Abstract
Enterotoxins of Staphylococcus aureus are among the most common causes of food poisoning. Acting as superantigens they intoxicate the organism by causing a massive uncontrolled T cell activation that ultimately may lead to toxic shock and death. In contrast to our detailed knowledge regarding their interaction with the immune system, little is known about how they penetrate the epithelial barrier to gain access to their targets. We therefore studied the uptake of two staphylococcal enterotoxins (SEs), SEA and SEB, using organ cultured porcine jejunal explants as model system. Attachment of both toxins to the villus surface was scarce and patchy compared with that of cholera toxin B (CTB). SEA and SEB also bound to microvillus membrane vesicles in vitro, but less efficiently than CTB, and the binding was sensitive to treatment with endoglycoceramidase II, indicating that a glycolipid, possibly digalactosylceramide, acts as cell surface receptor at the brush border. Both SEs partitioned poorly with detergent resistant membranes (DRMs) of the microvillus, suggesting a weak association with lipid raft microdomains. Where attachment occurred, cellular uptake of SEA and SEB was also observed. In enterocytes, constitutive apical endocytosis normally proceeds only to subapical early endosomes present in the actomyosin-rich “terminal web” region. But, like CTB, both SEA and SEB penetrated deep into the cytoplasm. In conclusion, the data show that after binding to the enterocyte brush border SEA and SEB perturb the apical membrane trafficking, enabling them to engage in transcytosis to reach their target cells in the subepithelial lamina propria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gram-positive bacterium Staphylococcus aureus (S. aureus) is a common opportunistic pathogen capable of colonizing both humans and domestic animals; thus about 20 % of the human population are persistent carriers and 60 % intermittent carriers (Kluytmans et al. 1997). S. aureus is known to produce a large number of different staphylococcal enterotoxins (SEs) of which SEA and SEB are those best characterized (Bohach et al. 1990; Pinchuk et al. 2010; Hennekinne et al. 2012). SEs are commonly classified as superantigens, defined by their ability to cross-link the major histocompatibility complex class II (MHC II) molecules with a variable portion of the β chain (Vβ) of the T cell receptor (TCR) without involvement of the antigen-specific site (Choi et al. 1989). In this antigen-independent way they may stimulate up to 25 % of the T cells at a time, resulting in the release of a massive cytokine bolus (Pinchuk et al. 2010). SEs are the causative agents of the second most commonly reported type of food-borne diseases, calculated to cause about 76 million illnesses, 325,000 hospitalizations, and 5,000 deaths annually in the US (Mead et al. 1999). These pyrogenic toxins are resistant to heat, acid and gastrointestinal proteases, and within a few hours of ingestion microgram quantities of SEs may cause food poisoning with classical symptoms that include vomiting, diarrhea, nausea and abdominal pain. In the majority of those afflicted, the disease is self-resolving, but in more severe cases it may lead to toxic shock and death.
The gastrointestinal injuries associated with SE food poisoning have been extensively studied for a number of years using various animal models, including monkeys, dogs, pigs, and rodents (Pinchuk et al. 2010). Generally, morphological and inflammatory changes are observed after exposure to the toxins all along the gastrointestinal tract, most severely in the segments of the small intestine. Thus, in Rhesus monkeys SEB fed by a gastric tube caused major histochemical changes including a shortening of villi, elongation of crypts, and a dense inflammatory infiltration of the lamina propria (Sheahan et al. 1970).
Intraperitoneal administration of SEB to mice caused a time- and dose-dependent enteropathy characterized by reduced jejunal villus height, increased depth of the crypts, and an increase in the number of T cells present in the lamina propria (Benjamin et al. 1998). SEA given orally to rats prompted a similar response in the overall crypt-villus architecture of the duodenum and included a leucocyte infiltration of the lamina propria (Beery et al. 1984). In the latter study, the toxin was detected in the kidneys already 15 min after exposure, showing that it can rapidly pass through an intact intestinal epithelium. In a current model of SE action in the gastrointestinal tract, the toxin first passes the epithelial barrier and subsequently encounters—and binds to—subepithelial MHC II-expressing antigen-presenting cells (macrophages, dendritic cells, or myofibroblasts) and TCR expressing CD4+ cells (Pinchuk et al. 2010). The resulting strong production and release of proinflammatory cytokines and chemokines in turn causes an increased chemotaxis of various immune cells from the gut associated lymphoid tissue (GALT) to the site of SE entry, thereby generating an uncontrolled burst of proinflammatory cytokines and chemokines that ultimately leads to acute inflammation and shock.
However, in contrast to our detailed knowledge of the SE-associated inflammation of the intestine, much less is yet known about how this process is initiated and the role played by epithelial non-immune cells in progression of disease (Pinchuk et al. 2010). In particular, insight into how SEs breach the epithelial barrier is scarce and has mainly been studied in model epithelial cell lines. For SEB and toxic shock syndrome toxin 1 (TSST-1), this was shown to occur in Caco-2 cells by transcytosis, i.e., by endocytic uptake from one cell membrane domain followed by exocytosis from the opposite domain (Hamad et al. 1997). Transcytosis was dose-dependent, facilitated, and occurred bidirectionally, but interestingly not in the case of SEA which only crossed the cell monolayer nonspecifically similar to a fluid phase marker (Hamad et al. 1997). However, in another study using T-84 cells, SEA and SEB exhibited comparable dose-dependent transcytosis whereas TSST-1 was more efficiently transported, and a 10-amino acid peptide conserved amongst the SEs was identified as a putative transcytosis epitope (Shupp et al. 2002). In kidney proximal tubule cells a glycolipid, digalactosylceramide, was proposed to act as a cell surface receptor for SEB but not for SEA or TSST-1 (Chatterjee and Jett 1992; Chatterjee et al. 1995), and a domain within the SEB sequence (amino acids 191–220) identified to be involved in the binding (Chatterjee et al. 2007).
The organization of the transcytotic membrane traffic in fully developed small intestinal enterocytes is much different from that occurring in model cell lines like those described above, particularly regarding the directionality. Thus, significant apical-to-basolateral transcytosis only takes place until “closure” soon after birth, mainly to secure uptake of luminal maternal immunoglobulin G to the neonate (Rodewald and Kraehenbuhl 1984), whereas later in life basolateral-to-apical transcytosis becomes an important pathway for secretion of dimeric immunoglobulin A into the gut lumen (Mostov 1994; Kraehenbuhl and Neutra 1992). Although constitutive apical endocytosis to some extent occurs from the mature brush border membrane, the resulting early endosomes largely remain in the subapical region known as the “terminal web”, a unique feature of cells with a fully developed brush border (Hansen et al. 2009). The cytoskeletal meshwork of this actomyosin-rich region (Mooseker et al. 1983) most likely acts as a diffusion barrier preventing endosomes from trafficking further into the enterocyte (Hansen et al. 2009).
In the present work, we studied luminal binding and uptake of fluorescein-conjugated SEA− and SEB in organ cultured mucosal explants of porcine jejunum with the aim to establish how these two toxins manage to cross the epithelial barrier en route to their targets in the lamina propria.
Materials and methods
Materials
S. aureus enterotoxins A− and B (SEA and SEB), cholera toxin B subunit (CTB), rabbit antibodies to the three toxins, and recombinant endoglycoceramidase II from Rhodococcus sp. expressed in E. coli, were obtained from Sigma–Aldrich (www.sigmaaldrich.com), Alexa Fluor 594-conjugated CTB, a fluorescein isothiocyanate (FITC) protein labeling kit, mouse monoclonal anti-fluorescein antibodies, a fixable FM lipophilic styryl dye (FM 1-43 FX), Alexa Fluor-conjugated secondary antibodies for immunofluorescence microscopy, and ProLong Gold antifade reagent with DAPI from Invitrogen (www.invitrogen.com), a rabbit antibody to intestinal alkaline phosphatase from AbD Serotec (www.biogenesis.co.uk/), and HRP-conjugated secondary antibodies for immunoblotting and secondary antibodies for immunogold electron microscopy from DAKO (www.dako.dk). A rabbit antibody to porcine intestinal aminopeptidase N was previously described (Hansen et al. 1987). Jejunal segments, taken 1–2 m from the pylorus of pig small intestines, were surgically removed by licensed staff from anaesthetisized animals that were fasted overnight at the Department of Experimental Medicine, the Panum Institute, University of Copenhagen.
Organ culture of mucosal explants
Freshly obtained jejunal segments were quickly rinsed in ice-cold MEM medium and mucosal explants of about 0.1 g were excised and cultured at 4 or 37 °C in MEM medium for 1 h, essentially as previously described (Danielsen et al. 1982). FITC-conjugated SEA− and SEB were added to the medium at a concentration of 10 μg/ml, Alexa-CTB at 5 μg/ml, and FM dye at 20 μg/ml.
FITC-conjugation of SEA and SEB
FITC-conjugated SEA− and SEB were prepared for use in fluorescence- and immunogold microscopy to eliminate problems with unspecific background labeling when using antibodies to the SEs. The conjugation was performed using a FITC protein labeling kit according to the protocol supplied by the manufacturer. Briefly, 200 μl of SEA or SEB (50–100 μg of toxin) in 50 mM HEPES-HCl, 150 mM NaCl, pH 7.1 (HB), was mixed with 20 μl 1 M sodium bicarbonate, pH 9.0, in a reaction tube before 10–20 μl freshly prepared dye stock solution was added. The mixture was incubated under magnetic stirring for 1 h protected from light. After incubation, unconjugated FITC was removed by extensive dialysis against PBS.
Fluorescence microscopy
Immediately after culture, mucosal explants were given a quick rinse in fresh medium and fixed in 4 % paraformaldehyde in 0.1 M sodium phosphate, pH 7.2 (buffer A) for 2 h at 4 °C. After a rinse in buffer A they were frozen in precooled 2-methylbutane and sectioned in a cryostat (Leica CM1850) at −20 °C. For immunolabeling of aminopeptidase N, sections were incubated for 1 h at room temperature with anti-aminopeptidase N (1:200 dilution) in 50 mM Tris–HCl, 150 mM NaCl, 0.5 % ovalbumine, 0.1 % gelatine, 0.005 % Tween 20, 0.2 % telostan gelatine, pH 7.2 (buffer B), followed by incubation for 1 h at room temperature with an Alexa-conjugated secondary antibody (1:200 dilution in buffer B). A control with omission of the primary antibody was routinely included in all labeling experiments. Sections were mounted in antifade medium with DAPI and examined in a fluorescence microscope (Leica DM 4000B) fitted with a digital camera (Leica DC 300FX). Images were obtained using Leica objectives with the following magnification/numerical aperture: 20×/0.40, 40×/0.65, and 63×/0.90. The following filter cubes were used: I3 (band pass filter, excitation 450–490 nm), TX2 (band pass filter, excitation 560/40 nm), and A4 (band pass filter, excitation 360/40 nm).
Immunogold electron microscopy
For ultracryosectioning, mucosal explants were fixed in 4 % paraformaldehyde in buffer A as described for fluorescence microscopy. After a rinse in buffer A and an overnight immersion in 2.3 M sucrose, 1 % paraformaldehyde, they were mounted on a metal pin and frozen in liquid nitrogen. Sections were cut in an ultramicrotome (RMC MT 6000-XL), collected with a sucrose droplet, and attached to formvar-coated nickel grids. For immunolabeling, the ultracryosections were incubated with anti-fluorescein antibodies (1:250–500 dilution) for 1 h at room temperature, followed by labeling with secondary gold-conjugated antibodies (1:100 dilution) for 1 h at room temperature, essentially as described previously (Hansen et al. 1999). Controls with omission of the primary antibody were included in the experiments, and the sections were examined in an electron microscope (Zeiss EM 900) fitted with a digital camera (Mega View II).
Subcellular fractionation and DRM analysis
Mucosa scraped from segments of porcine jejunum was homogenized and fractionated by the divalent cation precipitation method (Booth and Kenny 1974). Briefly, homogenization was performed in 10 volumes of 2 mM Tris–HCl, 50 mM mannitol, pH 7.1, containing 10 μg/ml of aprotinin and leupeptin. The homogenate was centrifuged at 500g, 5 min, and MgCl2 was added to the supernatant to give a final concentration of 10 mM. After 10 min on ice, the preparation was centrifuged at 1,500g, 10 min. The supernatant was collected and centrifuged at 48,000g, 30 min to yield a pellet of microvillus membrane vesicles.
For DRM analysis, microvillus membrane vesicles were resuspended in 0.5 ml of HB and incubated for 1 h at room temperature with 10 μg of SEA or SEB. After centrifugation at 20,000g, 30 min, the pellet was collected, resuspended in 0.9 ml HB and solubilized by addition of 0.1 ml 10 % Triton X-100. After incubation for 10 min on ice, the preparation was subjected to sucrose gradient ultracentrifugation overnight as previously described (Danielsen 1995).
Toxin binding to microvillus membrane vesicles
For binding experiments with SEA, SEB and CTB, microvillus membrane vesicles were resuspended in 200 μl of HB in the presence of 1 μg of toxin. After incubation for 1 h at room temperature, the preparation was centrifuged at 20,000g, 30 min. Pellets and supernatants were collected and analyzed by SDS/PAGE (Laemmli 1970). For removal of glycolipids, the microvillus membrane vesicles were first resuspended in 300 μl 0.1 M sodium acetate, 0.4 % Triton X-100, pH 5.0. 150 μl of the preparation was then incubated with 0.02 units of endoglycoceramidase II overnight at room temperature, and 150 μl incubated in parallel as a control without addition of enzyme. After incubation, the preparations were centrifuged at 20,000g, 30 min. The pellets were collected, resuspended in HB and incubated with toxin as described above.
SDS/PAGE and immunoblotting
Prior to electrophoresis, samples were denatured by boiling for 3 min in the presence of 1 % sodium dodecyl sulfate and 10 mM dithiothreitol. SDS/PAGE in 15 % gels was performed as described (Laemmli 1970). After electrophoresis and semi-dry blotting onto Immobilon PVDF membranes, immunoblotting was performed by incubation with primary antibodies to SEA, SEB, or CTB (all 1:400 dilution) or alkaline phosphatase (1:2,000), followed by HRP-conjugated secondary antibodies (1:2,000 dilution). Blots were developed with an electrochemiluminescence (ecl) reagent according to the protocol supplied by the manufacturer (GE Healthcare, www.gehealthcare.com), using a cooled camera TCam285 imaging system (Phase GmbH, Lübeck, Germany, www.phase-hl.com/). After immunoblotting, total protein was visualized by staining with Coomassie brilliant blue R250 (0.2 %) dissolved in an ethanol/H2O/acetic acid mixture (50:43:7).
Results
SEA and SEB attachment to the epithelial surface of organ cultured mucosal explants
Organ culture of mucosal small intestinal explants is an established in vivo-like model system that preserves both biological functionality and crypt-villus architecture for several hours (Danielsen et al. 1982). We have previously used it for studying secretory processes, for instance synthesis and luminal secretion of immunoglobulins (Hansen et al. 1999; Hansen et al. 2006), as well as for binding and endocytic uptake of cholera toxin B subunit (CTB) (Hansen et al. 2005). In the present work, we used this system for studying the interaction between the epithelium and the FITC-conjugated enterotoxins A− and B of S. aureus. As shown in Fig. 1, attachment of both SEA and SEB to the villus surface could be seen after 1 h of incubation. However, for both enterotoxins, the binding was scarce and patchy with large villus areas seemingly unaffected by exposure to the toxins. Often, binding was seen between closely aligned villi, suggesting that such “sheltered” areas favored entrapment of the toxins. No labeling in the intercellular space between neighboring enterocytes or in the lamina propria below the epithelium was detected, indicating that passage through tight junctions was excluded. In contrast, CTB efficiently coated the entire villus surface after only 15 min of incubation (Fig. 1), indicating a much higher avidity for the enterocyte brush border.
SEA, SEB and CTB attachment to organ cultured porcine jejunal epithelium. Fluorescent images showing binding of the toxins to villi after 1 h of culture in the presence of 10 μg/ml of SEA or SEB or 5 μg/ml of CTB. SEA and SEB binding was scarce and patchy whereas CTB lined the entire villus surface. Nuclei were stained with DAPI. E enterocytes, LP lamina propria. Bars 40 μm
Endocytic uptake of SEA and SEB into enterocytes
Fixable, lipophilic styryl FM dyes become fluorescent when they spontaneously insert into cell membranes. In addition, they are water soluble and nontoxic to cells and therefore suitable markers for endocytosis (Bolte et al. 2004). FM was previously used for analysis of endocytic membrane trafficking from the enterocyte brush border (Hansen et al. 2009), and as shown in Fig. 2, it visualized a temperature-dependent, constitutive internalization of membrane from the brush border. More specifically, the dye accumulated in distinct subapical punctae, previously shown to represent early endosomes in the terminal web (hence termed “TWEEs”). Most likely, this actomyosin-rich area of the cell acts as an intracellular diffusion barrier that restricts apical-to-basolateral transcytosis in the fully developed enterocyte. But as shown in Fig. 2, CTB induced trafficking of FM-labeled punctae deeper into the cytoplasm, indicating the ability of this toxin to perturb the apical membrane trafficking pathways of the intoxicated cells. The experiments with FM thus demonstrate that by fluorescence microscopy, we can distinguish three stages of toxin localization: (1) Surface binding, (2) Punctate uptake into the terminal web region (TWEE’s), and (3) punctuate uptake deeper into the cytoplasm.
Endocytic uptake of FM dye. The lipophilic dye was incorporated efficiently into the luminal brush border of the enterocytes (E) after 1 h at 4 °C, a temperature nonpermissive of membrane traffic. At 37 °C, FM was mainly seen in numerous bright punctae representing TWEEs narrowly lining the terminal web region of the enterocytes about 1 μm below the brush border. In the presence of CTB, FM-positive punctae had frequently penetrated deep into the enterocyte cytoplasm. Inserts show details in ~2 times higher magnification. Nuclei were stained with DAPI. LP lamina propria. Bars 20 μm
Figure 3 shows double labeling images of SEA and SEB, respectively, together with the brush border marker aminopeptidase N. For both enterotoxins it is evident that they not only attached to the brush border, but also appeared in intracellular punctae, indicative of an endocytic internalization. Immunolabeling with an anti-FITC antibody enhanced the sensitivity of detecting internalized enterotoxins and revealed that they were present not only in the subapical TWEEs but also in deeper-lying compartments (Fig. 3).
Internalization of SEA and SEB. Fluorescent images showing endocytic uptake into the enterocytes (E) of both SEA and SEB. Both toxins could be seen as punctae (some marked by arrows) well below the luminal surface that was visualized by immunolabeling for the brush border enzyme aminopeptidase N (top panel). Labeling with an antibody to fluorescein enhanced the sensitivity of detection and showed penetration of toxin-positive punctae deep into the cytoplasm (marked by arrows) (bottom panel). LP lamina propria. Bars 20 μm
Using the anti-FITC antibody, attachment and uptake of SEB was studied in closer detail by immunogold electron microscopy (Fig. 4). The patchy villus labeling observed by immunofluorescence microscopy was confirmed at the ultrastructural level, and altogether, the itinerary of events starting from binding at the tip of the microvilli of the brush border, uptake into TWEEs, and trafficking to deeper-lying compartments close to the lateral cell surface, could be visualized. Some of the labeling near the meandering lateral membranes between neighboring enterocytes might represent SEB awaiting transcytosis or alternatively, toxin already secreted into the intercellular space.
Binding and uptake of SEB analyzed by immunogold electron microscopy. Electron micrographs showing labeling with an anti-FITC antibody (marked by arrows). a Immunogold particles at the tip of microvilli (Mic) representing SEB adhesion to the brush border. b Immunogold particles near or at the base of microvilli (Mic) from where endocytosis takes place. c, d Immunogold labeling in TWEEs present in the subapical terminal web region 1–2 μm from the surface. e A labeled endosome below the terminal web region, approximately 6 μm from the apical cell surface. f Immunogold particles located near the nucleus (N) along the meandering lateral membrane (LM). Bars 0.2 μm (a, c–e), 0.5 μm (b, f)
Taken together, the above experiments show that lumenal SEA and SEB both attach to the brush border and subsequently are taken up by enterocytes. In comparison with CTB the initial binding to the brush border of both S. aureus enterotoxins was patchy, but in enterocytes where cell surface binding did occur, SEA and SEB, like CTB, perturbed the constitutive apical endocytosis. This was no longer confined to TWEEs, but progressed to a pathway leading deeper into the cells, including areas near the lateral surface from where transcytosis is likely to take place.
SEA and SEB binding to microvillus membrane vesicles
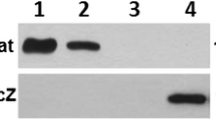
Upon homogenization brush border microvilli rupture and spontaneously form outside-out closed vesicles that are subsequently isolated from other cellular membranes by the divalent cation precipitation method (Booth and Kenny 1974). As shown in Fig. 5, both SEA and SEB bound to microvillus membrane vesicles after incubation for 30 min at room temperature. Under these conditions where 1 μg of toxin and ~0.2 mg of vesicles were mixed, only a minor part of both SEA and SEB was pelleted together with the microvillus membranes after incubation. In contrast, in a similar parallel experiment, CTB bound completely to the membranes.
SEA, SEB and CTB binding to microvillus membrane vesicles. The membranes were incubated with toxins as described in “Materials and Methods”. After centrifugation the pellets (P) and supernatants (S) were collected and subjected to SDS/PAGE, followed by immunoblotting with antibodies to the relevant toxin. After blotting, total protein was visualized by Coomassie brilliant blue as a loading control. In all experiments, only one quarter of the supernatant fraction was loaded onto the gel track. Molecular mass values (kDa) are indicated by arrows
Many bacterial toxins employ membrane glycolipids as their receptors for binding and subsequent intoxication of target cells (Sandvig and Van Deurs 2002; Ewers and Helenius 2011; Kulkarni et al. 2010), and for SEB digalactosylceramide has been identified as its cognate receptor in human kidney proximal tubule cells (Chatterjee et al. 1995). The domain of SEB involved in the binding was reported to be contained within amino acids 191–220, i.e., in or near the C-terminus of the toxin (Chatterjee et al. 2007). Endoglyceramidase II cleaves oligosaccharides from the ceramide moiety of glycolipids, and as shown in Fig. 6, digestion of microvillar vesicles with this enzyme prior to incubation with SEB markedly reduced the binding of toxin to the membranes. Likewise, binding of SEA was reduced by pretreatment with the endoglycosidase, albeit to a lesser extent. The main cell surface receptor for CTB is the ganglioside GM1 (Holmgren et al. 1973; Cuatrecasas 1973), and as shown in Fig. 6, this toxin also exhibited less binding after the pretreatment with the glycohydrolase.
Glycolipid-dependency of microvillus toxin binding. Prior to the toxin binding experiments, the microvillus membrane vesicles were incubated in the presence (+) or absence (−) of endoglycoceramidase II (EG) as described in “Materials and Methods”. In all experiments, only one quarter of the supernatant fractions was loaded onto the gel track. After immunoblotting with antibodies to the relevant toxin, total protein was visualized by Coomassie brilliant blue as a loading control. Molecular mass values (kDa) are indicated by arrows
CTB did not prevent binding of either SEA or SEB to microvillus membranes when added simultaneously, indicating that the toxins do not share receptors at the brush border (Fig. 7). In addition, binding was not affected by high salt conditions (1.5 M NaCl) for any of the three toxins, implying that adherence to the membrane is not simply mediated by weak ionic interactions (data not shown).
CTB does not compete with SEA or SEB in binding to microvillus membrane vesicles. The membranes were incubated with 1 μg of SEA or SEB either in the absence (−) or presence (+) of 1 μg of CTB as described in “Materials and Methods”. After binding the samples were analyzed as described in the legend to Fig. 6. Molecular mass values (kDa) are indicated by arrows
To summarize, the biochemical experiments performed confirm the ability of both SEA and SEB to bind to the enterocyte brush border membrane. In comparison with CTB they exhibited less efficient binding, in agreement with the scarce and patchy labeling observed by immuno microscopy. However, for neither of the toxins, the removal of microvillus glycolipids completely abolished binding, suggesting that other types of receptors may be present. For CTB, we have previously shown both by co-immunoprecipitation and by overlay experiments that sucrase-isomaltase, one of the major brush border enzymes (Sjostrom et al. 1983), may act as receptor as well (Hansen et al. 2005). Similar biochemical experiments were performed with SEA and SEB in the present work to detect potential protein receptors in the brush border for either of the two enterotoxins, but no likely candidates were revealed (results not shown). Nevertheless, binding to such receptors may occur with an affinity too low to be detected by the methods used.
DRM analysis of SEA and SEB
Mainly due to its high contents of glycolipids (Christiansen and Carlsen 1981) the porcine intestinal microvillus membrane to a high degree resists solubilization with ice-cold Triton X-100 and forms buoyant DRMs in a subsequent density gradient ultracentrifugation (Brown and Rose 1992; Braccia et al. 2003). Many of the major brush border enzymes are associated with DRMs, for instance the glycosylphosphatidylinositol-anchored alkaline phosphatase (AP) (Hansen et al. 2007), as shown in Fig. 8. By comparison, only a small fraction of both SEA and SEB partitioned in the DRM fractions, the major part appearing in the soluble fractions or in the pellet (Fig. 8). Since the glycolipids of the microvillus membrane are enriched in DRMs (Braccia et al. 2003), this result implies that any glycolipid receptor-toxin interaction is largely broken either by the detergent extraction or during the following overnight centrifugation. This is in contrast to CTB which in a similar experiment remained in the buoyant fractions (Hansen et al. 2005), but agrees well with the microscopic and other biochemical data presented above showing a relatively weaker avidity of SEA and SEB for the brush border epithelium. Alternatively, unlike glycolipids, other potential receptors for SEA and SEB may not resist the extraction with Triton X-100 and therefore partition in the two bottom fractions of the density gradient.
DRM analysis of SEA and SEB bound to microvillus membrane vesicles. After toxin binding to the membranes and extraction with ice-cold 1 % Triton X-100, the distribution of SEA and SEB in DRMs was analyzed by sucrose gradient centrifugation, followed by SDS/PAGE and immunoblotting of the gradient fractions, including the pellet (P). The distribution of alkaline phosphatase (AP), a endogeneous brush border marker, defined the DRM fractions of the gradient. Molecular mass values (kDa) are indicated by arrows
Discussion
SEA is the most common toxin associated with staphylococcal food poisoning and together with SEB the best characterized of the enterotoxins produced by S. aureus. The aim of the present study was to investigate the earliest stage of the process whereby SEA and SEB typically intoxicate the body: the penetration of the defensive barrier represented by the intact gut epithelium. This barrier comprises several features aimed to prevent pathogens from gaining access to the organism: First, a mucus coating up to a thickness of several hundred micrometer acts as an unstirred layer which hampers diffusion of macromolecules into close contact with the epithelium (Allen and Flemstrom 2005). Second, tight junctions between neighboring cells prevents macromolecular paracellular passage (Mrsny 2005), and third, “holes” in the epithelium left by apoptotic exfoliating cells are rapidly sealed (Watson et al. 2005). For transcellular passage, macromolecules need first to attach to the epithelial surface, and our fluorescence microscopy results showed that both SEA and SEB were capable of this, albeit in a scarce and patchy manner, in stark contrast to that achieved by CTB. To our knowledge, similar binding studies have not previously been performed, but the scarce attachment of SEs observed where sections of whole villi were often devoid of fluorescence labeling agrees well with a previous immunohistochemical localization of SEA in rat small intestine where the toxin was only detected in phagocytic cells in the lamina propria but not in the epithelial cells (Beery et al. 1984).
The low avidity towards the brush border membrane of the SEs relative to CTB was also indicated by the biochemical binding experiments, and may be reflected by the fact that the latter toxin belongs to the large group of pentameric AB5 toxins, whereas SEs are monomers. Even though pentameric toxins often have only a low affinity for glycolipids, they nevertheless exhibit high avidity because of the multivalency of their binding (Ewers and Helenius 2011). Digalactosylceramide has previously been identified as the cognate cell surface receptor for SEB in kidney proximal tubule cells (Chatterjee et al. 1995), and a dihexosylceramide containing galactose as the only carbohydrate is one of the major glycolipids of the porcine small intestinal brush border (Christiansen and Carlsen 1981). Therefore, the endoglycoceramidase-sensitivity of SEB, and to a lesser extent of SEA, for binding to enterocyte microvillus membranes suggests that this digalactosylceramide also acts as a SE-receptor in the gut. However, since the endoglycoceramidase cleaves a wide range of membrane glycolipids, including the receptor for CTB, ganglioside GM1, other glycolipids of the brush border might also be involved in SE binding as well. In fact, the high content of glycolipids of the porcine brush border membrane, about one-third of the total membrane lipid (Christiansen and Carlsen 1981), is the structural basis for the unique lipid raft organization of this membrane, which renders it resistant to the harsh environment of the gut lumen (Danielsen and Hansen 2008; Danielsen and Hansen 2006). Therefore, binding to any of the glycolipid components would be expected to cause a strong association with the DRM fraction of the membrane, as is the case for CTB (Hansen et al. 2005). Admittedly, the weak partition with DRMs observed (Fig. 8) does not readily agree with any glycolipid acting as receptor for the SEs. However, a plausible explanation for this apparent discrepancy may well be that the detergent extraction step of the protocol for preparing DRMs destroys the relatively weak binding between toxin and receptor. Finally, although we failed to detect SE interaction with any proteins of the microvillus membrane, such binding cannot be excluded.
Following binding to the brush border, internalization is the next step in the intoxication process. However, the enterocyte brush border membrane does not lend itself easily to endocytosis due to the microvillus architecture where formation of endocytic vesicles can only occur at the small membrane patches between neighboring microvilli (Hansen et al. 2003). A constitutive internalization of apical membrane by endocytosis nevertheless takes place into apical early endosomes (TWEEs), as demonstrated by uptake of the lipophilic FM dye (Fig. 2). Both SEA and SEB clearly appeared in subapical punctae corresponding to those visualized by FM dye, showing that they are indeed taken up by enterocytes via endocytosis. But as observed previously, membrane trafficking progressing from these endosomes further into the cytoplasm to connect with transcytotic and lysosomal pathways does not normally occur at any appreciable rate, probably because the dense terminal web acts as a physical diffusion barrier for membrane organelles (Hansen et al. 2009). Previous studies using different intestinal epithelial cell lines (Caco-2 and T-84) have reported somewhat diverging rates of transcytosis for SEA and SEB (Hamad et al. 1997; Shupp et al. 2002). Although our data would suggest that SEB attaches to the brush border more efficiently than SEA, the intracellular labeling patterns clearly demonstrated that both SEs managed to breach the barrier and penetrate deeper into cytoplasm, similarly to CTB. The localization of SEB by immunogold electron microscopy at or near the lateral cell surface suggests a transcytotic itinerary of events leading to the intercellular space between neighboring enterocytes.
In the case of CTB, endocytosis from the enterocyte brush border occurs by a clathrin-dependent mechanism, and in the presence of toxin this pathway is markedly increased above the constitutive rate (Hansen et al. 2005). Conceivably, a toxin-induced increase in the volume of apical endocytosis may be sufficient to overwhelm the terminal web barrier. Alternatively, the uptake of toxin may initiate a cytoskeletal rearrangement that permits/facilitates early endosomes to engage in membrane trafficking further into the cell in a way similar to that employed by other enterotoxins (Popoff 2011) and some viruses (Taylor et al. 2011). Hopefully, future studies will help to elucidate in greater detail the molecular mechanisms regulating this important early step in the intoxication process.
In conclusion, we found that in contrast to subunit B of cholera toxin (CTB), SEA and SEB attachment to the epithelial villus surface was only scarce and patchy. In agreement with this, SEs bound to microvillus membrane vesicles in vitro less efficiently than CTB, and unlike the latter toxin, they were largely absent from the detergent resistant membrane (DRM) fraction, indicating a weak association with lipid rafts in the brush border. However, the binding was sensitive to treatment with endoglycoceramidase II, suggesting that digalactosylceramide (or another microvillus glycolipid) may be a cell surface receptor for the SEs. Where attachment to the surface occurred, the SEs were also taken up by the enterocytes via endocytosis. Importantly, like CTB, they penetrated deep into the cytoplasm, indicating that they also have the capacity to push the endocytic membrane traffic through the terminal web barrier, thus allowing the SEs to reach the basolateral sides of the cell from where transcytosis subsequently occurs. A model depicting the proposed series of events whereby SEs penetrate the epithelial barrier is shown in Fig. 9.
A model depicting the proposed series of events used by SEs to penetrate the epithelial barrier. In the absence of toxin, a constitutive endocytosis occurs from the brush border to early endosomes in the terminal web region (TWEEs), possibly followed by recycling to the apical surface. During intoxication, luminal SEs attach to microvilli and move to the bottom of the “microcrypts” between neighboring microvilli from where endocytosis takes place. Once taken up by TWEEs, the SEs “highjack” the endosomes and force them across the terminal web into a transcytotic pathway which ultimately leads to exocytosis into the lamina propria
References
Allen A, Flemstrom G (2005) Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288:C1–C19
Beery JT, Taylor SL, Schlunz LR, Freed RC, Bergdoll MS (1984) Effects of staphylococcal enterotoxin A on the rat gastrointestinal tract. Infect Immun 44:234–240
Benjamin MA, Lu J, Donnelly G, Dureja P, McKay DM (1998) Changes in murine jejunal morphology evoked by the bacterial superantigen Staphylococcus aureus enterotoxin B are mediated by CD4+ T cells. Infect Immun 66:2193–2199
Bohach GA, Fast DJ, Nelson RD, Schlievert PM (1990) Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol 17:251–272
Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214:159–173
Booth AG, Kenny AJ (1974) A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 142:575–581
Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrom BT, Hansen GH, Danielsen EM (2003) Microvillar membrane Microdomains exist at physiological temperature—role of galectin-4 as lipid raft stabilizer revealed by “superrafts”. J Biol Chem 278:15679–15684
Brown DA, Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544
Chatterjee S, Jett M (1992) Glycosphingolipids: the putative receptor for Staphylococcus aureus enterotoxin-B in human kidney proximal tubular cells. Mol Cell Biochem 113:25–31
Chatterjee S, Khullar M, Shi WY (1995) Digalactosylceramide is the receptor for staphylococcal enterotoxin-B in human kidney proximal tubular cells. Glycobiology 5:327–333
Chatterjee S, Neill R, Shupp JW, Hammamieh R, Ionin B, Jett M (2007) Identification of staphylococcal enterotoxin B domains involved in binding to cultured human kidney proximal tubular cells: imparting proliferation and death. Exp Biol Med 232:1142–1151
Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J (1989) Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci USA 86:8941–8945
Christiansen K, Carlsen J (1981) Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim Biophys Acta 647:188–195
Cuatrecasas P (1973) Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry 12:3547–3558
Danielsen EM (1995) Involvement of detergent-insoluble complexes in the intracellular transport of intestinal brush border enzymes. Biochemistry 34:1596–1605
Danielsen EM, Hansen GH (2006) Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol 23:71–79
Danielsen EM, Hansen GH (2008) Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem 64:377–382
Danielsen EM, Sjostrom H, Noren O, Bro B, Dabelsteen E (1982) Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J 202:647–654
Ewers H, Helenius A (2011) Lipid-mediated endocytosis. Cold Spring Harb Perspect Biol 3:1–14
Hamad AR, Marrack P, Kappler JW (1997) Transcytosis of staphylococcal superantigen toxins. J Exp Med 185:1447–1454
Hansen GH, Sjostrom H, Noren O, Dabelsteen E (1987) Immunomicroscopic localization of aminopeptidase N in the pig enterocyte. Implications for the route of intracellular transport. Eur J Cell Biol 43:253–259
Hansen GH, Niels-Christiansen LL, Immerdal L, Hunziker W, Kenny AJ, Danielsen EM (1999) Transcytosis of immunoglobulin A in the mouse enterocyte occurs through glycolipid raft- and rab17-containing compartments. Gastroenterology 116:610–622
Hansen GH, Pedersen J, Niels-Christiansen LL, Immerdal L, Danielsen EM (2003) Deep-apical tubules: dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem J 373:125–132
Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM (2005) Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 44:873–882
Hansen GH, Niels-Christiansen LL, Immerdal L, Danielsen EM (2006) Antibodies in the small intestine: mucosal synthesis and deposition of anti-glycosyl IgA, IgM, and IgG in the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol 291:G82–G90
Hansen GH, Niels-Christiansen LL, Immerdal L, Nystrom BT, Danielsen EM (2007) Intestinal alkaline phosphatase: selective endocytosis from the enterocyte brush border during fat absorption. Am J Physiol Gastrointest Liver Physiol 293:G1325–G1332
Hansen GH, Rasmussen K, Niels-Christiansen LL, Danielsen EM (2009) Endocytic trafficking from the small intestinal brush border probed with FM dye. Am J Physiol Gastrointest Liver Physiol 297:G708–G715
Hennekinne JA, De Buyser ML, Dragacci S (2012) Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev 36:815–836
Holmgren J, Lonnroth I, Svennerholm L (1973) Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun 8:208–214
Kluytmans J, van BA, Verbrugh H (1997) Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520
Kraehenbuhl JP, Neutra MR (1992) Transepithelial transport and mucosal defence II: secretion of IgA. Trends Cell Biol 2:170–174
Kulkarni AA, Weiss AA, Iyer SS (2010) Glycan-based high-affinity ligands for toxins and pathogen receptors. Med Res Rev 30:327–393
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV (1999) Food-related illness and death in the United States. Emerg Infect Dis 5:607–625
Mooseker MS, Keller TC III, Hirokawa N (1983) Regulation of cytoskeletal structure and contractility in the brush border. Ciba Found Symp 95:195–215
Mostov KE (1994) Transepithelial transport of immunoglobulins. Annu Rev Immunol 12:63–84
Mrsny RJ (2005) Modification of epithelial tight junction integrity to enhance transmucosal absorption. Crit Rev Ther Drug Carrier Syst 22:331–418
Pinchuk IV, Beswick EJ, Reyes VE (2010) Staphylococcal enterotoxins. Toxins (Basel) 2:2177–2197
Popoff MR (2011) Multifaceted interactions of bacterial toxins with the gastrointestinal mucosa. Future Microbiol 6:763–797
Rodewald R, Kraehenbuhl JP (1984) Receptor-mediated transport of IgG. J Cell Biol 99:159s–164s
Sandvig K, Van Deurs B (2002) Membrane traffic exploited by protein toxins. Annu Rev Cell Dev Biol 18:1–24
Sheahan DG, Jervis HR, Takeuchi A, Sprinz H (1970) The effect of staphylococcal enterotoxin on the epithelial mucosubstances of the small intestine of rhesus monkeys. Am J Pathol 60:1–18
Shupp JW, Jett M, Pontzer CH (2002) Identification of a transcytosis epitope on staphylococcal enterotoxins. Infect Immun 70:2178–2186
Sjostrom H, Noren O, Danielsen EM, Skovbjerg H (1983) Structure of microvillar enzymes in different phases of their life cycles. Ciba Found Symp 95:50–72
Taylor MP, Koyuncu OO, Enquist LW (2011) Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol 9:427–439
Watson AJ, Chu S, Sieck L, Gerasimenko O, Bullen T, Campbell F, McKenna M, Rose T, Montrose MH (2005) Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology 129:902–912
Acknowledgments
Karina Rasmussen and Lise-Lotte Niels-Christiansen are thanked for excellent technical assistance. The work was supported by grants from Augustinus Fonden, Aase og Ejnar Danielsens Fond, Fonden til Lægevidenskabens Fremme, Brødrene Hartmanns Fond, and Hørslev Fonden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danielsen, E.M., Hansen, G.H. & Karlsdóttir, E. Staphylococcus aureus enterotoxins A− and B: binding to the enterocyte brush border and uptake by perturbation of the apical endocytic membrane traffic. Histochem Cell Biol 139, 513–524 (2013). https://doi.org/10.1007/s00418-012-1055-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-1055-8