Abstract
Tuftelin was originally discovered and mostly studied in the tooth, but later found also in other organs. Despite its wide distribution among tissues, tuftelin’s function has so far been specified only in the formation of enamel crystals. Nevertheless, in many cases, tuftelin was suggested to be associated with cellular adaptation to hypoxia and recently even with cell differentiation. Therefore, we aimed to investigate tuftelin expression along with hypoxia-inducible factors (HIFs) during the early development of the mandibular/alveolar (m/a) bone, when osteoblasts started to differentiate in vivo and to compare their expression levels in undifferentiated versus differentiated osteoblastic cells in vitro. Immunohistochemistry demonstrated the presence of tuftelin already in osteoblastic precursors which were also HIF1-positive, but HIF2-negative. Nevertheless, HIF2 protein appeared when osteoblasts differentiated, one day later. This is in agreement with observations made with MC3T3-E1 cells, where there was no significant difference in tuftelin and Hif1 expression in undifferentiated vs. differentiated cells, although Hif2 increased upon differentiation induction. In differentiated osteoblasts of the m/a bone, all three proteins accumulated, first, prenatally, in the cytoplasm and later, particularly at postnatal stages, they displayed also peri/nuclear localization. Such a dynamic time–space pattern of tuftelin expression has recently been reported in neurons, which, as the m/a bone, differentiate under less hypoxic conditions as indicated also by a prevalent cytoplasmic expression of HIF1 in osteoblasts. However, unlike what was shown in cultured neurons, tuftelin does not seem to participate in final osteoblastic differentiation and its functions, thus, appears to be tissue specific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuftelin is a highly evolutionary conserved molecule (Satchell et al. 2000), belonging to a class of acidic proteins called enamelins (Deutsch et al. 1991; Santo and Line 2015). Tuftelin was first identified in the enamel, produced by ameloblasts (Deutsch et al. 1991), and further investigated along tooth development (Diekwisch et al. 1997; Luo et al. 2004; Campos-Navarro et al. 2017), and also in other craniofacial structures (Shilo et al. 2019a). Regarding the brain, tuftelin was even found to be critical for neuronal differentiation in vitro (Shilo et al. 2019b).
Notably, along with hard tissues, tuftelin was detected in several other soft organs including lung, liver, kidney and testis (MacDougall et al. 1998; Mao et al. 2001) and it was present even in morula and embryonic stem cells (Deutsch et al. 2002; Leiser et al. 2007). Based on these observations, tuftelin mRNA levels were significantly higher in organs exposed to low oxygen tension/hypoxic conditions (Leiser et al. 2007). This possible relationship was further supported by in vitro experiments (Deutsch et al. 2011; Leiser et al. 2011). The majority of studies about association between hypoxia and osteogenesis are dealing with the endochondral long bones, where growth plate chondrocytes are considered as a physiological model of hypoxia (Provot and Schipani 2007). There is not much information about hypoxia in the intramembranous bones, such as the mandibular/alveolar (m/a) one.
Therefore, tuftelin expression during m/a bone formation was examined along with the presence of hypoxia-inducible factors, HIF1 and HIF2, to follow their subcellular localization and possible time–space co-localization in vivo. The expression patterns of tuftelin, HIF1 and HIF2 at protein level were investigated by immunohistochemistry. Quantification of tuftelin, Hif1 and Hif2 mRNA expression was performed by qPCR.
Materials and methods
Animals
Wild-type mice (strain CD-1) were purchased from the Breeding Units of Masaryk University, Brno. The experimental protocol was in agreement with the law and approved in the frame of the GA CR project 17-14886S. Heads of pups corresponding to embryonic/prenatal (E) days 13, 14, 15, 18 and postnatal (P) day 10 were collected. Heads (E13, 14, 15), or dissected quadrants of mandibles (E18, P10) were fixed in 4% buffered paraformaldehyde and decalcified in buffered EDTA (Sigma-Aldrich). After dehydration in ethanol series, the samples were treated with xylene and embedded in paraffin. Serial frontal histological sections were processed and split between slides for the following analyses: haematoxylin–eosin (HE), and immunohistochemistry (tuftelin, HIF1, HIF2 expression).
Culture of osteoblastic precursors: cell line MC3T3-E1
MC3T3-E1 cells (purchased from Sigma-Aldrich) were cultured under normoxic conditions (5% CO2) for 3 weeks (common differentiation period for MC3T3-E1 cells—e.g., Quarles et al. 1992) in non-differentiating and differentiating MEM Alpha medium (Gibco, United States) containing 10% fetal calf serum (FCS), penicillin/streptomycin (100 µg/ml) and for the differentiated cells, by adding the ascorbic acid (0.25 mM). Cells were harvested using RLT buffer (Qiagen, United States) for further RNA isolation by RNeasy Mini Kit (74106, Qiagen, United States). Expression levels of the investigated genes in the MC3T3-E1 cells were used as reference values.
Immunohistochemistry
Serial sections of heads or mandibular quadrants were deparaffinized in xylene and rehydrated in a gradient series of ethanol ending with water. The antigen retrieval was applied at embryonic heads for HIF1, HIF2 and tuftelin antibodies (citrate buffer, pH = 6.0/98 °C/5–15 min/water bath). Endogenous peroxidase activity was eliminated in all sections by 3% hydrogen peroxidase in phosphate-buffered saline (RT/5 min) to eliminate possible background. The primary antibodies anti-tuftelin 1:50 (MyBioSource; MBS2034040) and anti-HIF2 1:100 (GeneTex, GTX30114) were applied overnight at 4 °C; anti-HIF1 1:100 (Novus Biologicals, NB100-479SS) was applied for 1 h at room temperature. To visualize the primary antibody, a peroxidase-conjugated streptavidin–biotin system (Vectastain PK-4002; Vector Laboratories, Inc. Burlingame, CA) followed by chromogen substrate (diaminobenzidine (DAB); K3466; Dako, Copenhagen, Denmark) reaction was used. Positive cells (brown) were counterstained with haematoxylin to visualize the nuclei (blue). Negative control was achieved by omitting primary antibody from the protocol. Well documented presence of tuftelin in ameloblasts and enamel (Deutsch et al. 1991), as well as odontoblasts and dentin (Zeichner-David et al. 1995) was used as a positive control.
Immunofluorescence and microscopy
Immunofluorescence analysis of tuftelin, HIF1 and HIF2 expression was performed for MC3T3-E1 cell line to determine the subcellular localization of the investigated proteins. The primary antibodies: anti-tuftelin (MyBioSource; MBS2034040), anti-HIF1 (Novus Biologicals, NB100-479SS) and anti-HIF2 (GeneTex, GTX30114) were applied in dilution: 1:50 for tuftelin or 1:100 for HIF1 and HIF2. Then, Alexa Fluor 488-conjugated secondary antibodies (Thermo Fisher Scientific, United States) were used 1:200 for 40 min at RT. Nuclei were detected by ProLong Gold Antifade reagent with DAPI (Thermo Fischer Scientific, United States). To document immunostainings, a Leica DMLB2/Leica DFC320 microscope and camera computer set was used (Leica PL APO 40x/0.75 objective, Leica Application Suite 4.7.1 acquisition software). Adobe Photoshop® was applied to adjust brightness, contrast and color balance, always for the whole images.
Separation of the alveolar bone for RNA isolation
Organ slices (250 µm) were prepared from the mouse mandibles at E13, E14 and E15 utilizing a standard table McIlwain™ Tissue Chopper (Mickle, Guildford, UK) as described earlier (e.g., Diep et al. 2009; Minarikova et al. 2015). The alveolar bones were removed from the slices containing molar tooth germs using micromanipulations (as described previously, e.g., Vesela et al. 2019). Isolated alveolar bone was lysed by solution composed of RLT buffer containing 1% 2-Mercaptoethanol (Sigma-Aldrich, M3148-100ML).
qPCR
Total RNA was extracted from the alveolar bone (at the stage E13, E14, E15) and osteoblastic precursor cell line MC3T3-E1 using Mini RNeasy Kit (Qiagen, Germany). The total RNA concentration and purity of each sample were assessed by NanoDrop (Thermo Fischer Scientific, United States) and the first-strand cDNA was synthesized using the reverse Master Mix (Generi Biotech, Czech Republic). The qPCR was performed in 10-µl final reaction volumes containing the one-step master mix gb Ideal PCR Master Mix (Generi, Biotech, Czech Republic) using LightCycler 96 (Roche, Switzerland) with preheating at 95 °C for 10 min, followed by 40 cycles of 95 °C/15 s and 62,5 °C/1 min. Hif1, Hif2 and tuftelin expression levels (Mouse Hif1, Mm00468869_m1; Hif2, Mm01236112_m1; tuftelin, Mm01261414_m1; Glut1 Mm00441480_m1; Pdk1 Mm00554300_m1 primers and probes TaqMan Gene expression Assay, Thermo Fisher Scientific, United States) were calculated using relative gene expression ∆∆CT method, with normalization against actin levels (mouse Actb, Mm02619580_g1, TaqMan Gene Expression Assay, Thermo Fischer Scientific, United States). As a reference level, undifferentiated osteoblastic cell line MC3T3-E1 was used for all genes of interest. For the analysis, three biological replicates of m/a bone were performed at each stage. The qPCR reactions were performed in triplicates for each sample for statistical analysis.
Results
Immunolocalization of tuftelin, HIF1 and HIF2 in vivo
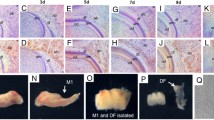
Mandibular/alveolar (m/a) bone develops from mesenchymal cells condensation, and, in the region of the mouse first molar tooth germ, becomes morphologically apparent at E13 (Fig. 1a). At that stage, the staining for tuftelin protein was apparent in the mesenchymal cells within the forming m/a bone (Fig. 1b). These mesenchymal cells showed a positivity also for HIF1 (Fig. 1c) but were negative for HIF2 (Fig. 1d).
Immunolocalization of tuftelin, HIF1 and HIF2 in the intramembranous mandibular bone at E13, E14 and E15. The alveolar/mandibular bone in the first mandibular molar (M1) region at E13 (a–d), at E14 (e–h), E15 (i–l). Histological view, haematoxylin–eosin (a, e, i), immunohistochemistry (b–d, f–h, j–l). The osteoblasts were tuftelin-positive at E13 (b), E14 (f) as well as E15 (j). Osteoblasts were positive for HIF1 at E13 (c), at E14 (g) as well as at E15 (k). At E13, early osteoblasts were negative for HIF2 (d). Osteoblasts were slightly positive for HIF2 at E14 (h), as well as osteoblasts and future osteocytes at E15 (i). Scale bar = 50 μm. Red arrows show examples of positive cells. AB alveolar bone, M1 first mandibular molar, MC Meckel’s cartilage
At E14 (Fig. 1e), differentiating osteoblasts were positive for tuftelin (Fig. 1f), as well as for HIF1 (Fig. 1g). At this stage, osteoblasts became positive also for HIF2 (Fig. 1h).
At E15 (Fig. 1i), first osteocytes started to appear and the presence of multinucleated osteoclasts increased the complexity of cell populations in the bone anlage. Osteoblasts and osteocytes at this stage were positive for tuftelin (Fig. 1j) as well as for HIF1 (Fig. 1k) and HIF2 (Fig. 1l).
At E18 (Fig. 2a), the m/a bone encapsulated the developing tooth. Tuftelin-positive osteoblasts and osteocytes showing a cytoplasmic and perinuclear/nuclear staining (Fig. 2b) were present in the bone at this stage. A similar pattern was observed for HIF1 in the alveolar bone (Fig. 2c). The staining for HIF2 in the alveolar bone was more scattered and restricted mostly to osteoblasts (Fig. 2d).
Immunolocalization of tuftelin, HIF1 and HIF2 in the intramembranous mandibular bone at E18 and P10. The alveolar/mandibular bone in the first mandibular molar region at E18 (a–d) and P10 (e–h). Histological view, haematoxylin–eosin (a, e), immunohistochemistry (b–d, f–h) and control staining (i–l). Tuftelin was detected in the cytoplasm and peri/nuclear regions of osteoblasts and osteocytes at E18 (b). Osteoblasts and osteocytes at E18 show cytoplasmic and perinuclear/nuclear positivity for HIF1 (c). HIF2 antigenicity was detected in osteoblasts and osteocytes at E18 (d). At the postnatal stage (P10), osteoblasts and osteocytes were positive for tuftelin which was detected in the cytoplasm and peri/nuclear regions (f). The osteoblasts and osteocytes were all positive for HIF1 in the cytoplasm, some even in their nuclei (g). Osteoblasts and osteocytes were positive for HIF2 at P10 (h). Staining was mostly cytoplasmic, but some perinuclear/nuclear signal was also observed (c, d, g, h). An example of negative control at E18 (i). Positive controls for tuftelin (j) enamel of developing molar, HIF1 (k) kidney and HIF2 (l) intestine. Scale bar = 50 μm. Red arrows show examples of positive cells
Postnatally, at P10, the developing tooth is tightly surrounded by the m/a bone (Fig. 2e). At that stage, osteoblasts and osteocytes were positive for tuftelin in both cytoplasmic and perinuclear/nuclear regions (Fig. 2f). The cells were also positive for HIF1 (Fig. 2g) and HIF2 (Fig. 2h).
These immunohistochemical findings are summarized in Fig. 3.
Localization of positive signal in the specific parts of the cells. Table shows stage, antibody and specific parts of the cell where the positive signal was detected: “+” positive signal, “++” strong signal, “±” weak signal, “–” no signal. Pictures below the table show examples of the specific signals: CYT cytoplasmic, PER perinuclear, NUC nuclear
Quantification of tuftelin, Hif1 and Hif2 expression
During m/a bone development (E13–E15), tuftelin mRNA (Fig. 4a) expression detected at E13 (~ 3.5-fold of the reference) slightly dropped (to ~ 2.0-fold) at E14 to return back a day later. A similar trend was observed in the case of Hif1 expression (Fig. 4b) where there was a slight decrease (to ~ 2.1-fold of the reference) at E14, to move back at E15 (to ~ 2.5-fold). Hif2 mRNA (Fig. 4c) increased between the day E13 and E14 (to ~ 30-fold of the reference level) and the expression was maintained at E15. To evaluate the expression of bona fide HIF1 and HIF2 target genes, and thus their activity, Glut1 (Suppl. Fig. 1a) and Pdk1 (Suppl. Fig. 1b) expressions were investigated at E13, E14 and E15.
qPCR of tuftelin, Hif1 and Hif2 in the intramembranous/alveolar bone at E13, E14, E15. Immunofluorescent staining of differentiated MC3T3-E1 cells for tuftelin, HIF1 and HIF2. Tuftelin expression at E13, E14, E15 in intramembranous alveolar/mandibular bone development, and in undifferentiated/differentiated MC3T3 cells (a). Hif1 expression in the forming intramembranous alveolar/mandibular bone at E13, E14, E15, and in the undifferentiated/differentiated MC3T3 cells (b). Hif2 expression at E13, E14, E15 in the intramembranous alveolar/mandibular bone and in the undifferentiated/differentiated MC3T3 cells (c). The expression level in undifferentiated MC3T3-E1 cells is displayed as fold 1.0. Statistically significant differences are highlighted (ANOVA, *p < 0.01; **p < 0.0001). The results are representative of three independent experiments. Immunofluorescent localization of tuftelin, HIF1 and HIF2 is shown in differentiated MC3T3-E1 cells. The positive signal is observed in green and the nuclei are visible in blue. Scale bar = 100 μm
Additionally, tuftelin mRNA was evaluated in pre-osteoblastic MC3T3-E1 cells (Fig. 4a,b,c), which are derived from an intramembranous bone. The expression levels of tuftelin in undifferentiated cells were used as the reference value along with levels after 21 days of differentiation. MC3T3-E1 were positive for tuftelin as well as for HIF1 and HIF2 proteins—as shown for differentiated cells (Fig. 4d).
To follow the expression of Hif1 (Suppl. Fig. 2a) and Hif2 (Suppl. Fig. 2b) in the time course of MC3T3-E1, Hif1 and Hif2 expressions were quantified at day 3, 10 and 21, and also the expression of Glu1 (Suppl. Fig. 2c) and Pdk1 (Suppl. Fig. 2d) was investigated in the samples. Simultaneously, tuftelin expression was measured (Suppl. Fig. 3).
Discussion
Bones are formed by two alternative basic osteogenic mechanisms producing intramembranous (the majority of bones in the skull) or endochondral (typically long bones of the limbs) type of bones. Mandibular/alveolar (m/a) bone belongs to the first category, where osteoblasts directly differentiate from mesenchymal cells.
The avascular mesenchymal condensations of the future m/a bone in the region of the first mouse molar become histologically visible at the E13 (Alfaqeeh et al. 2013) and tuftelin protein was already detected at this stage. Two days later, when the complex set of bone cells (osteoblasts, osteocytes, osteoclasts) could be observed and vascularization network appeared (Vesela et al. 2019), tuftelin positivity in osteoblasts was maintained. Unlike what was originally described in the tooth (Bashir et al. 1998; Deutsch et al. 2002), tuftelin in the m/a bone was intracellular and did not appear to be a matrix constituent. Nevertheless, Delgado et al. (2017) suggested that the presence of tuftelin in the enamel may be in fact associated with Tomes’ processes of secretory ameloblasts rather than the extracellular matrix itself. Indeed, the lack of a signal peptide at its N-terminus (Paine et al. 2000) still remains as an open question.
The intracellular localization of tuftelin in the m/a bone changed during development. At early stages, tuftelin was abundant in differentiating/differentiated osteoblasts and displayed a cytoplasmic localization. Whereas, at later pre- and at postnatal stages, the number of tuftelin-positive osteoblasts showed a decreasing trend, and the localization expanded into peri/nuclear regions. Such an observation was reported in cerebral neurons and other craniofacial structures (Shilo et al. 2019a; b), although this phenomenon has not been explained yet.
As suggested for soft tissues (MacDougall et al. 1998; Mao et al. 2001), tuftelin might be connected with physiological development, particularly in tissues/organs, where a low oxygen tension is important (Chen et al. 1999; Giaccia et al. 2004). The expression patterns of tuftelin, HIF1 and HIF2 in osteoblasts were almost identical including initial cytoplasmic, and later also perinuclear or nuclear localizations. HIF1 is known to regulate the differentiation of mesenchymal cells into chondrocytes (Amarilio et al. 2007), which requires the translocation of the HIF1α into the nucleus (e.g. Suzuki et al. 2017). Similar activation mechanism was described in the case of HIF2 and both HIFs act as transcription factors (e.g., Pangou et al. 2016).
The function of tuftelin in osteoblasts is not known. Nevertheless, HIF1 can support the differentiation of mesenchymal cells not only into chondrocytes, but also into osteoblasts (Provot and Schipani 2007; Provot et al. 2007; Wan et al. 2010), and HIF2 was also identified as a crucial osteogenic factor (Merceron et al. 2019). Moreover, tuftelin was recently demonstrated as a molecule required for NGF-induced differentiation of PC12 cells (Shilo et al. 2019b).
Since tuftelin accumulated in early (differentiating) osteoblasts from the mandibular bone, its expression in differentiated vs. undifferentiated osteoblastic MC3T3-E1 cells of intramembranous origin (Czekanska et al. 2012) was examined as a reference model. These cells were positive for tuftelin as well as for HIF1 and HIF2 proteins. Despite Hif1 mRNA expression peaked during the proliferation phase of MC3T3-E1 differentiation process (Quarles et al. 1992) and tuftelin expression followed this trend, the level of both mRNAs did not significantly differ when comparing undifferentiated and fully differentiated cells. Notably, Hif2 was elevated in the last phase of the differentiation process and was significantly increased in differentiated cells. These results are in agreement with the in vivo findings indicating a significant increase of HIF2 expression from E13 and E14 in the mandibular/alveolar bone development when the number of differentiated osteoblasts increased. Notably, in vivo, Hif2 in the forming m/a bone is expressed at ×10 higher relative level than Hif1. Actually, HIF1/Hif1 expression was evidenced in the prenatal intramembranous m/a bone for the first time. The only study related to HIFs in fetal bones was performed in a porcine model (De Spiegelaere et al. 2010); however, the developmental stage was not specified in their study.
The mostly cytoplasmic localization of tuftelin and HIFs as well as expression of Glut1 and Pdk1 in the prenatal m/a bone suggested functions unrelated to hypoxia during osteogenesis. This was further supported by the fact that in the intramembranous m/a bone, the blood vessels quickly extended within the forming structure (Vesela et al. 2019), so that the hypoxic conditions within the original mesenchymal condensations were not as dramatic as in the avascular part of the growth plate during long bone development (Araldi and Schipani 2010).
Hypoxia within mesenchymal condensations was expected to be critical for early angiogenesis of intramembranous bones; however, this could not be confirmed (Filipowska et al. 2017). There is a striking difference in endochondral and intramembranous bone development in dermo1-Cre ± , HIF-1 fl/fl mutants with a much milder impact in the latter case (Wan et al. 2010). These findings could be explained by our results showing that HIF1 is present in the developing m/a bone cells, but does not translocate into their nuclei. The complex mechanism stabilizing HIFs in a specific cell compartment in not yet clear, but HIF’s entry into the nucleus is not expected to be the only event that controls its stability (Berra et al. 2001). The inhibition of prolyl hydroxylase has been proposed to be the most probable stabilization step under hypoxic conditions (Chua et al. 2010). This causes prevention of HIF1a hydroxylation, disables Von Hippel Lindau-dependent ubiquitination and HIF1α degradation in proteasome.
Although the complex interplay between HIF1 and HIF2 in cells of the osteoblast lineage still needs to be specified (Merceron et al. 2019), the results presented here showed that tuftelin colocalizes with HIF1 already in the mesenchymal condensation and later with both HIFs in differentiated osteoblasts. However, such a time–space correlation does not necessarily mean functional interactions. Furthermore, the correlation between tuftelin expression levels and hypoxia remains unclear. The possibility of a direct regulation of tuftelin expression by HIF1 was recently reported in cancer cells (Dou et al. 2019), where the binding of HIF1 to the promoter of Tuft1, the gene encoding tuftelin, was demonstrated. The influence of hypoxia on tuftelin and HIF1 expression in mesenchymal precursors has previously been described as biphasic: the decreased level of tuftelin after 6 and 12 h of hypoxia was followed by an elevation after 24 h (Deutsch et al. 2011). In the in vivo m/a bone, tuftelin significantly decreased at E14, when compared to E13, and again increased at E15 and a similar trend was observed in the case of Hif1. However, both molecules retained their cytoplasmic localization during the investigated period.
The specific functions of tuftelin and the underlying mechanisms have been investigated recently. Notably, elevated levels of tuftelin were shown to be associated with interactions with TIP39 (Delgado et al. 2017) and Akt-mTOR/GSK3β signaling pathway (Kawasaki et al. 2018), which is involved in the proliferation and apoptosis suppression (Liu et al. 2018). Despite this yet limited knowledge, attempts are being made to search for possible clinical issues. In association with enamel formation, tuftelin was reported among parameters contributing to hypomineralization of teeth (Jeremias et al. 2013) and the formation of caries (Shimizu et al. 2012; Sovik et al. 2015; Hu et al. 2019). In chondrogenesis, tuftelin was recently reported as a novel candidate gene for metatarsophalangeal osteoarthritis (Sliz et al. 2017). Tuftelin was shown to be a regulator of cancer cell proliferation, invasion and apoptosis (Zhou et al. 2016; Liu et al. 2018; Yu et al. 2018). Tuftelin was even proposed as a promising biomarker for tumor progression (Kawasaki et al. 2018) as being a key regulator of the mTORC1 pathway also involved in osteoblast growth, proliferation, survival and differentiation (McGonnell et al. 2012) and in osteosarcoma metabolism and development (Wang et al. 2017).
References
Alfaqeeh SA, Gaete M, Tucker AS (2013) Interactions of the tooth and bone during development. J Dent Res 92:1129–1135. https://doi.org/10.1177/0022034513510321
Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E (2007) HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Dev Camb Engl 134:3917–3928
Araldi E, Schipani E (2010) Hypoxia, HIFs and bone development. Bone 47:190–196
Bashir MM, Abrams WR, Tucker T, Sellinger B, Budarf M, Emanuel B, Rosenbloom J (1998) Molecular cloning and characterization of the bovine and human tuftelin genes. Connect Tissue Res 39:13–24
Berra E, Roux D, Richard DE, Pouysségur J (2001) Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes O(2)-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. EMBO Rep 2(7):615–620. https://doi.org/10.1093/embo-reports/kve130
Campos-Navarro PM, Guerrero-Barrera AL, Avelar-González FJ, Navarro-Rincón-Gallardo ME, Mariel-Murga H, Mareil-Cárdenas J, Gutiérrez-Cantú FJ (2017) Expression of tuftelin in human dental germs. Int J Morphol 35(1):293–298
Chen EY, Fujinaga M, Giaccia AJ (1999) Hypoxic microenvironment within an embryo induces apoptosis and is essential for proper morphological development. Teratology 60:215–225
Chua YL, Dufour E, Dassa EP, Rustin P, Jacobs HT, Taylor CT, Hagen T (2010) Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J Biol Chem 285(41):31277–31284. https://doi.org/10.1074/jbc.M110.158485
Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for in vitro research. Eur Cell Mater 9(24):1–17
De Spiegelaere W, Cornillie P, Casteleyn C, Burvenich C, Van den Broeck W (2010) Detection of hypoxia inducible factors and angiogenic growth factors during foetal endochondral and intramembranous ossification. Anat Histol Embryol 2010(39):376–384
Delgado S, Deutsch D, Sire JY (2017) Evolutionary analysis of the mammalian tuftelin sequence reveals features of functional importance. J Mol Evol 84:214–224. https://doi.org/10.1007/s00239-017-9789-5
Deutsch D, Palmon A, Fisher LW, Kolodny N, Termine JD, Young MF (1991) Sequencing of bovine enamelin (“tuftelin”) a novel acidic enamel protein. J Biol Chem 266:16021–16028
Deutsch D, Leiser Y, Shay B, Fermon E, Taylor A, Rosenfeld E, Dafni L, Charuvi K, Cohen Y, Haze A et al (2002) The human tuftelin gene and the expression of tuftelin in mineralizing and nonmineralizing tissues. Connect Tissue Res 43:425–434
Deutsch D, Silverstein N, Shilo D, Lecht S, Lazarovici P, Blumenfeld A (2011) Biphasic influence of hypoxia on tuftelin expression in mouse mesenchymal C3H10T1/2 stem cells. Eur J Oral Sci 119:55–61. https://doi.org/10.1111/j.1600-0722.2011.00861.x
Diekwisch TG, Ware J, Fincham AG, Zeichner-David M (1997) Immunohistochemical similarities and differences between amelogenin and tuftelin gene products during tooth development. J Histochem Cytochem 45(6):859–866
Diep L, Matalova E, Mitsiadis TA, Tucker AS (2009) Contribution of the tooth bud mesenchyme to alveolar bone. J Exp Zool B Mol Dev Evol 312B(5):510–517
Dou C, Zhou Z, Xu Q, Liu Z, Zeng Y, Wang Y, Li Q, Wang L, Yang W, Liu Q, Tu K (2019) Hypoxia-induced TUFT1 promotes the growth and metastasis of hepatocellular carcinoma by activating the Ca2 +/PI3K/AKT pathway. Oncogene 38:1239–1255
Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T (2017) The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 20(3):291–302. https://doi.org/10.1007/s10456-017-9541-1
Giaccia AJ, Simon MC, Johnson R (2004) The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev 18:2183–2194
Hu XP, Song TZ, Zhu YY, Wu LL, Thang X, Zhou JY, Li ZG (2019) Association of ENAM, TUFT1, MMP13, IL1B, IL10 and IL1RN gene polymorphism and dental caries susceptibility in Chinese children. J Int Med Res 47(4):1696–1704
Jeremias F, Koruyucu M, Küchler EC (2013) Genes expressed in dental enamel development are associated with molar-incisor hypomineralization. Arch Oral Biol 2013(58):1434–1442
Kawasaki N, Isogaya K, Dan S, Yamori T, Takano H, Yao R, Morishita Y, Taguchi L, Morikawa M, Heldin CH, Noda T, Ehata S, Miyazono K, Koinuma D (2018) TUFT1 interacts with RABGAP1 and regulates mTORC1 signaling. Cell Discov 4(1):1
Leiser Y, Blumenfeld A, Haze A, Dafni L, Taylor AL, Rosenfeld E, Fermon E, Gruenbaum-Cohen Y, Shay B, Deutsch D (2007) Localization, quantification, and characterization of tuftelin in soft tissues. Anat Rec Adv Integr Anat Evol Biol 290:449–454
Leiser Y, Silverstein N, Blumenfeld A, Shilo D, Haze A, Rosenfeld E, Shay B, Tabakman R, Lecht S, Lazarovici P et al (2011) The induction of tuftelin expression in PC12 cell line during hypoxia and NGF-induced differentiation. J Cell Physiol 226:165–172
Liu H, Zhu J, Mao Z, Zhang G, Hu X, Chen F (2018) Tuft1 promotes thyroid carcinoma cell invasion and proliferation and suppresses apoptosis through the Akt-mTOR/GSK3β signaling pathway. Am J Transl Res 10(12):4376–4384
Luo W, Wen X, Wang HJ, MacDougall M, Snead ML, Paine ML (2004) In vivo overexpression of tuftelin in the enamel organic matrix. Cells Tissues Organs 177(4):212–220
MacDougall M, Simmons D, Dodds A, Knight C, Luan X, Zeichner-David M, Zhang C, Ryu OH, Qian Q, Simmer JP et al (1998) Cloning, characterization, and tissue expression pattern of mouse tuftelin cDNA. J Dent Res 77:1970–1978
Mao Z, Shay B, Hekmati M, Fermon E, Taylor A, Dafni L, Heikinheimo K, Lustmann J, Fisher LW, Young MF et al (2001) The human tuftelin gene: cloning and characterization. Gene 279:181–196
McGonnell M, Grigoriadis AE, Lam EW, Price JS, Sunters A (2012) A specific role for phosphoinositide 3-kinase and AKT in osteoblasts? Front Endocrinol 20:3–88
Merceron C, Ranganathan K, Wang E, Tata Z, Makkapati S, Khan MP, Mangiavini L, Yao AQ, Castellini L, Levi B, Giaccia AJ, Schipani E (2019) Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res 7:7
Minarikova M, Oralova V, Vesela B, Radlanski RJ, Matalova E (2015) Osteogenic profile of mesenchymal cell populations contributing to alveolar bone formation. Cells Tissues Organs 200:339–348
Paine CT, Paine ML, Luo W, Okamoto CT, Lyngstadaas SP, Snead ML (2000) A tuftelin-interacting protein (TIP39) localizes to the apical secretory pole of mouse ameloblasts. J Biol Chem 275(29):22284–22292
Pangou E, Befani C, Mylonis I, Samiotaki M, Panayotou G, Simos G, Liakos P (2016) HIF-2α phosphorylation by CK1δ promotes erythropoietin secretion in liver cancer cells under hypoxia. J Cell Sci 129(22):4213–4226. https://doi.org/10.1242/jcs.191395
Provot S, Schipani E (2007) Fetal growth plate: a developmental model of cellular adaptation to hypoxia. Ann N Y Acad Sci 1117:26–39
Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E (2007) Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol 177:451–464
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res 7:683–692
Santo ARE, Line SRP (2015) The enamel organic matrix: structure and function. Braz J Oral Sci 4:716–724
Satchell PG, Shuler CF, Diekwisch TG (2000) True enamel covering in teeth of the Australian lungfish Neoceratodus forsteri. Cell Tissue Res 299:27–37
Shilo D, Blumenfeld A, Haze A, Sharon S, Goren K, Hanhan S, Gruenbaum-Cohen Y, Ornoy A, Deutsch D (2019a) Tuftelin’s involvement in embryonic development. JEZ-B 332:125–135. https://doi.org/10.1002/jezb.22855
Shilo D, Cohen G, Blumenfeld A, Goren K, Hanhan S, Sharon S, Haze A, Deutsch D, Lazarovici P (2019b) Tuftelin is required for NGF-induced differentiation of PC12 cells. J Mol Neurosci 68(1):135–143
Shimizu E, Jong G, Patridge N, Rosenberg PA, Lin LM (2012) Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod 38(9):1293–1297
Sliz E, Taipale M, Welling M, Skarp S, Alaraudanjoki V, Ignatius J, Ruddock L, Nissi R, Männikkö M (2017) TUFT1, a novel candidate gene for metatarsophalangeal osteoarthritis, plays a role in chondrogenesis on a calcium-related pathway. PLoS One 12:e0175474
Sovik JB, Vieira AR, Tveit AB, Mulic A (2015) Enamel formation genes associated with dental erosive wear. Caries Res 49(3):236–242
Suzuki N, Gradin K, Poellinger L, Yamamoto M (2017) Regulation of hypoxia-inducible gene expression after HIF activation. Exp Cell Res 2017(356):182–186. https://doi.org/10.1016/j.yexcr.2017.03.013
Vesela B, Svandova E, Bobek J, Lesot H, Matalova E (2019) Osteogenic and angiogenic profiles of mandibular bone-forming cells. Front Physiol 10:124. https://doi.org/10.3389/fphys.2019.00124
Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL (2010) Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci 1192:322–326
Wang DW, Wu L, Cao Y, Yang L, Liu W, Xiao-Qiang E, Ji G, Bi ZG (2017) A novel mechanism of mTORC1-mediated serine/glycine metabolism in osteosarcoma development. Cell Signal 29:107–114
Yu Y, He J, Li P, Qiu N, Wang L, Feng H (2018) TUFT1 promotes osteosarcoma cell proliferation and predicts poor prognosis in osteosarcoma patients. Open Life Sci 13(1):396–403. https://doi.org/10.1515/biol-2018-0048
Zeichner-David M, Diekwisch T, Fincham A, Lau E, MacDougall M, Moradian-Oldak J, Simmer J, Snead M, Slavkin HC (1995) Control of ameloblast differentiation. Int J Dev Biol 39:69–92
Zhou B, Zhan H, Tin L, Liu S, Xu J, Dong Y, Li X, Wu L, Guo W (2016) TUFT1 regulates metastasis of pancreatic cancer through HIF1-Snail pathway induced epithelial-mesenchymal transition. Cancer Lett 382:11–20
Acknowledgement
This work was supported by the Grant Agency of the Czech Republic (17-14886S).
Author information
Authors and Affiliations
Contributions
J. B. samples, analysis (immunohistochemistry), evaluation of results, contribution to manuscript preparation; V. O. samples, analysis (qPCR/bones, immunofluorescence), evaluation of results, contribution to manuscript preparation; A. K. analysis (qPCR/MC3T3-E1 cells); I. Z. preliminary data; H. L. critical review and contribution to manuscript preparation; E. M. head of the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2019_1813_MOESM1_ESM.tif
Supplementary material 1 (TIFF 71 kb). qPCR of Glut1 and Pdk1 in the intramembranous/alveolar bone at E13, E14, E15. Glut1 expression in the forming intramembranous alveolar/mandibular bone at E13, E14, E15, and in the undifferentiated/differentiated MC3T3 cells (a). Pdk1 expression at E13, E14, E15 in the intramembranous alveolar/mandibular bone (b). The results are representative of three independent experiments
418_2019_1813_MOESM2_ESM.tif
Supplementary material 2 (TIFF 109 kb). qPCR of Hif1, Hif2, Glut1 and Pdk1 during differentiation of MC3T3-E1 cells. Intervals of 3, 10 and 21 days of cultivation, expression of Hif1 (a), Hif2 (b), Glut1 (c), Pdk1 (d). Statistically significant differences are highlighted (ANOVA, *p < 0.01; **p < 0.0001). The results are representative of three independent experiments
418_2019_1813_MOESM3_ESM.tif
Supplementary material 3 (TIFF 24 kb). qPCR of tuftelin during differentiation of MC3T3-E1 cells. Intervals of 3, 10 and 21 days of cultivation. The results are representative of three independent experiments
Rights and permissions
About this article
Cite this article
Bobek, J., Oralova, V., Kratochvilova, A. et al. Tuftelin and HIFs expression in osteogenesis. Histochem Cell Biol 152, 355–363 (2019). https://doi.org/10.1007/s00418-019-01813-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-019-01813-4