) Does a stressful psychosocial environment mediate the effects of shift work on cardiovascular risk factors? Scand J Work Environ Health 25:376–381
Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 248:151–153
Rimoldi O, Pierini S, Ferrari A, Cerutti S, Pagani M, Malliani A (1990) Analysis of short-term oscillations of R–R and arterial pressure in conscious dogs. Am J Physiol 258:H967–H976
Sayers BM (1973) Analysis of heart rate variability. Ergonomics 16:17–32
Smith L, Folkard S, Poole CJM (1994) Increased injuries on night shift. Lancet 344:1137–1139
Yehuda R, Halligan SL, Yang RK, Guo LS, Makotkine I, Singh B, Pickholtz D (2003) Relationship between 24-hour urinary-free cortisol excretion and salivary cortisol levels sampled from awakening to bedtime in healthy subjects. Life Sci 73:349–358
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitani, S., Fujita, M. & Shirakawa, T. Circadian variation of cardiac autonomic nervous profile is affected in Japanese ambulance men with a working system of 24-h shifts. Int Arch Occup Environ Health 79, 27–32 (2006). https://doi.org/10.1007/s00420-005-0026-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-005-0026-y