Abstract
Objectives
To measure eye blink frequency as a measure of trigeminal stimulation of the eye. Human subjects were exposed to oxidation mixtures representative of reactive indoor pollutants and clean air, from which relative changes in blink frequencies were measured.
Method
Male subjects (n=8) were exposed for 20 min to limonene oxidation products (LOPs), isoprene oxidation products (IOPs), nitrate radicals (NO3), their residual reactants, and clean air at 20% relative humidity. A baseline blink frequency was measured prior to and following each exposure (2×8 min). The subjects were exposed locally in the non-dominant eye and single blind in random order. Blinking was video-recorded and evaluated for full sessions of 36 min while the subjects viewed an educational film. The initial terpene concentrations were one to two orders of magnitude higher than mean indoor concentrations.
Results
The mean blink frequency increased significantly during subjects’ exposure to gas-phase oxidation products at lower part-per-billion (ppb) levels of LOPs, 42% (P<0.0001) and NO3, 21% (P<0.022), compared with that at baseline. Neither the residual reactants nor clean air changed the blink frequency significantly. The findings coincided with qualitative reporting of weak eye irritation symptoms.
Conclusions
Changes in blink frequency appear to be a promising measure of trigeminal stimulation from exposure to eye irritants in ppb levels. Gaseous products of limonene and ozone, and reactive radicals may cause eye irritation indoors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eye complaints, such as burning, dry and itching eyes (i.e. eye irritation) are among the most common symptoms reported in the indoor environment. The prevalence has been reported to be approximately 20% to 40% in offices (Brightman and Moss 2000). The cause(s) of eye irritation in the indoor environment is unknown, although a number of suspected indoor factors have been evaluated (Mendell 1993). Indoor air pollution has been hypothesised as being one cause of eye irritation (Norn 1992; Versura et al. 1999; Saxena et al. 2003). For example, reported eye irritation has been associated with the use of specific cleaning products for flooring materials. A number of identified correlations between reported eye irritation and measured signs in the outer eye, both in human exposure studies with indoor air pollutants and in field investigations, has been found [cf. (Wolkoff et al. 2003)]. It has been proposed that volatile organic compounds (VOCs) could cause thinning and alteration of the pre-corneal tear film (PTF) and epithelial damage of conjunctiva (Norn 1992). In particular, lipophilic and/or surface-active pollutants have been hypothesised to cause thinning of PTF, which finally results in reduced stability of the PTF (Norn 1992). This is unlikely, because both lipophilic and hydrophilic indoor VOCs may alter the PTF only at industrial levels. This has been shown for decane (Kjaergaard et al. 1989) and propylene glycol (Wieslander et al. 2001), which are representative of a lipophilic VOC and a hydrophilic VOC, respectively.
However, a number of glycol ethers and esters have not been shown to cause any alteration effects of the PTF, even at industrial concentrations (Emmen et al. 2003; Iregren et al. 1993; Nihlén et al. 1998a, 1998b). Similarly, a mixture of 22 different VOCs dominated by butyl acetate and toluene did not alter PTF at a total level of 24 mg m−3 (Kjaergaard et al. 1991). The indoor concentrations of such VOCs are generally 3–4 orders of magnitude lower than industrial levels, occupational exposure limits or estimated thresholds for airway irritation. It is doubtful whether these compounds cause eye irritation (Wolkoff and Nielsen 2001). However, some VOCs are discriminated against by the analytical methods and are not measured, i.e. chemically unstable and/or short-lived species. Such species are produced in the reaction of ozone (O3) with some terpenes and cause airway irritation in a mouse bioassay, unlike the reactants themselves (Wolkoff et al. 2000). The bio-response is only partly understood, and the reaction mixture comprises both residual (unreacted) reactants and terpene oxidation products, which are complex mixtures of intermediate compounds, radicals, organic acids and carbonyl compounds (Seinfeld and Pandis 1998).
Ultrafine particles (i.e. of size 1–100 nm) are also formed in terpene oxidation reactions with O3 (Bonn et al. 2002), e.g. during cleaning activities that use terpene-based agents (Long et al. 2000). Whether these or the gaseous oxidation products are responsible for the biological response remains unknown. Although the above bioassay in addition to some field studies supports the reactive chemistry as a plausible hypothesis for eye and airway irritation in non-industrial settings (Wolkoff and Nielsen 2001), there is a need for further substantiation by a human model. There is not yet convincing evidence that particles can alter the PTF at typical indoor levels and result in eye complaints. The results from human exposure studies in climate chambers with exposure to approximately 400 μg m−3 airborne office floor dust are inconclusive about eye irritation (Hauschildt et al. 1999; Pan et al. 2000).
The abundance and, hence, the exposure of terpene oxidation products indoors, depends on the identity and concentration of the reactants (e.g. O3 and limonene), their reaction rate and the air exchange rate, which determine the build-up of reaction products. Terpenes are emitted from vegetation and certain wood-based building materials and are added to household products. The terpene, limonene, is predominantly an indoor source, and high concentrations can be obtained at a low air-exchange rate in the absence of oxidants. A typical, mean, indoor concentration is less than 10 parts per billion (ppb), but values as high as 70 ppb have been reported (Wolkoff et al. 2000), e.g. in cleaning agents or in materials in new housing (Hodgson et al. 2000). O3 has been measured in concentrations of a few ppb to hundreds of ppb and is, typically, 20%–70% of outdoor levels (Weschler 2000). The main indoor source of isoprene is human exhalation and environmental tobacco smoke; indoor measurements of isoprene concentrations are sparse but are generally below 10 ppb (e.g. Fenske and Paulson 1999; Wolkoff et al. 2000). Indoor nitrogen dioxide (NO2) concentrations are similar to outdoor values, i.e. up to approximately 25–50 ppb (Wolkoff et al. 2000).
A number of eye physiology methods have been used to characterise eye signs, such as the stability of the PTF (Kjaergaard and Hodgson 2001). They are, however, not a direct measure of eye irritation. Eye blink frequency (BF) has been proposed as a measure of eye irritation (Walker et al. 2001). There are three types of blinking. First, reflex blinking is a rapid closure movement of the eyelids. It is of short duration as response to a variety of external stimuli, usually auditory, cognitive, trigeminal or visual, including a component of other motor behaviours. Generally, trigeminal stimulation dictates most reflex blinks. Second, voluntary (conscious) blinking, and third, involuntary (spontaneous) blinking occur without external stimuli and at a fairly constant frequency (Sibony and Evinger 1998). The dominance of reflex blinking supports the above proposal.
Increase of BF has been found for some VOCs, though at industrial concentrations. For example, 1,400 ppm butyl acetate resulted in a significant increase from 9 to 12 min−1 (Iregren et al. 1993). Exposure to a VOC mixture (total 12–24 mg m−3) resulted in a dose/time-dependent increase in BF associated with reported eye irritation (Prah et al. 1993). The BF increased from approximately 21 to 35 min−1 from 35 min of exposure to 0.03 to 3.2 ppm formaldehyde, most noticeably in the range 1.2 to 3.2 ppm and concurrently with increase of eye irritation (Weber-Tschopp et al. 1977). Recently, a positive correlation has been observed between BF and environmental tobacco smoke particles at low levels (Junker et al. 2001). However, comparison of the results is difficult because the period of measurement has generally been approximately 2 min or less, much shorter than the individual cycle of involuntary blinking found in this study.
The purpose of this study was to investigate whether exposure of the human eye to limonene oxidation products (LOPs), isoprene oxidation products (IOPs), and nitrate radicals (NO3) at low relative humidity could increase the BF reflected as a trigeminal stimulation. The reaction mixtures represented a juvenile mixture of O3 and a fast-reacting terpene (LOPs), and an old mixture of O3 and a slow-reacting hemi-terpene (IOPs). NO3 is a model compound of a reactive species, which we included to see if a reactive radical in low concentration triggers trigeminal stimulation. We chose a relative humidity of 20% to obtain as potent a response as possible, as observed in a mouse bioassay study (Wilkins et al. 2003).
Materials and methods
Chemicals
Oxygen (99.999% pure), nitrogen (>98% pure, O2+H2O<2 ppm) and certified nitrogen dioxide (NO2) of concentration 94.6 ppm (98% pure) were from Hydrogas, Norway. O3 was generated photochemically by the irradiation of pure oxygen with a thermostat-controlled mercury lamp with a high performance power supply (for details see Wolkoff et al. 2000). Limonene (>99.9% pure) and isoprene (>98% pure) were from Fluka. A gas generator (model 350, AID, Avondale, Pa., USA) generated limonene and isoprene vapours by evaporation of the liquids into a filtered nitrogen stream. Clean humidified air was generated as a separate air supply from charcoal-filtered air of medical grade. In the supply unit the air stream was split between a dry channel and one passing a sparger with clean water. We adjusted the relative humidity by combining the two channel flows (see Fig. 1).
Generation of single compounds/mixtures
The experimental set-up is shown in Fig. 1. The measured concentrations of the reactants (i.e. starting concentrations before mixing, sampling point I) and their residual concentrations (i.e. after mixing and oxidation reaction, sampling point II) for all exposures are shown in Table 1. O3 was partly consumed in surface reactions in the reaction flow tubes and the reaction chamber. We measured a first-order rate constant in order to address the wall loss of O3. After that, we calculated the chemical loss of O3 in the three systems; see “Modelling” and “Appendix” for details.
We generated LOPs by mixing flows of limonene (215 ml min−1), O3 (50 ml min−1), and air (725 ml min−1) in a Teflon tube [length (l) 1.3 m, internal diameter (i.d.) 2 mm] connected to a wide-bore polyethylene (PE) reaction flow tube (l 4.7 m, i.d. 2.2 cm). The flow through the PE reaction tube (193 ml min−1) was adjusted by regulation of a pump (model s2500, Dupont, Del., USA) connected by a T-union to the Teflon tube downstream and the PE reaction tube upstream. The chemical extent of the reaction was calculated to be 50%, based on the consumption of O3 after 10 min of reaction, excluding wall loss; see Table 1.
We generated IOPs by mixing flows of isoprene (2,020 ml min−1), O3 (50 ml min−1), NO2 (8.5 ml min−1) and air (1,965 ml min−1) in a Teflon tube (l 1.3 m, i.d. 2 mm) connected to a 183 l stainless-steel chamber with hand-polished inner walls (l 48.6 cm, width 48.6 cm, height 77.6 cm). The inlet and outlet of the chamber were connected to two separate Dupont pumps, which regulated the flow into the chamber (1,700 ml min−1) and the flow out of the chamber to the eyepiece (193 ml min−1). The chemical extent of the reaction was calculated to be 67%, based on the O3 consumption after 108 min of reaction, excluding wall loss.
NO3 radicals were prepared from O3 (50 ml min−1) and NO2 (8.5 ml min−1), which were diluted with air (4,550 ml min−1) and aged for 40 min in the stainless-steel chamber described above. The outlet of the chamber was connected to a Dupont pump, which reduced the flow to the eyepiece to 193 ml min−1. The chemical extent of the reaction was calculated to be 23%, based on the O3 consumption after 40 min of reaction, excluding wall loss.
We generated the limonene and isoprene/NO2 by turning off the O3 generator in the respective systems and produced O3, as a single component, by omitting the limonene supply in the LOPs set-up. The concentrations were slightly higher than their residual concentrations in the reaction mixtures (see Table 1). Exposure to isoprene/NO2 was carried out as a binary mixture. Any perception or change of BF from this mixture was assumed to be the summarised effect of isoprene and NO2, because it could be modelled, from the associated rate constants, that less than 1% of the NO2 reacted with isoprene during the 108 min of reaction time.
All the flow tubes and the chamber were cleaned prior to each session with 500 ppb O3 (4 l min−1) for 2 h. All exposure concentrations were adjusted to 20%±3% relative humidity and 21°C±2°C. The mixing ratio of O2 was 26% in sessions 1, 4 and 5;otherwise, it was 21%.
Human eye exposure to mixtures and clean air
Eight male subjects aged between 30 and 63 years (mean 48 years) participated in the study. Inclusion criteria were: non-smoker, absence of any pathological eye history and otherwise healthy. One wearer of contact lenses was requested not to use them for 2 weeks prior to exposure. The subjects used no systemic medication likely to provoke dry eyes (e.g. (Craig 2002; Doughty et al. 1997). Prior written consent was obtained from the subjects and the study was approved by the local Danish Research Ethics Committee (Copenhagen and Frederiksberg, KF01-005/02).
A specially designed glass eyepiece was utilised to restrict the exposure to the eye. The eyepiece was connected to the reaction flow tube or the stainless-steel chamber via a Teflon tube (l 1.8 m, i.d. 1 cm); see Fig. 1. The eyepiece was designed to fit the facial curve, which ensured an effective air supply at atmospheric pressure. This gave an unnoticeable, low, linear velocity, which was estimated to be less than 4 cm s−1 in the eyepiece, based on geometric calculations. A digital video-camera (Sony DCR-PC110E PAL; 25 frames/s) recorded the subject’s blinking; in addition, comments by the subjects about perception were recorded. The temperature was 21°C±2°C and the relative humidity was 31%±9% in the laboratory.
The subjects were exposed single-blind in their non-dominant eye, which sometimes caused minor visual disturbances through the eyepiece. The dominant eye was, however, unaffected, and generally, after a few minutes the subjects did not pay attention to the eyepiece. They were neither informed about the nature of stimulus nor could they smell it. An educational video-film was shown during the session to prevent the subjects from cat-napping. Each of the seven sessions was made up of four successive stages (see Table 2): an acclimatisation stage, which included clean air for 3 min; initial baseline recording, with clean air for 8 min (stage A); one of the compounds/mixtures BA–BG (stage B) for 20 min; and finally a baseline recovery stage of clean air for 8 min (stage C). The shift from stages A to B and B to C involved a manual change of eyepieces and interrupted the stages for ca. 1 min (omitted on the graphs in Figs. 1 and 2). The subjects were exposed in the morning in random order of sessions; there was a minimum of 2 days between each session so that adaptation to the exposures could be avoided.
Measurements
All flows of oxygen, nitrogen, and NO2 were adjusted daily by flow controllers and measured before and after sampling with a traceable electronic bubble flow meter (Gillian Instrument Corporation, N.J., USA). Temperature and relative humidity were measured by a calibrated hygrometer (model Testo 601, Testoterm GmbH & Co., Germany). The reactant concentrations of limonene and isoprene were measured prior to their reaching the reaction chamber or the PE reaction flow tube at sample point I; see Fig. 1. The residual concentrations of limonene and isoprene were measured at the end of the Teflon tube at sample point II, which connected the steel chamber or PE flow tube with the eyepiece. Limonene and isoprene were measured weekly by being sampled on Tenax TA adsorbent tubes followed by thermal desorption–gas chromatographic flame ionisation detection. The sampling rate was 200±3 ml min−1 with Alpha-1 pumps (Ametek, Pa., USA). Analytical details are described elsewhere (Wolkoff 1998). A calibrated photo ionisation detector (PID) (model PGM-7240, RAE Systems, Calif., USA) was used for daily quality control of the limonene or isoprene concentrations. O3 was monitored continuously in the reaction mixture with a newly calibrated chemiluminescence monitor (model 265 A, API, San Diego, Calif., USA). In the same manner, NO2 was measured continuously with a newly calibrated chemiluminescence monitor (model 200A, API). The limit of detection for both instruments was 2 ppb. The loss of O3 to the walls of the PE reaction flow tube was measured at the inlet (sampling point I) and outlet (sampling point II) for the applied flow and O3 concentration and similarly for the steel chamber.
The total number of ultrafine particles was measured at sampling point II, once for a 10 min period by a condensation particle counter in the range 7–1,000 nm and with a detection limit of 2 particles cm−3 (model 3022A, TSI, Minn., USA). The data were averaged over 10 min.
Calculation of the blink frequency
A blink was defined as a downward movement of the upper eyelid discernible by the researcher, but twitches and incomplete blinks were excluded. An incomplete blink was defined as a movement of less than one half of the total distance of the upper lid. All video-recordings were critically viewed by the same researcher, and each complete blink in stages A, B and C was stored, together with the time, on a computer. The BF was calculated in sequences of non-overlapping 4 min averages. An analysis showed that variation increased with the BF, and, hence, the data were not normally distributed. Therefore, we transformed the data into a normal distribution by taking the logarithm of the BF. The change of BF caused by exposure to a reaction mixture or single compound was modelled by linear regression analysis (SAS, version 8.1). The estimated changes of BF in stage B relative to the baseline BF of stages A and C, and P values obtained from a t-test, are shown in Table 3.
A possible effect of clean air was tested in session 7 to enable us to see if the set-up itself would influence the BF.
Modelling
We developed a simple one-compartment model with 19 reactions in order to calculate the extent of the oxidation reactions, which were expressed in terms of chemically consumed O3. The reactions involved and their rate constants are listed in the Appendix. We applied VISSIM, version 3.0 (Visual Solutions, Westford, Mass., USA) for this purpose, using the Backword Euler ( (Stiff) integration method with a 0.001 minimum stepsize.
The O3 concentrations in the reaction flow tube and the reaction chamber were measured at sample points I and II in the absence of other species; see Fig. 1. The measurements were used to calculate the rate constants for the wall loss designated in reactions 7 and 8 in the Appendix as:
The wall loss was modelled for LOPs, IOPs, and NO3 by the implementation of first-order rate expressions using the above derived rate constants. The percentage of consumed O3 in oxidation reactions, excluding wall loss, was calculated as:
Results
The influence of exposure on blink frequency
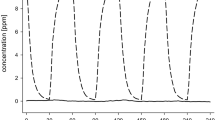
Examples of low, medium and high BFs, where the subjects have been exposed to clean air (session 7), are shown in Fig. 2. The BF/time curves for all sessions showed sinus-like cycles of about 5 min. Moreover, the amplitude was larger for subjects with high baseline BF. The baseline BF showed a variation between different days of exposure. This was calculated as the standard deviation of the BF in stage A for all sessions (the mean standard deviation was 3.1 min−1) and appeared to increase linearly with the baseline BF (R2=0.97). In addition, the BF showed a small positive or negative trend averaging 0.05 min−2 in session 7; see also Fig. 2.
Exposures of 20 min to LOPs caused a significant increase of 42%, while exposure to IOPs and NO3 resulted in increases of approximately 20% relative to the baseline; see Table 3. The increase was significant for LOPs and NO3, but that for IOPs was on the borderline of significance. Examples of BF/time curves for one of the subjects are shown in Fig. 3.
LOPs, IOPs and NO3 contained residual concentrations of reactants, i.e. O3 and limonene; O3, isoprene and NO2; and O3 and NO2, respectively, in addition to the oxidation products. The oxidation reactions had not reached completion, as a consequence of the chosen reaction times. Therefore, we tested whether the residual reactants could be responsible for the measured increase of BF; see Tables 1 and 3. The effects of O3, limonene, and isoprene/NO2, respectively, were negligible or showed an insignificant decrease of BF, like clean air.
The formation of LOPs, IOPs and NO3 was modelled in order for the amount of chemically consumed O3 to be estimated. The wall loss of O3 in the reaction tube and chamber followed first-order rate expressions, of which the rate constants are listed in Table 1. The wall loss was included in the model as an O3-competing reaction. The percentages of O3 that were chemically consumed in the LOPs, IOPs and NO3 mixtures were 50, 67 and 23%, respectively. The ultrafine particle counts were negligible; see Table 1.
Discussion and conclusion
A session of exposure to clean air (session 7) was included in order for us to study the variation of BF for each subject during exposure to a blank. Irrespective of the subjects, the BF/time curves showed similar sinus-like patterns reflecting involuntary blinking. The individual variations of BF with time, which is designated the baseline blink cycle, have been observed by other researchers (B. Piccoli, personal communication; Monster et al. 1978). Since the BF alternates on a minute scale in a sinus-like cycle, the length of the baseline BF recording must be larger than or equal to a cycle, for purposes of comparison. A shorter baseline recording will not be representative, and a longer one may tire the subject unnecessarily. A baseline of 8 min was chosen to include a minimum of one cycle. Moreover, the baseline BF showed a positive or negative trend in a particular session, which implies that a baseline recording prior to an exposure (stage A) might not be representative for a full session. A second baseline after the exposure (stage C) was, therefore, included. The application of stage C is valid only if the BF is unaffected by the intervening exposure. This was not expected, since the reactants were present in low concentrations. If the assumption were incorrect, the difference in the baseline BF from stage A to stage C should be more pronounced in sessions 1–6 relative to the session of clean air. The baseline BF level changed during the exposure, on average a small positive trend. However, this trend did not exceed that of the session with clean air, and it is, therefore, unlikely that the final baseline would be affected by the intervening exposure.
The baseline BF is also subject to variation on different days of exposure. Moreover, the linear dependence of the standard deviation on the BF implies that subjects with high BF show larger variation. Therefore, a baseline is only representative when it is recorded immediately prior to an exposure.
The significant increases in the BF by exposure to LOPs and NO3 can be ascribed to neither their reactants nor clean air. On the contrary, clean air, limonene and isoprene/NO2 lowered the BF, although not significantly. The effect is, therefore, ascribed to the oxidation products, which are formed in the reactions. A larger study is necessary to clarify the effects of IOPs and its reactants, due to the borderline P value. Qualitatively, the increases of BF coincided with self-reported eye irritation symptoms, such as smarting, stinging, burning, and warming at the lower lid and/or the inferior part of the conjunctiva. Seven out of eight subjects, six out of eight subjects, and five out of eight subjects reported weak irritation from LOPs, IOPs, and NO3, respectively. Less frequently, two out of eight, one out of eight and one out eight subjects, respectively, reported weak irritation from limonene, isoprene/NO2, and clean air, while four out of eight subjects reported irritation from O3, though BF was unaffected. Whether O3 plays a role in the perceived irritation of the oxidative mixtures must be further studied.
Ultrafine particles were virtually absent in the exposure to the reaction mixtures and in the pure reactants. Their low yield in this study might be due to the combination of low concentrations and short reaction time and to the wall sink effect of the reaction flow tube or steel chamber, both of which have large surface-to-volume ratios. Hence, the observed increases of BF and the reporting of weak irritation can solely be ascribed to the gaseous oxidation products. Since the identity of the oxidation products that are responsible for the observed effects remains unknown, the reaction mixtures and the extent of the reaction have been characterised by the amount of O3 reacted; see Table 1.
The observed increases in BF are dominated by reflex blinking as a result of trigeminal stimulation by the gaseous oxidation products. The reason is that the subjects were exposed to constant temperature, relative humidity, and air velocity during each session. In addition, the subjects watched an educational film during each session in order to maintain a neutral mental state that minimised external stimuli affecting the reflex blinking. The film did not excite the subjects but served to prevent their cat-napping.
The purpose of the study was to measure BF changes in humans exposed to LOPs and IOPs, which are suspected to cause eye and airway irritation in office environments. The concentrations applied were somewhat higher than those typically measured indoors. LOPs can be formed in a short time in high yields, due to the fast reaction between limonene and O3. The reaction time of 10 min implies that the LOPs could be formed at air changes as high as 6 h−1, which is at the high end in European office buildings (Bluyssen et al. 1996). There would be sufficient time for LOPs to build up and result in an increase of BF, provided there are high-enough steady-state concentrations. Optimal conditions for the formation of LOPs indoors depend on the outdoor flux of O3 and that of limonene indoors (Weschler and Shields 2000). The build-up of IOPs is slower than that of LOPs, due to a smaller rate constant between isoprene and O3 as well as that of NO2 and O3 (Atkinson et al. 1992). Moreover, isoprene is less abundant than limonene. The yield of IOPs will decrease at high air exchange rates, and if limonene or other unsaturated VOCs, which react faster with O3, are present. The age of the IOPs mixture corresponded to 0.6 h−1, which is at the lower end of mechanically ventilated office buildings in Europe (Bluyssen et al. 1996). The NO3-producing reaction caused a significant increase in BF when moderate concentrations of O3 and NO2 were present. However, the NO3 radical will only build up fast in an environment with high concentrations of O3 and NO2 in the absence of unsaturated VOCs, such as terpenes, and a low concentration of NO (Weschler et al. 1994). For this reason, NO3 can play a role in the above mentioned (atypical) environments; however, it may explain a minor fraction of the effect observed by IOPs. The session with NO3 was included in the study because it is a reactive radical. It serves as a model compound for the reactive radicals that are formed in the LOPs and IOPs mixtures and are reactive like NO3. Since exposure to NO3 causes a significant increase in BF, the reactive radicals might, therefore, be partly responsible for the effects observed in the exposures to LOPs and IOPs.
In conclusion, changes in human BF during exposure to low concentrations of irritants appear to be a promising measure of trigeminal stimulation. The measurements must, however, include an initial and a final baseline recording, which contains a minimum of one full cycle of involuntary blinking. Exposure to mixtures of LOPs and NO3 increased the human BF significantly, which was seen for neither the reactants nor clean air. LOPs might be responsible for trigeminal stimulation of the eye by an increase in BF under certain indoor conditions, whereas IOPs might not be relevant. NO3 was included as a model compound for reactive radicals, which increased the BF even at low concentrations. The findings support the hypothesis about reactive chemistry in indoor air.
References
Atkinson R (1986) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem Rev 86:169–201
Atkinson R (1991) Kinetics and mechanisms of the gas-phase reactions of the NO3 radical with organic compounds J Phys Chem Ref Data 20:459–507
Atkinson R, Aschmann SM, Arey J, Shorees B (1992) Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes. J Geophys Res 97:6065–6073
Atkinson R, Baulch DL, Cox RA, Hampson RF Jr, Kerr JA, Rossi MJ, Troe J (1997) Evaluated kinetic and photochemical data for atmospheric chemistry: supplement VI. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry J Phys Chem Ref Data 26:1329–1499
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF Jr, Kerr JA, Rossi MJ, Troe J (2001) Summary of evaluated kinetic and photochemical data for atmospheric chemistry. IUPAC Subcommittee on gas kinetic data evaluation for atmospheric chemistry. Web version, pp 1–56
Bluyssen PM, De Olivera Fernandes E, Groes L, Clausen G, Fanger PO, Valbjørn O, Bernhard CA, Roulet CA (1996) European indoor air quality audit project in 56 office buildings. Indoor Air 6:221–238
Bonn, B, Schuster G, Moortgat GK (2002) Influence of water vapour on the process of new particle formation during monoterpene ozonolysis, J Phys Chem A 106:2869–2881
Brightman HS, Moss N (2000). Sick building syndrome studies and the compilation of normative and comparative values. In: Spengler JD, Samet JM, McCarthy JF (eds) Indoor air quality handbook. McGraw-Hill, New York, pp 3.1–3.32
Craig JP (2002) Structure and function of the preocular tear film. The tear film: structure, function and clinical examination. Butterworth-Heinemann, Oxford, pp 18–50
DeMore WB, Golden DM, Hampson RF, Howard CJ, Kurylo MJ, Molina MJ. Ravishankara AR, Sander SP (1987) Chemical kinetics and photochemical data for use in stratospheric modeling evaluation, no 8. JPL Publication, pp 87–41
Doughty MJ, Fonn D, Richter D, Simpsom T, Caffery B, Gordon K (1997) A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci 74:424–431
Emmen HH, Muijser H, Arts JH, Prinsen MK (2003) Human volunteer study with PGME: eye irritation during vapour exposure. Toxicol Lett 140–141:249–259
Fenske JD, Paulson SE (1999) Human breath emissions of VOCs. J Air Waste Manag Assoc 49:594–598
Grosjean E, Grosjean D (1996) Rate constants for the gas-phase reaction of ozone with 1,1-disubstituted alkenes. Int J Chem Kinet 28:911–918
Hauschildt P, Mølhave L, Kjaergaard SK (1999) Reactions of healthy persons and persons suffering from allergic rhinitis when exposed to office dust. Scand J Work Environ Health 25:442–449
Hodgson AT, Rudd AF, Beal D, Chandra S (2000) Volatile organic compound concentrations and emission rates in new manufactured and site-built houses. Indoor Air 10:178–192
Iregren A, Löf A, Toomingas A, Wang Z (1993) Irritation effects from experimental exposure to n-butyl acetate. Am J Ind Med 24:727–742
Junker MH, Danuser B, Monn C, Koller T (2001) Acute sensory responses of nonsmokers at very low environmental tobacco smoke concentrations in controlled laboratory settings. Environ Health Perspect 109:1045–1052
Kjaergaard SK, Hodgson M (2001) The assessment of irritation using clinical methods and questionnaires. AIHAJ 62:711–716
Kjaergaard SK, Mølhave L, Pedersen OF (1989) Human reactions to indoor air pollutants: n-decane. Environ Int 15:473–482
Kjaergaard SK, Mølhave L, Pedersen OF (1991) Human reactions to a mixture of indoor air volatile organic compounds. Atmos Environ 25A:1417–1426
Long CM, Suh HH, Koutrakis P (2000) Characterization of indoor particle sources using continuous mass and size monitors. J Air Waste Manag Assoc 50:1236–1250
Mendell MJ (1993) Non-specific symptoms in office workers: a review and summary of the epidemiologic literature. Indoor Air 3:227–236
Monster AW, Chan HC, O’Connor D (1978) Long-term trends in human eye blink rate. Biotel Patient Monit 5:206–222
Nihlén A, Löf A, Johanson G (1998a) Controlled ethyl tertiary-butyl ether (ETBE) exposure to male volunteers II. Acute effects. Toxicol Sci 46:143–150
Nihlén A, Wålinder R, Löf A, Johanson G (1998b) Experimental exposure to methyl tertiary-butyl ether. II. Acute effects in humans. Toxicol Appl Pharmacol 148:281–287
Norn MS (1992) Pollution keratoconjunctivitis. Acta Ophthalmol 70:269–273
Pan Z, Mølhave L, Kjaergaard SK (2000) Effects on eyes and nose in humans after experimental exposure to airborne office dust. Indoor Air 10:237–245
Paulson SE, Flagan RC, Seinfeld JH (1992) Atmospheric photooxidation of isoprene. Part I: the hydroxyl radical and ground state atomic oxygen reactions. Int J Chem Kinet 24:79–101
Prah JD, Hazucha M, Horstman D, Garlington R, Case M, Ashley D, Tepper J (1993) Pulmonary, respiratory, and irritant effects of exposure to a mixture of VOCs at three concentrations in young men. In: Jakkola JJK, Ilmarinen R, Seppänen O (eds) Proceedings of the 6th International Conference on Indoor Air Quality and Climate, vol 1, Indoor Air ‘93, Helsinki, pp 607–612
Saxena R, Srivastava S, Trivedi D, Anand E, Joshi S, Gupta SK (2003) Impact of environmental pollution on the eye. Acta Ophthalmol Scand 81:491–494
Seinfeld JH, Pandis SN (1998) Atmospheric chemistry and physics—from air pollution to climate change. Wiley, New York, chapt V
Shu Y, Atkinson R (1994) Rate constants for the gas-phase reactions of O3 with a series of terpenes and OH radical formation from the O3 reactions with sesquiterpenes at 296? 2 K. Int J Chem Kinet 26:1193–1205
Sibony PA, Evinger C (1998) Anatomy and physiology of normal and abnormal eyelid position and movement. In: Miller NR, Newman NJ (eds) Walsh & Hoyt’s clinical neuro-ophthalmology. Williams & Wilkins, Baltimore, pp 1509–1592
Versura P, Profazio V, Cellini M, Torreggiani A, Caramazza R (1999) Eye discomfort and air pollution. Ophthalmologica 213:103–109
Wahner A, Mentel TF, Sohn M (1998) Gas-phase reaction of N2O5 with water vapor: importance of heterogeneous hydrolysis of N2O5 and surface desorption of HNO3 in a large Teflon chamber. Geophys Res Lett 25:2169–2172
Walker JC, Kendall-Reed M, Utell MJ, Cain WS (2001) Human breathing and eye blink rate. Responses to airborne chemicals. Environ Health Perspect 109:507–512
Weber-Tschopp A, Fischer T, Grandjean E (1977) Reizwirkungen des formaldehyds (HCHO) auf den menschen. Int Arch Occup Environ Health 39:207–218
Weschler CJ (2000) Ozone in indoor environments: concentrations and chemistry. Indoor Air 10:269–288
Weschler CJ, Shields HC (2000) The influence of ventilation on reactions among indoor pollutants: modeling and experimental observations. Indoor Air 10:92–100
Weschler CJ, Shields HC, Naik DV (1994) Indoor chemistry involving O3, NO and NO2 as evidenced by 14 months of measurements at a site in Southern California. Environ Sci Technol 28:2120–2132
Wieslander G, Norbäck D, Lindgren T (2001) Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58:649–655
Wilkins CK, Wolkoff P, Clausen PA, Hammer M, Nielsen GD (2003) Upper airway irritation of terpene/ozone oxidation products (TOPS). Dependence on reaction time, relative humidity and initial ozone concentration. Toxicol Lett 143:109–114
Wolkoff P (1998) Impact of air velocity, temperature, humidity, and air on long-term VOC emissions from building products. Atmos Environ 32:2659–2668
Wolkoff P, Nielsen GD (2001) Organic compounds in indoor air—their relevance for perceived indoor air quality. Atmos Environ 35:4407–4417
Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD (2000) Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air 10:82–91
Wolkoff P, Skov P, Franck C, Pedersen LN (2003) Eye irritation and environmental factors in the office environment. Hypotheses, causes, and a physiological model. Scand J Work Environ Health 29:411–430
Acknowledgements
We thank all subjects for participating in this study. Discussions with Dr. C.K. Wilkins are gratefully acknowledged, as is statistical assistance from Dr. Jacob Bue Bjørner and Karl Bang Christensen, Ph.D. We thank Dr. W.S. Cain for an eyepiece prototype.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Chemical reactions included in the modeling section and references to the rate constants are as follows:
Reaction | Rate constant | Reference |
|---|---|---|
(1) Isoprene + OH = products | 2.54×10−11 exp (3401/RT) | Atkinson 1986 |
(2) Isoprene + O3 = products | 5.6×10−15 exp (−15049/RT) | Grosjean and Grosjean 1996 |
(3) Isoprene + NO3= products | 3.02×10−12 exp (−3708/RT) | Atkinson 1991 |
(4) Isoprene + NO2 = products | 1.81×10−19 | Paulson et al. 1992 |
(5) NO2 + O3 = NO3 + O2 | 1.40 10−13 exp (−20537/RT) | Atkinson et al. 2001 |
(6) HO2 + O3 = OH + 2 O2 | 1.4×10−14 exp (−4989/RT) | Atkinson et al. 1997 |
(7) O3 + walla = 1.5 O2 (polyethylene) | 9.1 10−4 s−1 | This work |
(8) O3 + wall = 1.5 O2 (stainless steel) | 1.8 10−5 s−1 | This work |
(9) NO2 + OH = HNO3 | 7.51×10−11 (T/298 K)−0.60 | Atkinson et al. 1997 |
(10) NO2 + HO2 = HO2NO2 | 1.8×10−31 (T/298 K)−3.20 | Demore et al. 1987 |
(11) HO2NO2 = NO2 + HO2 | 5.7×1015 exp (−93122/RT) | Atkinson et al. 1997 |
(12) NO2 + NO3 = N2O5 | 2.01×10−12 (T/298 K)0.20 | Atkinson et al. 1997 |
(13) N2O5 = NO2 + NO3 | 9.71×1014 (T/298 K)0.10 exp(−92291/RT) | Atkinson et al. 1997 |
(14) HO2 + NO3 = OH + NO2 + O2 | 4 10−12 | Atkinson et al. 1997 |
(15) OH + NO3 = HO2 + NO2 | 2.01×10−11 | Atkinson et al. 1997 |
(16) HO2 + HO2 = O2 + H2O2 | 2.19×10−13 exp (4989/RT) | Atkinson et al. 1997 |
(17) N2O5 + H2O = 2 HNO3 | 2.51×10−22 | Wahner et al. 1998 |
(18) Limonene + O3 = 0.86 OH | 2.01×10−16 | Shu and Atkinson 1994 |
(19) Limonene + OH = products | 1.69×10−10 | Atkinson 1986 |
Rights and permissions
About this article
Cite this article
Klenø, J., Wolkoff, P. Changes in eye blink frequency as a measure of trigeminal stimulation by exposure to limonene oxidation products, isoprene oxidation products and nitrate radicals. Int Arch Occup Environ Health 77, 235–243 (2004). https://doi.org/10.1007/s00420-003-0502-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-003-0502-1