Abstract
Immunocytochemistry is a powerful tool for detection and visualization of specific molecules in living or fixed cells, their localization and their relative abundance. One of the most commonly used fluorescent DNA dyes in immunocytochemistry applications is 4′,6-diamidino-2-phenylindole dihydrochloride, known as DAPI. DAPI binds strongly to DNA and is used extensively for visualizing cell nuclei. It is excited by UV light and emits characteristic blue fluorescence. Here, we report a phenomenon based on an apparent photoconversion of DAPI that results in detection of a DAPI signal using a standard filter set for detection of green emission due to blue excitation. When a sample stained with DAPI only was first imaged with the green filter set (FITC/GFP), only a weak cytoplasmic autofluorescence was observed. Next, we imaged the sample with a DAPI filter set, obtaining a strong nuclear DAPI signal as expected. Upon reimaging the same samples with a FITC/GFP filter set, robust nuclear fluorescence was observed. We conclude that excitation with UV results in a photoconversion of DAPI that leads to detection of DAPI due to excitation and emission in the FITC/GFP channel. This phenomenon can affect data interpretation and lead to false-positive results when used together with fluorochrome-labeled nuclear proteins detected with blue excitation and green emission. In order to avoid misinterpretations, extra precaution should be taken to prepare staining solutions with low DAPI concentration and DAPI (UV excitation) images should be acquired after all other higher wavelength images. Of various DNA dyes tested, Hoechst 33342 exhibited the lowest photoconversion while that for DAPI and Hoechst 33258 was much stronger. Different fixation methods did not substantially affect the strength of photoconversion. We also suggest avoiding the use of mounting medium with high glycerol concentrations since glycerol showed the strongest impact on photoconversion. This photoconversion effect cannot be avoided even when using narrow bandpass filter sets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunocytochemistry is a powerful tool in cell biology. It allows one to see the distribution and localization of biomolecules within a single cell. Obtaining results from immunocytochemistry is a multistep process. These steps are: preparation of the cells, fixing the cells, staining the cells, data acquisition and data analysis. Preparation of cells has been well established. Cell confluence and cell adherence prior to fixation have to be optimized according to the cell type and the purpose of the study. The most commonly used fixatives are methanol, ethanol, acetone (coagulants) and formalin or paraformaldehyde (crosslinking agents). Coagulants precipitate antigens and permeabilize the cell at the same time. They cause cellular shrinkage and destruction of most organelles within the cell, thus are not suitable for electron microscopy (Melan 1999). Formaldehyde and paraformaldehyde crosslink proteins by addition of various chemical groups (Melan 1999). Fixatives have a substantial effect on the cell morphology, immunoreactivity of antigens and stability of different molecular complexes. Therefore, an appropriate fixation procedure should be carefully chosen depending on the cell components being investigated (Suthipintawong et al. 1996; Skoog and Tani 2011). Fixation time is also among the many important parameters that should be taken into consideration when fixing cells. The duration of fixation should be long enough to complete the chemical process without over fixing the cells (Grizzle 2009; Oyama et al. 2007; Skoog and Tani 2011). The analytical criteria include the working pH, osmolarity, temperature and the solvents used (Bacallao et al. 2006). Detection of intracellular antigens requires a cell permeabilization step prior to staining to allow antibodies to enter the cell. This can be facilitated by a wide range of detergents, such as SDS or nonidet NP-40. Throughout these steps antigen availability and cell morphology is altered depending on treatment duration and the chemicals used. Therefore the duration, the reagents and the concentrations of the reagents have to be optimized for a particular set of antigens. Staining can be improved by blocking the non-specific binding of the antibody via bovine serum albumin, milk or serum. Antibody selection is the key for quality immunostaining. The primary antibody has to be specific and should have high affinity to its antigen. The secondary antibody should be stable and better yet cross-adsorbed for good species specificity (Fritschy 2008; Saper 2009).
If the specimen is prepared on a coverslip, mounting the coverslip onto a slide is an essential intermediary step before data acquisition. An ideal mounting medium should be optically clear and stable. It should have anti-fading ability, which should be preserved during storage. Finally, it should maintain fluorescence during the microscopic examination and also inhibit photobleaching. There are many commercially available mounting media and lab-made versions, which are mainly composed of glycerol, buffered glycerol with anti-fade, fructose syrup, Apathy’s gum-syrup, and polyvinylpyrrolidone (Longin et al. 1993; Ono et al. 2001). Among these media the most commonly used ones are glycerol or a specified glycerol–water (or PBS, Phosphate buffered saline) mixture. The glycerol–water or glycerol–PBS mixture can be adjusted to an appropriate refractive index. Adding more water/PBS to the mixture lowers the refractive index of the specimen (Diaspro et al. 2002; Barcellona et al. 1990).
4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) has been widely used in multicolor co-localization studies (Waggoner et al. 1996), for detecting DNA in various organisms (Kapuscinski 1995), in multicolor flow cytometry experiments (Douglas et al. 1998), for quantitative DNA analysis (Maciorowski et al. 1997) and for cell cycle analysis (Darzynkiewicz et al. 2008). DAPI can also be used to stain double stranded DNA in agarose or polyacrylamide gels (Buel and Schwartz 1993; Nairn et al. 1982). One of its advantages is that the cells do not need to be permeabilized so DAPI can be used to stain live or fixed cells. It migrates through the cell membrane less efficiently in live cells and therefore the higher DAPI concentrations are needed for live-cell staining (Zink et al. 2003).
Another group of widely used DNA probes are blue-fluorescent bisbenzimidazole derivatives: Hoechst 33258, Hoechst 33342 and Hoechst 34580. When bound to DNA molecules, Hoechst 33258 and Hoechst 33342 exhibit spectral properties very similar to those of DAPI (Latt and Stetten 1976; Otto and Tsou 1985). Because of their superior membrane permeability properties and lesser toxicity, they are often used instead of DAPI as live nuclear stains (Arndt-Jovin and Jovin 1977; Reeve and Kelly 1983). Hoechst dyes could also be used for all other previously described DAPI applications (Mocharla and Mocharla 1987; Brunk et al. 1979; Crissman and Steinkamp 1982; Latt et al. 1977).
There are known fluorophores in which the fluorescent and spectral properties can be altered due to specific conditions. Excitation and emission spectra of several fluorescent proteins can be altered by an exposure to a light of a specific wavelength (Lukyanov et al. 2005). In this manner, proteins can be changed irreversibly from a quiescent state to a fluorescent one—photoactivation (Patterson and Lippincott-Schwartz 2002), irreversibly to a state with a different fluorescence spectrum—photoconversion (Wiedenmann et al. 2004) and reversibly between two different fluorescence states—photoswitching (Ando et al. 2004). All these fluorescent proteins are used for highlighting individual molecules, organelles or cells and tracking them with live-cell microscopy techniques (Lukyanov et al. 2005).
Recently, Piterburg et al. 2012 showed photoconversion of DAPI. When exposing fixed cells stained with DAPI and mounted in different commercial mounting media to UV excitation for longer periods (2–4 min) they observed sensitivity to a blue/cyan excitation. Here we extend these observations and further investigate the role of glycerol on photoconversion of DAPI. We show that photoconversion of DAPI leads to a change in DAPI fluorescence characteristics and induces a robust fluorescence signal upon imaging in the FITC/GFP (fluorescein isothiocyanate/green fluorescent protein) channel. The intensity of the photo-converted signal depends on DAPI concentration, duration of DAPI excitation, cell fixation method and the composition of the mounting medium. We also noticed that photoconversion is present even when cells are stained with Hoechst 33258 or Hoechst 33342 though at different intensity. Our observations suggest that caution needs to be exerted when acquiring fluorescence images of nuclear proteins on samples counterstained with DAPI.
Materials and methods
Sample preparation
Human testicular embryonal carcinoma cells (NTERA-2, CRL-1973, American Tissue Culture Collection) were cultured on glass coverslips (Fisher, thickness no. 1) in DMEM with 10 % FBS. Cells were washed once in PBS and fixed in formalin-free fixative Accustain® (ACC, 20 min) (Sigma-Aldrich, USA), ice-cold methanol (MeOH, 10 min) or fresh 4 % paraformaldehyde (PFA, 10 min). After fixation, cells were washed with PBS and stained with DAPI or Hoechst 33258 or Hoechst 33342 for 5 min at room temperature in the dark. After staining, the samples were washed three times. Coverslips were mounted with appropriate glycerol concentration and sealed with nail polish. For staining nuclei, 1,000, 300 and 100 nM DAPI or 1,000 nM Hoechst 33258 or 1,000 nM Hoechst 33342 concentrations were used. Samples were then mounted in 100, 80, 50 % glycerol and PBS or in ProLong® Gold mounting medium (Invitrogen). Each experiment was repeated twice.
Image acquisition
Images were acquired with a 63× air objective in a specific order. After a phase contrast image, the cells were imaged first with the FITC/GFP filter set for 1,000 ms followed by DAPI excitation. The duration of DAPI excitation with UV light prior to taking the second FITC/GFP image was set for 10 s. The exposure of the second FITC/GFP image was again 1,000 ms. Images for experiments where we were determining the rate of DAPI photoconversion with FITC/GFP filter sets depending on the length of pre-illumination with DAPI light were acquired after 1, 2, 4, 8, 16, 32 and 64 s of excitation with UV. DAPI exposure times were adjusted depending on the dye concentrations and mounting media used. The series was repeated on two independent cell samples and three visual fields from each were analyzed. Images were acquired with Axio Observer.Z1 (Zeiss) fluorescence microscope equipped with DAPI filter set 49 (excitation 365 nm, emission 445/50 nm, beam splitter 395 nm) and GFP filter set 38HE eGFP (excitation 470/440 nm, emission 525/50 nm, beam splitter 495 nm) (Zeiss, detailed information at https://www.micro-shop.zeiss.com). Images were acquired using AxioCam MRm camera (Zeiss) and processed with AxioVision Rel. 4.7 software. A halogen lamp for phase contrast and a 100 W mercury arc lamp for fluorescence imaging were used.
Signal quantification and data analysis
Fluorescence intensity was quantified using ImageJ (http://rsbweb.nih.gov/ij/). Mean fluorescence intensity was determined by calculating the mean gray value within the selected image area. Three areas without cells were selected for mean background signal quantification. Average nuclear fluorescence intensity represents the mean of all nuclei analyzed corrected for background intensity. Data are represented as mean ± SD. Statistical t test was applied for normally distributed data or its nonparametric equivalent Sign test (related samples) or Mann–Whitney U test (independent samples) was applied otherwise. In order to verify assumptions of t test, a Kolmogorov–Smirnov test was applied to test normality of analyzed data. A selected risk level was α = 0.05.
Results
DAPI photoconversion
Excitation spectra for DAPI and FITC/GFP are discrete and they do not overlap. Their emission spectra, however, significantly overlap (from 490 to 600 nm). DAPI has excitation maximum at 370 nm and emission maximum at 470 nm; while GFP’s excitation maximum is at 490 and emission maximum at 525 nm. The overlap in the emission spectrum is normally not problematic; if the images are acquired sequentially starting with the “reddest” dye (Kapuscinski 1995).
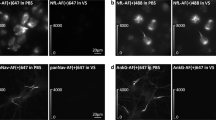
Pursuing an initial observation for the possibility of a DAPI photoconversion, we stained human testicular embryonal carcinoma cells (NTERA-2) with 1,000 nM DAPI. We tested DAPI stained cells for green fluorescence before and after DAPI image acquisition. As a negative control, DAPI-stained samples were first imaged with the FITC/GFP filter set for green fluorescence. As expected, we only observed minor cytoplasmic autofluorescence. Next, we imaged the sample with DAPI filter set, retrieving a strong nuclear DAPI signal as blue fluorescence. Subsequently, we acquired images of the same view field using a FITC/GFP filter set. Contrary to our previous observation without pre-DAPI imaging, we observed a robust nuclear signal (Fig. 1c). Only the field of view that was excited with DAPI filter set displayed green nuclear fluorescence (Fig. 1d). When the field of view was moved, no green fluorescence was observed in the neighboring cells that were not first exposed to DAPI filter set excitation (Fig. 1g), although cells were present throughout the viewing fields. Once we have confirmed that DAPI photoconversion could be detected with FITC/GFP filter set and that its intensity depends on the time of DAPI excitation, we examined other variables that could be affecting DAPI photoconversion.
DAPI photoconversion. Human testicular embryonal carcinoma cells (NTERA-2) stained with 1,000 nM DAPI and mounted in 100 % glycerol. a Phase contrast, b UV excitation with DAPI filter at 100 ms exposure and c is FITC/GFP filter with 1,000 ms exposure following prior excitation using DAPI filter for 10 s. c becomes d, where view field was shifted to the right and FITC/GFP image taken with 1,000 ms exposure time without first exposing this field to DAPI. e Phase contrast, f UV excitation with DAPI filter at 100 ms exposure and g FITC/GFP filter with 1,000 ms exposure following prior excitation using DAPI filter for 10 s. Scale bar 20 μm
The effect of DAPI excitation duration on DAPI emission in FITC/GFP
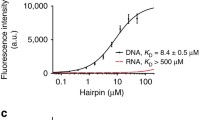
Next, we wanted to determine the effect of excitation time on DAPI photoconversion in samples stained with a standard 1,000 nM DAPI concentration. We tested 1, 2, 4, 8, 16, 32 and 64 s exposure times. An increase in nuclear fluorescence in the FITC/GFP channel was observed even after 1 s of DAPI excitation. Exposure times longer than 32 s did not further increase fluorescence intensity, indicating saturation of the system (Fig. 2b). Mean FITC/GFP fluorescence intensity of NTERA-2 cell nuclei enhanced with increasing DAPI excitation times and with increasing glycerol concentration in the mounting medium used (Fig. 2).
Time dependence of fluorescence intensity for FITC/GFP cube signal on the length of prior excitation with UV. a NTERA-2 nuclei stained with 1,000 nM DAPI and mounted in 100 % glycerol after 0, 1, 8 and 32 s of UV excitation. b Mean fluorescence intensity using FITC/GFP filter set of NTERA-2 nuclei corrected for background stained with 1,000 nM DAPI after 0–64 s of UV excitation in four different mounting solutions (50, 80, 100 % glycerol, and PBS). Scale bar 20 μm
Since relatively short DAPI exposure times resulted in a substantial amount of signal (Fig. 2), we set 10 s as the DAPI excitation before taking the second image with FITC/GFP filter set as the optimal excitation time for the following sets of experiments.
The effect of mounting media and DAPI concentration on DAPI photoconversion
Mounting media can cause optical aberrations so we next tested the effect of different glycerol concentrations in mounting media on DAPI photoconversion. Coverslips were mounted in 100, 90 (not shown), 80, 50 % glycerol and in PBS. Post-exposure mean GFP fluorescence intensities were significantly different from the mean intensity before DAPI excitation (p < 0.001) (Fig. 3). DAPI photoconversion was the strongest in 100 % glycerol. Briefly, the photoconversion was quite strong in 80 and 90 % glycerol (we did not see a noticeable difference between 80 and 90 %). The signal was still detectable in 50 % glycerol. When we used only PBS as mounting medium, the effect of DAPI photoconversion was very weak and barely noticeable at the set exposure time (Fig. 3).
Mean fluorescence intensity for FITC/GFP filter set of NTERA-2 cell nuclei at various DAPI concentrations or Hoechst 33258 and Hoechst 33342 in different mounting media. Mean fluorescence intensity measured following imaging with FITC/GFP filter set of NTERA-2 cell nuclei was corrected for background before and after 10 s of DAPI excitation for various nuclear stains (100, 300, 1,000 nM DAPI, and 1,000 nM Hoechst 33528 and Hoechst 33342) and mounting media with different glycerol (GLY) concentrations (PBS, 50, 80, 100 % GLY). For each BEFORE/AFTER pair all the differences were statistically significant (p < 0.001)
The most commonly used DAPI concentration is 300 nM (Life Technologies 2006). 100 nM DAPI concentration is not sufficient for imaging and longer exposure times are required to obtain better quality images. Therefore, we tested the effect of DAPI concentration on DAPI photoconversion. We compared three DAPI concentrations: 100, 300 and 1,000 nM and an unstained control sample. We found that the higher the DAPI concentration, the more pronounced the DAPI photoconversion (Fig. 4). At 100 nM DAPI, mean FITC/GFP fluorescence intensity in all glycerol-based mounting media was significantly different than the PBS mounting medium (p < 0.001). At 300 and 1,000 nM DAPI concentrations, mean intensities of cell nuclei in 50, 80 and 100 % glycerol-based media further increased (p < 0.001). DAPI photoconversion was most severe for the samples stained with 1,000 nM DAPI and mounted in 100 % glycerol, showing a synergy between DAPI and glycerol concentration. We did not observe any fluorescence in DAPI or FITC/GFP channel in our control unstained sample. Increased nuclear fluorescence of fine structures correlates with DAPI concentration.
Mean fluorescence intensity for FITC/GFP filter set of NTERA-2 cell nuclei at different glycerol concentrations. a Mean fluorescence intensity for FITC/GFP filter set of NTERA-2 cell nuclei was corrected for background after 10 s of UV excitation for various glycerol concentrations in mounting media (PBS, 50, 80, 100 % glycerol) and different DAPI concentrations (100, 300, and 1,000 nM). b The effect of DAPI and glycerol concentrations on photoconversion of DAPI. Detection of DAPI photoconversion increased with both DAPI and glycerol concentration. At each DAPI concentration, all differences in fluorescence intensity measurements between mounting media were statistically significant (p < 0.001). Scale bar 20 μm
Comparison of DNA binding dyes
Three DNA dyes were studied: DAPI, Hoechst 33258 and Hoechst 33342. DAPI exhibited the strongest photoconversion under described conditions. An alternative nuclear stain, Hoechst 33258 with alike fluorescence properties to DAPI, exhibited a similar pattern of photoconversion as DAPI, although the signal intensity was lower. The lowest degree of photoconversion was observed when nuclei were stained with Hoechst 33342. The photoconversion was minimal in mounting medium containing 80 % and less glycerol (Fig. 3).
The effect of fixative on DAPI photoconversion
Three fixatives (ACC, MeOH and PFA) were tested for their effect on DAPI photoconversion. The photoconversion was present regardless of the fixative used. The strongest photoconversion happened when cells were fixed with ACC. These cells also exhibited the strongest autofluorescence. Cells fixed with MeOH and PFA exhibited lower photoconversion. The differences in photoconversion in cells fixed with different fixatives were statistically significant (Fig. 5).
Effect of fixative on DAPI photoconversion. NTERA-2 cells were fixed with ACC, MeOH or 4 % PFA and stained with 1,000 nM DAPI and mounted in 100 % GLY. Mean fluorescence intensity for FITC/GFP filter set of NTERA-2 cell nuclei was corrected for background before and after 10 s of DAPI excitation for various fixatives. For each BEFORE/AFTER pair and between fixatives all the differences were statistically significant (p < 0.001). ACC Accustain® fixative, MeOH methanol, PFA paraformaldehyde, GLY glycerol
Photoconversion and ProLong® Gold mounting medium
Next, we wanted to see if we could observe DAPI photoconversion with other mounting media. We mounted unstained samples in commercially available ProLong® Gold (Invitrogen) that already contains DAPI and is also glycerol based. We could see DAPI photoconversion in nuclei and cytoplasm with ProLong® Gold (Fig. 6). In samples pre-stained with 300 nM DAPI and mounted in ProLong® Gold without DAPI, the photoconversion was comparable with that seen when slides were mounted in ProLong® Gold containing DAPI (data not shown).
Photoconversion in samples mounted in ProLong® Gold. NTERA-2 cells were mounted in ProLong® Gold with DAPI (Invitrogen). Images in FITC/GFP channel were taken with 1,000 ms exposure. Post-UV image was taken after 10 s of DAPI excitation with UV. Unstained sample mounted in ProLong® Gold without DAPI was used as a control. Scale bar 20 μm
We have also repeated all of these experiments using an Olympus IX81 microscope equipped with DAPI and FITC filter sets (SemRock, Brightline®). Then, the images were acquired using a Hamamatsu C4742-80-12AG CCD camera and processed using Slidebook®. In addition, we tested different cell lines, namely mouse embryonic fibroblasts and NCCIT (ATCC). DAPI photoconversion was successfully recapitulated in all these experiments (data not shown).
Discussion
The blue-fluorescent DAPI nucleic acid stain preferentially stains double stranded DNA and when bound its fluorescence increases by approximately 20-fold apparently due to the displacement of water molecules from both DAPI and the minor groove (Barcellona et al. 1990). DAPI also binds to RNA but this binding is not strongly fluorescent (Tanious et al. 1992). The DAPI/RNA complex exhibits a longer-wavelength fluorescence emission maximum than the DAPI/dsDNA complex (500 vs. 461 nm) and a quantum yield that is only about 20 % as high (Kapuscinski 1990). The mechanism of binding to DNA was not clear for a long time. It appears that DAPI associates preferably with AT clusters (Kubista et al. 1987). When it binds to dsDNA its absorption maximum is at a wavelength of 358 nm and its emission maximum is at 461 nm. DAPI blue color is convenient because it can be used in multicolor fluorescent experiments together with yellow, green and red fluorophores. For this it is important to use fluorescent labels with spectral characteristics that can be separated from one another. The emission peak of DAPI is rather broad and overlaps with FITC and FITC emission. DAPI and FITC or FITC-like dyes are often used together. Since they have discrete excitation spectra, it is assumed that when using typical fluorescein or GFP filter cubes, DAPI will not be observed.
Recently, DAPI photoconversion was detected in samples mounted with Eukitt quick-hardening mounting medium (Fluka 03989), Fluoromount-G (SouthernBiotech) and ProLong® Gold (Invitrogen) (Piterburg et al. 2012). Authors did not observe this phenomenon in live cells stained with Hoechst 33342, which is consistent with our results using PBS as a mounting medium for fixed cells.
Our data showed DAPI photoconversion in samples mounted with varying concentrations of glycerol and also with ProLong® Gold. With ProLong® Gold the effect is less robust and slightly different than what we have been observing with glycerol. With the ProLong® Gold, the signal observed after DAPI excitation was more diffused than what we observed for glycerol. We could see DAPI photoconversion in nuclei and cytoplasm with ProLong® Gold; whereas with glycerol the photoconversion was more pronounced in nuclei (Fig. 6). We also observed photoconversion with fluorescent dyes Hoechst 33258 and Hoechst 33342. Compared to the DAPI exposure times (minutes) used by Piterburg et al., the excitation times of DAPI used in our experiments were significantly shorter (seconds). These differences could be due to different microscope configurations or differences in excitation source and excitation path efficiencies. In both studies, a similar DAPI concentration dependency was observed for fluorescence detected in the FITC/GFP channel.
In this study, DAPI-stained cells were first imaged using the FITC/GFP filer set and displayed no fluorescence other than some expected cytoplasmic autofluorescence. Second, the same field of view was excited with DAPI filter set. Third the same field was again imaged using the FITC/GFP filter set, and now we observed a strong nuclear signal. From the spectral properties of DAPI we can see that DAPI (and also Hoechst 33258 and 33342) is a remarkable green emitter; but it needs to be excited in the UV-blue range (peak at 358 nm). The standard GFP excitation at 480–490 nm does not allow DAPI excitation and bleeding through the GFP channel would not be possible, if spectral properties of DAPI are unchanged. We have also ruled out the possibility of prolonged DAPI fluorescence, since we have not observed any residual fluorescence post-DAPI excitation, if we imaged sample using GFP/FITC filter set with closed excitation path (data not shown). Therefore, we suspect that the exposure to the UV light might be causing the photoconversion of DAPI, shifting the excitation spectra toward the red and resulting in the detection of fluorescence when using the GFP/FITC filter set. It is difficult to say whether any other molecules or products of photocatalytic reactions are involved in photochemical modification of DAPI.
The strength of DAPI photoconversion is dependent on DAPI concentration, mounting media used and DAPI excitation time. We concluded that mounting in glycerol strongly enhances the photoconversion. The higher the glycerol concentration, the more pronounced is the apparent DAPI photoconversion. Glycerol enhanced the brightness of converted DAPI in GFP channel approximately 10 times stronger than if PBS was used as the mounting medium. We used different fixation methods and different reagents and yet the photoconversion was still observed. We were also able to observe the photoconversion in cells that were not fixed (data not shown).
Mean fluorescence intensity for FITC/GFP filter set post-exposure to UV light was the strongest when cells were fixed with ACC, and lower when cells were fixed in MeOH and PFA (Fig. 5). It has been shown that binding of Hoechst or DAPI to DNA is changed as a consequence of changed DNA structure after various fixation methods (Stokke and Steen 1985; Srinivasan et al. 2002).
The role of glycerol in this photoconversion is unclear, but may be related to the reduction in the activity of water in solutions with increasing glycerol concentrations. By reducing the hydration shell of the molecules, the equilibrium between the two binding modes of DAPI to DNA may change (Barcellona et al. 1990; Manzini et al. 1983). This can possibly affect the intensity of the photoconversion and cause the stronger effect in mounting medium with higher glycerol concentrations.
The possibility of DAPI photoconversion must be taken into account by anyone using DAPI together with another FITC-like dye or GFP. Widely recommended DAPI concentration for counterstaining nuclei is 300 nM (Life Technologies 2006). Most commonly used homemade mounting media consist of 80 % glycerol (100 % glycerol is rarely used due to high viscosity). If this or even higher concentrations of DAPI are used, the described effect could be strong enough to be mistaken for the fluorescence of studied nuclear protein stained with green fluorescence-conjugated antibody (FITC, Alexa488) or expressing GFP. Automated microscopy followed by computerized image processing together with data evaluation has become an important screening instrument in various experiments (Zeder and Pernthaler 2009). Since the DAPI fluorescence is often used as a tool to focus on cells and to find appropriate area for imaging, the photoconversion effect can present a serious problem reporting false-positive results. Suggestions to avoid this phenomenon would be to take extra precaution while preparing DAPI solution in the right concentration and to scan/capture the other channels first and DAPI last. Also DAPI can be substituted with Hoechst 33342 since this dye exhibited very low photoconversion. It is also important to choose an appropriate mounting medium. Alternatively, when nuclear antigens are the target for detection, counterstaining could be performed using a cytoskeletal protein such as F-actin using fluorescently conjugated molecules such as phalloidin.
Bleed-through of fluorescence emission complicates the interpretation of the acquired image and can lead to false results particularly in cases of co-localization studies and fluorescence quantification. Use of alternative DNA dyes, such as DRAQ5 (Martin et al. 2005) can alleviate the problem. DRAQ5 is a far-red fluorescent DNA dye (excitation/emission 647/670). It is a good choice for nuclear staining of fixed and live cells. DRAQ5 is useful for most bench-top, wide-field and confocal systems and due to its far-red emission it is spectrally ideally compatible with FITC-based fluorochromes.
References
Ando R, Mizuno H, Miyawaki A (2004) Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science 306(5700):1370–1373. doi:10.1126/science.1102506
Arndt-Jovin DJ, Jovin TM (1977) Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem 25(7):585–589. doi:10.1177/25.7.70450
Bacallao R, Sohrab S, Phillips C (2006) Handbook of biological confocal microscopy, 3rd edn. Springer, Berlin
Barcellona ML, Cardiel G, Gratton E (1990) Time-resolved fluorescence of DAPI in solution and bound to polydeoxynucleotides. Biochem Biophys Res Commun 170(1):270–280
Brunk CF, Jones KC, James TW (1979) Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem 92(2):497–500
Buel E, Schwartz M (1993) DAPI, a simple sensitive alternative to ethidium bromide staining of DNA in agarose gels. Appl Theor Electrophor 3(5):253–255
Crissman HA, Steinkamp JA (1982) Rapid, one step staining procedures for analysis of cellular DNA and protein by single and dual laser flow cytometry. Cytometry 3(2):84–90
Darzynkiewicz Z, Galkowski D, Zhao H (2008) Analysis of apoptosis by cytometry using TUNEL assay. Methods 44(3):250–254. doi:10.1016/j.ymeth.2007.11.008
Diaspro A, Federici F, Robello M (2002) Influence of refractive-index mismatch in high-resolution three-dimensional confocal microscopy. Appl Opt 41(4):685–690
Douglas RS, Pletcher CH Jr, Nowell PC, Moore JS (1998) Novel approach for simultaneous evaluation of cell phenotype, apoptosis, and cell cycle using multiparameter flow cytometry. Cytometry 32(1):57–65
Fritschy JM (2008) Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur J Neurosci 28(12):2365–2370. doi:10.1111/j.1460-9568.2008.06552.x
Grizzle WE (2009) Special symposium: fixation and tissue processing models. Biotech Histochem 84(5):185–193. doi:10.3109/10520290903039052
Kapuscinski J (1990) Interactions of nucleic acids with fluorescent dyes: spectral properties of condensed complexes. J Histochem Cytochem 38(9):1323–1329
Kapuscinski J (1995) DAPI: a DNA-specific fluorescent probe. Biotech Histochem 70(5):220–233
Kubista M, Akerman B, Norden B (1987) Characterization of interaction between DNA and 4′,6-diamidino-2-phenylindole by optical spectroscopy. Biochemistry 26(14):4545–4553
Latt SA, Stetten G (1976) Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem 24(1):24–33. doi:10.1177/24.1.943439
Latt SA, George YS, Gray JW (1977) Flow cytometric analysis of bromodeoxyuridine-substituted cells stained with 33258 Hoechst. J Histochem Cytochem 25(7):927–934
Life Technologies (2006) DAPI Counterstaining Protocols. http://www.invitrogen.com/site/us/en/home/References/protocols/cell-and-tissue-analysis/dapi-protocol/Basic-DAPI-Counterstaining-Protocols.html
Longin A, Souchier C, Ffrench M, Bryon PA (1993) Comparison of anti-fading agents used in fluorescence microscopy: image analysis and laser confocal microscopy study. J Histochem Cytochem 41(12):1833–1840
Lukyanov KA, Chudakov DM, Lukyanov S, Verkhusha VV (2005) Innovation: photoactivatable fluorescent proteins. Nat Rev Mol Cell Biol 6(11):885–891. doi:10.1038/nrm1741
Maciorowski Z, Veilleux C, Gibaud A, Bourgeois CA, Klijanienko J, Boenders J, Vielh P (1997) Comparison of fixation procedures for fluorescent quantitation of DNA content using image cytometry. Cytometry 28(2):123–129
Manzini G, Barcellona ML, Avitabile M, Quadrifoglio F (1983) Interaction of diamidino-2-phenylindole (DAPI) with natural and synthetic nucleic acids. Nucleic Acids Res 11(24):8861–8876
Martin RM, Leonhardt H, Cardoso MC (2005) DNA labeling in living cells. Cytometry A 67(1):45–52. doi:10.1002/cyto.a.20172
Melan MA (1999) Overview of cell fixatives and cell membrane permeants. Methods Mol Biol 115:45–55
Mocharla R, Mocharla H (1987) A novel, sensitive fluorometric staining technique for the detection of DNA in RNA preparations. Nucleic Acids Res 15(24):46223
Nairn RS, Dodson ML, Humphrey RM (1982) Comparison of ethidium bromide and 4′,6-diamidino-2-phenylindole as quantitative fluorescent stains for DNA in agarose gels. J Biochem Biophys Methods 6(2):95–103
Ono M, Murakami T, Kudo A, Isshiki M, Sawada H, Segawa A (2001) Quantitative comparison of anti-fading mounting media for confocal laser scanning microscopy. J Histochem Cytochem 49(3):305–312
Otto F, Tsou KC (1985) A comparative study of DAPI, DIPI, and Hoechst 33258 and 33342 as chromosomal DNA stains. Stain Technol 60(1):7–11
Oyama T, Ishikawa Y, Hayashi M, Arihiro K, Horiguchi J (2007) The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. Breast Cancer 14(2):182–188
Patterson GH, Lippincott-Schwartz J (2002) A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297(5588):1873–1877. doi:10.1126/science.1074952
Piterburg M, Panet H, Weiss A (2012) Photoconversion of DAPI following UV or violet excitation can cause DAPI to fluoresce with blue or cyan excitation. J Microsc 246(1):89–95. doi:10.1111/j.1365-2818.2011.03591.x
Reeve WJ, Kelly FP (1983) Nuclear position in the cells of the mouse early embryo. J Embryol Exp Morphol 75:117–139
Saper CB (2009) A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem 57(1):1–5. doi:10.1369/jhc.2008.952770
Skoog L, Tani E (2011) Immunocytochemistry: an indispensable technique in routine cytology. Cytopathology 22(4):215–229. doi:10.1111/j.1365-2303.2011.00887.x
Srinivasan M, Sedmak D, Jewell S (2002) Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol 161(6):1961–1971
Stokke T, Steen HB (1985) Multiple binding modes for Hoechst 33258 to DNA. J Histochem Cytochem 33(4):333–338
Suthipintawong C, Leong AS, Vinyuvat S (1996) Immunostaining of cell preparations: a comparative evaluation of common fixatives and protocols. Diagn Cytopathol 15(2):167–174
Tanious FA, Veal JM, Buczak H, Ratmeyer LS, Wilson WD (1992) DAPI (4′,6-diamidino-2-phenylindole) binds differently to DNA and RNA: minor-groove binding at AT sites and intercalation at AU sites. Biochemistry 31(12):3103–3112
Waggoner A, Taylor L, Seadler A, Dunlay T (1996) Multiparameter fluorescence imaging microscopy: reagents and instruments. Hum Pathol 27(5):494–502
Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, Spindler KD, Nienhaus GU (2004) EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc Natl Acad Sci USA 101(45):15905–15910. doi:10.1073/pnas.0403668101
Zeder M, Pernthaler J (2009) Multispot live-image autofocusing for high-throughput microscopy of fluorescently stained bacteria. Cytometry A 75(9):781–788
Zink D, Sadoni N, Stelzer E (2003) Visualizing chromatin and chromosomes in living cells. Methods 29(1):42–50
Acknowledgments
This research was financially supported by the Slovenian Research Agency: research grant P-0371 and in part by the united States Army Medical Research Materiel Command (USAMRMC) Grant10782106, and Worcester Polytechnic Institute. We thank Klemen Ziberna for all the help with the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Jež and T. Bas contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jež, M., Bas, T., Veber, M. et al. The hazards of DAPI photoconversion: effects of dye, mounting media and fixative, and how to minimize the problem. Histochem Cell Biol 139, 195–204 (2013). https://doi.org/10.1007/s00418-012-1039-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-1039-8