Abstract
Adult human jaw muscles differ from limb and trunk muscles in enzyme-histochemical fibre type composition. Recently, we showed that the human masseter and biceps differ in fibre type pattern already at childhood. The present study explored the myosin heavy-chain (MyHC) expression in the young masseter and biceps muscles by means of gel electrophoresis (GE) and immuno-histochemical (IHC) techniques. Plasticity in MyHC expression during life was evaluated by comparing the results with the previously reported data for adult muscles. In young masseter, GE identified MyHC-I, MyHC-IIa MyHC-IIx and small proportions of MyHC-fetal and MyHC-α cardiac. Western blots confirmed the presence of MyHC-I, MyHC-IIa and MyHC-IIx. IHC revealed in the masseter six isomyosins, MyHC-I, MyHC-IIa, MyHC-IIx, MyHC-fetal, MyHC α-cardiac and a previously not reported isoform, termed MyHC-IIx′. The majority of the masseter fibres co-expressed two to four isoforms. In the young biceps, both GE and IHC identified MyHC-I, MyHC-IIa and MyHC-IIx. MyHC-I predominated in both muscles. Young masseter showed more slow and less-fast and fetal MyHC than the adult and elderly masseter. These results provide evidence that the young masseter muscle is unique in MyHC composition, expressing MyHC-α cardiac and MyHC-fetal isoforms as well as hitherto unrecognized potential spliced isoforms of MyHC-fetal and MyHC-IIx. Differences in masseter MyHC expression between young adult and elderly suggest a shift from childhood to adulthood towards more fast contractile properties. Differences between masseter and biceps are proposed to reflect diverse evolutionary and developmental origins and confirm that the masseter and biceps present separate allotypes of muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human body contains more than 600 skeletal muscles, all with the same basic contractile function based on the interaction of the thick and thin myofilaments composed mainly of myosin and actin (Engel and Franzini-Armstrong 2004). In the past, muscles were classified as red and white, which correlated to their speed of contraction and fatiguability. Later, individual muscle fibre types were distinguished and could be correlated to different functional properties such as speed of contraction, twitch characteristics and different metabolic characteristics (Pette and Staron 2000; Schiaffino 2010). Several schemes for classification have been proposed. For human muscle, the most common one is based on the myofibrillar ATPase (mATPase) activity that reflects the myosin heavy-chain (MyHC) composition of the myofibrils and gives information of slow-twitch (type I) fibres and fast-twitch (type II) fibres, which can be further subdivided into IIA and IIB fibres (Dubowitz 2007). The mATPase reflects the enzyme activity of the MyHC molecule, which exists as several isoforms. In human limb skeletal muscles, three major MyHCs are expressed: slow (I), fast IIa and fast IIx (Schiaffino 2010). In the last decade, all genes in the human genome have been characterized and an evolutionary tree has been created which shows that in human muscles 14 genes are linked to the expression of the different MyHC isoforms and have temporarily over the years evolved for specific purposes (Berg et al. 2001; Desjardins et al. 2002; Stedman et al. 2004; Weiss et al. 1999). Hoh (2002) introduced the term allotype for muscles that were developmentally, structurally and functionally distinct. The first group of muscles described to have an allotype distinct from limb and trunk muscles was the jaw muscles (Hoh 2002).
Ringqvist (1973) was the first to observe that the human masseter muscle contained fibre types that differed from those in human limb muscles by being intermediate in myosin ATPase activity at pH 9.4 to that of type I and type II fibres, type ATPase IM fibres. Moreover, human masseter fibres are typically smaller in diameter compared with limb and trunk muscle fibres, especially the type IIB fibres (Eriksson 1982; Eriksson and Thornell 1983; Monemi et al. 1998). Later it was shown that type ATPase IM fibres contain both slow and fast MyHC isoforms (Eriksson 1982; Thornell et al. 1984) and in addition isoforms characteristic of developing muscles (Butler-Browne et al. 1988; Soussi-Yanicostas et al. 1990), as well as MyHC-α cardiac (Bredman et al. 1991; Pedrosa-Domellöf et al. 1992). Thus, human masseter isomyosin expression is very different from that of limb muscles.
In our laboratory, we have over the years studied the masseter muscle also during fetal development and ageing in comparison with a limb muscle, the biceps, and shown that the masseter muscle indeed has unique properties during the life span that differs from that of limb muscles (Monemi et al. 1999; Stål et al. 1994). Recently, we also showed marked differences in the mATPase fibre type composition between the human masseter in childhood, implying age-related changes in contractile properties in parallel with skeletal growth, teeth eruption and improvement of jaw-motor skills (Österlund et al. 2011). To explore in further detail how the muscle fibre types of the human masseter in childhood are related to isomyosin expression, we have evaluated whole muscle sample extracts and individual fibres of the young masseter and biceps for their content and distribution of MyHC isoforms. These findings will be put into the perspective of the evolution of the MyHC genes (MYH).
Materials and methods
Muscle specimens
The muscle specimens used in this study were from the anterior (sup ant) and posterior (sup post) regions of the superficial portion, and the deep portion (deep) of the human masseter muscle of seven previously healthy subjects, four males between 3 and 6 years (3, 4, 4 and 6 years), and three females between 3 and 7 years (3, 7 and 7 years), all with normal bite and jaw–face morphology. One specimen was obtained from the short head of the biceps brachii muscle (biceps) from each subject. For biochemistry analyses, samples were missing from biceps of 4-year-old male and 7-year-old female, from sup ant of 3-year-old female and 4-year-old male and from sup post of 3-year-old female. For immuno-histochemical (IHC) analyses, samples were missing for biceps 7-year-old female and for sup ant 4-year-old male. The samples were collected prior to 1990 according to prevailing directions issued by the National Board of Health and Welfare Stockholm, Sweden. Details on myosin ATPase enzyme-histochemical, fibre-type classification and results have been reported in our previous study (Österlund et al. 2011).
Gel electrophoresis (GE)
Muscle extracts were prepared from frozen muscle cross-sections of samples from the sup ant (n = 5), sup post (n = 6) and deep (n = 7) masseter regions and from the biceps brachii muscle (n = 5), and as reference muscle extract from one adult subject. Crushed frozen muscle samples were extracted on ice in Guba–Straub buffer solution (0.3 M NaCl, NaH2PO4·H2O 0.1 M, 0.05 M 1 Mm Na2HPO4, 1 mM MgCl2·6H2O, 10 mM Na4P2O7.10H2O and 10 mM EDTA.2H20 10 Mm, pH 6.7) for 1 h and centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatants were mixed with glycerol (1:2) and stored at −20 °C until further use. Protein concentration for each sample was determined using the Bio-Rad Protein Assay (Bio-Rad). MyHC isoform composition was determined by one dimension sodium dodecyl sulphate–polyacrylamide gel electrophores (SDS-PAGE) using the mini PROTEAN3 Cell GE system (Bio-Rad). Two 8 % acrylamide:bis (50:1) mini-gels (Talmadge and Roy 1993) were polymerized for >2 h at room temperature the same day as the run. Immediately before gel loading, samples diluted in Laemmeli sample buffer (Bio-Rad) to a final concentration of 0.05 μg/μl were heated at 90 °C for 3 min. The gels were loaded with either 2.0 μl or ≥3.0 μl of the muscle extract. The gels were run at a constant voltage (70 V) and temperature (7 °C) for 24 h and one gel was stained with SYPRO® Ruby protein gel stain (Bio-Rad) according to the manufacturer’s instructions (amount of protein per sample run on the gel, 0.10 μg) while the other gel was silver stained (amount of protein per sample run on the gel, 0.15 μg). The image from the SYPRO® Ruby stained gel was recorded by a Typhoon 9400 scanner (Amersham Biosciences) and subsequently used to determine the relative content of the corresponding MyHC isoforms using the image analysis software, ImageQuant TL (Amersham Biosciences). The densitometric quantification of MyHC isoforms was performed in the 0.10 μg loaded GE. For each subject and muscle portion the quantification was based on the mean of three repeated measurements. For the group, mean (%) and SD were calculated for the sup ant, sup post and deep portions of the masseter, and for biceps.
The presence of MyHC-fetal was determined using Western blot analysis and the monoclonal antibody NCL-MHCn (Novocastra Laboratories Ltd). This analyse included muscle specimens from fetal masseter and biceps brachii (22 weeks), perinatal masseter from sup ant, sup post and deep portions (3 months), young masseter from sup ant, sup post and deep portions (4 and 7 years), adult masseter from sup ant, post and deep portions (age 22 years), adult biceps (18 years) and elderly deep masseter (83 years). These gels were loaded with 0.4 μg protein/sample. Protein transfer to a nitrocellulose membrane (Bio-Rad, cat nr162-0090) was performed for 17 h at 14 °C in Towbin transfer buffer containing 10 % methanol using the Mini-Trans-Blot® electrophoretic transfer cell (Bio-Rad). Bound anti-MHCn was visualised using the Western Breeze® chromogenic Kit (Invitrogen, Cat No. WB7103).
Immuno-histochemistry
Cross-sections, 7-μm thick, were used for IHC with indirect peroxidase-anti-peroxidase (PAP) technique (Sternberger 1979) to visualize bound monoclonal antibodies (mAbs), each recognizing distinct MyHC. The antibodies used and their positive reaction against different MyHC isoforms is seen in Table 1.
Details
Cross-sections, 7 μm, serial to those used for myosin ATPase enzyme-histochemical fibre typing, were mounted on glass slides, air dried, and incubated with 5 % normal rabbit serum for 15 min to inhibit non-specific staining. All sections were incubated with primary MyHC mAbs and simultaneously treated with primary laminin mAb, a major non-collagenous component of the basement membrane, to improve the visualization of the cell border and diluted to appropriate concentrations with phosphate-buffered saline (PBS) containing 0.1 % bovine serum albumin (BSA) at +4 to +8 °C over the night. Double labelling for both MyHC and laminin was achieved by incubating the sections with the two different primary mAbs. After washing in 0.01 M PBS for 15 min, sections were again incubated with 5 % normal rabbit serum for 15 min. All sections were then incubated with rabbit-anti mouse antibodies (RAM) diluted to appropriate concentrations with 0.01 M PBS containing 0.1 % BSA for 30 min in room temperature. After washing in 0.01 M PBS for 15 min, all sections were incubated with mouse PAP diluted to appropriate concentration with 0.01 M PBS containing 0.1 % BSA for 30 min in room temperature. After repeated washes, the peroxide binding was revealed by applying a solution containing 3,3′-diaminobenzidine and hydrogen peroxide (H2O2) for 10 min. Finally, the sections were rinsed with running water for 5 min, dehydrated in graded concentrations of ethanol, followed by xylene treatment and mounted with DPX.
Morphometric analysis
The serial cross-sections were viewed under a light microscope (Leica DMR), equipped with a camera (Leica DC 220) connected with an image-analyses system (Leica QWin). In the masseter, two muscle samples from each of the sup ant, sup post and deep portions, respectively, were analysed. Eight random fields were analysed in each sample. In the biceps brachii, one muscle sample was analysed in eight random fields. Analysis of the antibody staining of individual fibres was performed in all serial sections. Incomplete sections were excluded from the analyses. The complete analyses embraced for the masseter 4,853 fibres of mATPase enzyme-histochemical fibres types that included type I (n = 2,751), type IM (n = 741), type IIC (n = 211), type IIAB (n = 417) and type IIB (n = 733). The corresponding number in the biceps was 4,558 fibres of mATPase enzyme-histochemical fibres type that included type I (n = 2,680), type IIA (n = 1,190), type IIAB (n = 636) and type IIB (n = 52). The fibre populations were equivalent with those in a previous report (Österlund et al. 2011). Due to the low frequency of the type IIA fibres in the masseter (n = 3), and types IM and IIC fibres in the biceps brachii (n = 5 and n = 0, respectively), these fibres were excluded from the analysis. Individual fibres expressing one MyHC isoform were defined as single, and fibres expressing two or more MyHC isoforms were defined as combinations or mixed fibres.
Statistical methods
Mean and standard deviation were calculated for descriptive statistics. Paired t tests were used to test the null hypothesis (H0) of no difference in proportion and relative content of various MyHCs between masseter regions and between masseter and biceps brachii muscles. The H0 was rejected at the level of significance p ≤ 0.05. The relationship between the mean values from GE and IHC analyses was described with correlation coefficient (Pearson) (r). Comparison between present results for young masseter, and previous data for adult and elderly (Monemi et al. 1999) masseter of relative content (%) of different MyHC isoforms in the SDS-PAGE gels was presented as 95 % confidence intervals.
Results
Gel electrophoresis (GE)
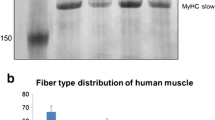
For both the young masseter and young biceps, the GE results showed an apparent inter-individual variability in density of the different MyHC bands. In both muscles, three bands were identified in the gels loaded with 0.10-μg muscle extract as MyHC-I, MyHC-IIa and MyHC-IIx (Table 2), when compared with data from the previous studies using the same GE technique (Liu et al. 2002). The MyHC-I band, with the highest mobility, was the most prominent in both muscles. It was larger in the sup ant masseter than in the sup post masseter (p = 0.032). In the young masseter, a faint band with somewhat lower mobility than that of the MyHC-I band was detected, in gels loaded with 15 μl, in subjects number 3, 5 and 6 in all regions, and in sup ant and sup post in adult samples (Fig. 1), although not resolved as separate bands by densitometry. This band reflected the presence of MyHC-α cardiac (Liu et al. 2002). No band representing MyHC-fetal was detected but this isoform is expected, since this developmental MyHC isoform is known to be present in the masseter of adults and elderly (Monemi et al. 1999). Therefore, the GE analysis was extended to include gels loaded with >3.0 μl muscle extract to allow low content isoforms to appear. The >3.0 μl gels revealed more details for the low-mobility bands (Fig. 2). In samples number 2 and 6, there were three low-mobility bands including a band in between the MyHC-IIa and the MyHC-IIx bands. This band corresponded to MyHC-fetal (Liu et al. 2002). In sample number 1, a faint band was seen with lower mobility than that of the MyHC-IIx, corresponding to MyHC-embryonic (Liu et al. 2002). To confirm the presence of MyHC-fetal, a Western blot analysis using the mAb MHCn was performed (Fig. 3). In this analysis, we included muscle samples from fetal masseter and biceps muscles (22 weeks) and from perinatal masseter (3 months), and also from adult masseter (22 years) and biceps (18 years) and elderly masseter (83 years). In the fetal samples, the result showed one band approximately at the level of MyHC-IIa. Notably, two bands were detected in the masseter samples aged 3 months and 4 and 7 years. One band appeared at the level of the band in the fetal samples. This band was seen also in the elderly masseter. The “extra” band was located in between the MyHC-IIa and MyHC-IIx bands. This “extra” band was also seen in the adult masseter (sup ant). No fetal band was seen in the adult biceps samples.
SDS-PAGE of the biceps and the deep, sup ant and sup post portions of the masseter loaded with 0.15 μg muscle extract. Samples from seven subjects (1–7) aged 3–7 years and two adult subjects aged 18 years (biceps) and 22 years (masseter). In both muscles, three bands correspond to MyHC-I, MyHC-IIa and MyHC-IIx (arrows). In the masseter (subjects 3, 5, 6 and 7 and adult), faint bands with somewhat lower mobility than the MyHC-I band corresponds to MyHC-α cardiac (arrow). Note marked inter-individual variability
SDS-PAGE of the deep masseter loaded with >3.0 μl muscle extract. Samples from seven subjects (1–7) aged 3–7 years and one adult subject aged 18 years. In the gel from the deep masseter, the band seen in between the MyHC-IIa and MyHC-IIx bands in subject number 2 and 6 corresponds to MyHC-fetal (arrows). In subject 1, a faint band corresponds to MyHC-embryonic (arrow). Note marked inter-individual variability
Western blots using the mAb MHCn. Samples from fetal masseter and biceps muscles (22 weeks), from the sup ant, sup post and deep portions of perinatal (3 months), young (4 and 7 years) and adult (22 years) masseter, elderly deep masseter (83 years) and adult biceps (18 years). In the fetal masseter and biceps and the elderly masseter, one band is seen (arrows). In the young and perinatal masseter, two bands are seen. One is located at the level of the band in the fetal samples (arrows). The “extra” band is located in between the MyHC-IIa and MyHC-IIx bands (arrow heads), present also in the adult sup ant masseter (arrow head)
Immuno-histochemistry
Figure 4 shows the staining pattern with the different mAbs of mATPase fibre types in the young masseter and biceps muscles. In the biceps, the mAbs A4.840 and A4.951 stained the type I fibres weakly to strongly. The mAb N2.261 stained the type IIA fibres moderately to strongly, type IIAB generally strongly and left half of the type IIB fibres unstained. The A4.74 and SC71 mAbs generally stained types IIA and IIAB fibres strongly and type IIB fibres weakly. The mAb BF35 stained the types I, IIA and IIAB moderately and left type IIB unstained. No young biceps fibres were stained with mAbs MHCn, F88, F1.652 and ALD19. In the masseter there was a marked variability in staining with all antibodies and of all fibre types (Fig. 4). The mAbs A4.840 and A4.951 stained all types I and IM fibres, less than half of the type IIC fibres, and occasionally, type IIAB and IIB fibres. The mAb N2.261 stained the type I fibres weakly, types IM, IIC and IIAB fibres moderately to strongly but generally left type IIB unstained. The mAb A4.74 stained the types IM, IIC, IIAB and IIB and a few type I. The mAb SC71 stained types IIC, IIAB and IIB and half of the type IM. The mAb BF35 stained all fibre types except half of the type IIB fibres. All fibre types, but preferentially types IM and IIC were stained with mAbs MHCn and F88. No fibres were stained with the mAbs ALD19 and F1.652.
Fibres were judged to contain MyHC-I when stained with mAbs A4.840, A4.951; MyHC-IIa when they were moderately to strongly stained with mAb N2.261 and weakly to strongly stained with mAbs A4.74, SC71 and BF35; MyHC-IIx when they were unstained with mAb BF 35 and weakly to strongly stained with mAbs A4.74 and SC71. Fibres that were unstained with mAb N2.261 and weakly to strongly stained with mAbs A4.74, SC71 and BF 35 were judged to contain a separate MyHC isoform, tentatively termed MyHC-IIx′. Both MyHC-fetal and MyHC-α cardiac were all discriminated by their specific antibodies, MHCn and F88, respectively.
Figure 5 shows examples of antibody stainings and detected MyHC isoforms in young biceps and masseter fibres. Table 3 shows group values of the proportion of different MyHC isoforms, as evaluated from the antibody stainings, in the young masseter and biceps-fibre populations. Figure 6 is based on pooled data for all subjects in the masseter (4,853 fibres) and in the biceps (4,558 fibres). In the masseter, 44 % contained one MyHC isoform, either MyHC-I, MyHC-IIa, MyHC-IIx or MyHC-IIx′. The rest of the fibres, 56 %, showed mixtures of two to four isoforms in 26 combinations, or 19 when less-frequent combinations (<1 %) were excluded. MyHC-fetal and MyHC-α cardiac isoforms always occurred in combinations with other isoforms. In the biceps, 99 % of the fibres contained only one isoform. Table 4 displays for the masseter the proportion of mATPase fibre types expressing one MyHC isoform or combinations of isoforms. In type I fibres, one single isoform and seven combinations of two to four isoforms were detected, in type IM four combinations, in type IIC one single isoform and six combinations, in type IIAB one single isoform and five combinations, and in type IIB three single isoforms and eight combinations of two to three isoforms. Matching the mATPase fibre types with MyHC isoforms revealed a systematic continuum of single and combinations of MyHC-I, MyHC-IIa, MyHC-IIx′and MyHC-IIx in the mATPase fibre types I, IM, IIC, IIAB and IIB, respectively.
Serial muscle cross-sections from the deep masseter (left) and the biceps (right) muscles (subject 3 years), stained for mATPase at pH 10.3 and 4.3 and with mAbs A4.951, N2.261, A4.74, BF35, MHCn and F88. Examples of MyHC isoforms in mATPase fibre types numbered 1–8. MyHC-I in type I (fibre number 1), MyHC-I + fetal + α-cardiac in type I (number 2), MyHC-I + IIa + fetal + α-cardiac in type IM (number 3), MyHC-I + IIa + α-cardiac in type IM (number 4), MyHC-IIx′ + fetal in type IIB (number 5), MyHC-IIx in type IIB (number 6), MyHC-IIa + IIx in type IIB (number 7) and MyHC-IIa in type IIA (number 8). Bar 50μm
Analysis of masseter regional differences for slow and fast MyHCs was performed by combining the present MyHC data with the previous data for proportion of different enzyme-histochemical fibre types (Österlund et al. 2011). The result showed larger proportion of fibres containing fast MyHC in the sup ant and sup post masseter regions than in the deep masseter (p = 0.026 and p = 0.012, respectively). The proportion of MyHC-IIx was larger in the sup post than in the deep masseter (p = 0.039).
Correlation between gel (GE) and immuno-histochemical results
To compare the outcome of the GE examination with the result of the IHC examination concerning the presence of different MyHC isoforms in the young masseter muscle, a correlation analysis was performed. In the masseter, there was a strong correlation between the GE data and the IHC data for MyHC-I (r = 0.895, p ≤ 0.0001), moderate correlation for MyHC-IIx (r = 0.540, p = 0.021) and no correlation for MyHC-IIa (r = 0.184 p > 0.05) (Fig. 7). Young biceps was not included in the analysis due to few samples.
Comparison young muscles with adult and elderly muscles
The 95 % confidence intervals for proportion (%) of the different isoforms in the SDS-PAGE gels of young, adult and elderly masseter is shown in Fig. 8. No overlap in confidence intervals between young masseter versus adult and elderly masseter indicates larger proportion of MyHC-I and smaller proportion of MyHC-IIa, MyHC-IIx and MyHC-fetal isoforms in young masseter than in adult and elderly (Monemi et al. 1999) masseter. Furthermore, the young masseter contained more MyHC mixed fibres (63 %) than in adults (23 %) (Stål et al. 1994) and elderly (41 %) (Monemi et al. 1999). For the biceps, data indicate larger content of MyHC-I and smaller amounts of MyHC-IIa and MyHC-IIx in young and elderly than in adults (Monemi et al. 1999).
95 % confidence intervals for relative content (%) of MyHC-I, MyHC-IIa, MyHC-IIx and MyHC-fetal in SDS-PAGE gels of young masseter versus adult and elderly masseter (Monemi et al. 1999). MyHC-I in upper panel (a), MyHC-IIa, MyHC-IIx and MyHC-fetal isoforms in lower panel (b). No overlap in confidence intervals between young versus adult and elderly indicates larger proportion of MyHC-I and smaller proportions of MyHC-IIa, MyHC-IIx and MyHC-fetal isoforms in young masseter
Discussion
As expected, the masseter and the biceps muscles showed pronounced differences in MyHC expression during childhood. Predominance of MyHC-I in both muscles was in good agreement with results from the enzyme-histochemical evaluation using myosin ATPase as a discriminator for the same samples (Österlund et al. 2011). Furthermore, MyHC-α cardiac and MyHC-fetal were both present in the masseter, co-expressed with the conventional slow and fast isoforms. Therefore, more than half of the young masseter fibres contained mixtures of up to four MyHC isoforms in great contrast to 1 %, and maximally two isoforms in the young biceps. Young masseter MyHC heterogeneity was further emphasized by as many as four different isoforms in the most common combination of MyHC isoforms, and 19 MyHC-based fibre types, and a continuum of single and combinations of MyHC isoforms systematically related to mATPase fibre types.
In the present study, we have used two methods to visualize the MyHC isoforms, IHC, with a panel of mAbs recognizing different MyHC isoforms in individual fibres, and GE, which visualizes MyHC bands on the basis of electrophoretic mobility. The identity of the GE bands can be verified on the basis of immuno-reactivity with the MyHC recognizing antibodies (Western immunoblots). Nevertheless, one has to be aware that all MyHCs are quite similar in amino-acid composition reflecting their similar organization and function. The amino-acid identity among the full-length skeletal MyHCs ranges from 78.9 to 94.8 %, however, as related to the evolutionary MyHC gene tree, the ancient MyHC isoforms show only 77 to 81 % identity with the more recently developed MyHC isoforms, whereas the fast isoforms IIa and IIx show 94.8 % identity and MyHC-α cardiac and MyHC-β cardiac 93.2 % identity (Weiss et al. 1999). This reflects the fact that it is very difficult to obtain truly specific antibodies for just one MyHC isoform and to separate the individual isoforms by GE, as discussed below.
For fibres expressing MyHC-I in the young masseter, there was generally a good agreement between the present IHC and GE results (73 and 86 %, respectively) and the proportion of enzyme-histochemical fibres expressing MyHC-I (83 % in types I, IM and IIC) (Österlund et al. 2011). This was not the case for fibres expressing fast MyHCs. IHC stained 45 % of the young masseter fibres for fast MyHC in accordance with the proportion of enzyme-histochemical fibre types expressing fast MyHC (55 % in types II and IM) (Österlund et al. 2011). However, fast MyHC content on the gels was only 13 %. This apparent disparity in results of the two methods can, however, be explained. Firstly, the mAbs against MyHCs used have very high sensitivity. Thus, individual fibres, which contain relatively small amounts of the fast isomyosins, as reflected in the gels, will nevertheless appear as stained fibres by IHC. Secondly, in young masseter, the type II fibre diameter is much smaller than the type I type diameter (Österlund et al. 2011) which means that a given proportion of fibres detected with mAbs will correspond to a relatively smaller muscle mass quantified by GE.
The results of the two methods with respect to the amount and localization of MyHC-fetal and MyHC-α cardiac in the young masseter also seem to be at variance. By IHC, both isoforms were detected in about half the young masseter fibre population, dispersed throughout the complete fibre type population though preferentially expressed in IM and IIC fibres. By GE, they were detected in the overloaded gels (silver stained) but only faintly visible in the Supro-ruby stained gels. Like the result for fast MyHC content, the finding for fetal and α-cardiac isoforms may be due to the high mAb sensitivity. With respect to the specificity of these mAbs, we are aware that Western blots of theses mAbs have revealed that some have a broader reactivity pattern than the originally published or proposed by manufacturer. This is the case for the mAbs SC71, A4.74 and N2.261. The first two are reported to identify MyHC-IIa, but show affinity, albeit weaker, also for MyHC-IIx (Smerdu and Soukup 2008). The mAb N2.261 not only shows very strong affinity for MyHC-IIa but also detects MyHC-I and MyHC-extra ocular (Liu et al. 2002), whereas the mAb BF35 reacts with MyHCs I, α-cardiac, IIa, slow tonic and extra ocular (Liu et al. 2002). By taking this into account, when classifying MyHC staining in relation to mATPase fibre typing, we have found it is necessary to introduce a new fibre type MyHC-IIx′, since the staining pattern of these fibres was not in agreement with the presumptive staining patterns expected from these mAbs. The mAb BF35 negative fibres are supposed to reflect fibres expressing MyHC-IIx, since this is the only MyHC which this antibody does not detect. However, in the present study, a proportion of the mAb BF35 unstained fibres were also unstained for the mAb N2.261, indicating also the absence of MyHC-IIa in these fibres. On the other hand, these fibres were stained with the mAbs SC71 and A4.74, which preferentially detect MyHC-IIa according to the manufacturer and researchers introducing them (Hoh et al. 1993; Liu et al. 2002; Schiaffino et al. 1989). However, these antibodies also have been shown to have affinity for MyHC-IIx, albeit clearly weaker than for MyHC-IIa. Thus, unfortunately, these mAbs do not reliably separate fibres containing MyHCs IIa and IIx (Smerdu and Soukup 2008). Until further information is available, we tentatively interpret the MyHC-IIx′ fibre to reflect the occurrence of a novel spliced isoform of MyHC-IIx. Interestingly, the young biceps lacked this isoform, which may again reflect some disparity in molecular structure between cranial and limb fast MyHC isoforms. This is most probably related to divergence between the masseter and biceps in evolutionary and developmental origins (Sambasivan et al. 2011). It should be noted that the existences of MyHC splice variants is not unique. Indeed isoforms of MyHC-I have already been described for MyHC-I in human muscle during development (Hughes et al. 1993) and variants of embryonic MyHC has also been reported by Lucas and Hoh (2003).
Our identification of two bands stained for MyHC-fetal using the Western blot technique is unexpected but might be of considerable interest in terms of MyHC expression and phylogenetic regulation. One band corresponded in mobility with a band in the fetal sample known to reflect the MyHC fetal isoform (Butler-Browne et al. 1988). This band co-migrated with the MyHC-IIa band in our gels. The other band had slower mobility and co-migrated with the MyHC-IIx band. We were able to exclude the possibility that the extra band was related to MyHC-embryonic, both on the basis of the known mobility of MyHC-embryonic in our gel system (Kjellgren et al. 2003) and the fact that the mAb F1.652, specific for human embryonic MyHC (Karsch-Mizrachi et al. 1989), stained our fetal-control samples but left young masseter samples unstained. Additional studies are needed to confirm the existence of the two fetal isoforms and their functional significance, but our results suggest the possibility of the existence of a so far unrecognized spliced variant of MyHC-fetal in human skeletal muscles. Recent molecular studies using mRNA techniques indicate adaptive changes in MyHC-fetal expression in the human masseter muscle in response to altered biomechanics from dental and surgical treatments (Harzer et al. 2010; Oukhai et al. 2011). The role of MyHC α-cardiac in human jaw-muscle function has not yet been revealed. Experimental results on rat cardiac myocytes indicate that even small amounts of MyHC-α cardiac significantly increase myocyte power output (Herron and McDonald 2002). An age-related decrease of this isoform is suggested when the present result in young masseter is compared with data from adult and elderly masseter.
The finding of more slow MyHC and less fast MyHC in young masseter as compared with the masseter in adults and elderly (Monemi et al. 1999) extends our previous finding of a smaller proportion of type IIB fibres in young masseter than in adult and elderly masseter (Österlund et al. 2011). Taken together, these results suggest a marked age-related plasticity of the masseter MyHC composition during growth, maturation, and aging. This, in turn, reflects a shift from childhood to adultdhood by a decrease of slow twitch and an increase of fast twitch motor units. Such a dynamic change toward a wider span of motor-unit properties would probably benefit the maturation and improvement of refined jaw-motor skills for eating and speech behaviour. However, the functional implication of a change toward less-slow and more-fast MyHC in adult and elderly masseter (Monemi et al. 1999) than in young masseter does not seem to match physiological data indicating relatively slow contraction properties in elderly masseter rather than fast (see Monemi et al. 1999).
Differences in MyHC expression between young masseter and biceps can be traced to different patterns of evolution and development of craniofacial muscles versus limb and trunk muscles. Recent studies have provided evidence that the skeletal muscles of the head that control mastication, facial expression and eye movements are evolutionary, morphologically and molecularly distinct from trunk and limb muscles (Harel et al. 2009; Noden and Francis-West 2006). In evolutionary terms, the head of vertebrates is thought to be a novel structure, and muscles associated with the head, e.g., extra ocular, jaw and facial muscles are also novel, being derived from the cranial mesoderm, an embryonic tissue that is unique to vertebrates. Thus, it has been suggested that the head muscles have arisen independently of limb and trunk muscles, which means that the progenitors from the cranial mesoderm are evolutionary distinct from the somatic muscle progenitor pool. Furthermore, current knowledge suggests that different genetic regulatory cascades operate within the individual craniofacial muscle groups, and that there is a remarkable relationship with cardiomyogenesis (Sambasivan et al. 2011).
Hoh (2002) has studied the evolutionary origins of vertebrate jaw closing and limb and trunk muscles in detail. He attributes the phenomenon of phylogenetic muscle plasticity to adaptive changes in muscle properties in response to changes in functional load during phylogeny. As the functional properties of jaw-closing muscles across species are varied and complex, jaw muscles show a much greater phylogenetic plasticity than limb and trunk muscles. In terms of myosin expression it fits with the extensive repertoire of myosin expression across species in jaw closers, whereas in limb muscles, differences in myosin expression only manifest in relation to scaling of body mass (Schmidt-Nielson 1984).
With the characterization of the genome of several species, it has become apparent that the most phylogenetically ancient striated muscle MyHC gene is the gene encoding for a MyHC isoform which appeared some 400 million years ago, initially referred to as superfast MyHC and found in carnivores (Rowlerson et al. 1981). This MyHC has been renamed MyHC-masticatory/IIM (Hoh 2002; Qin et al. 2002). Hoh (2002) has shown that during mammalian evolution, some taxa (carnivores, chiropterans, primates, dasyurids and diprotodonts) have retained the masticatory myosin expression in jaw-closers while others have replaced it with different MyHCs more adapted to their particular diet and eating habits (Hoh 2002; Kang et al. 1994). In humans, a gene on chromosome 7 encodes MyHC-masticatory/IIM, but the gene is not expressed. Silencing of this gene has been calculated to have occurred about 2.4 million years ago. This down regulation has been proposed to have had a major impact on the development and growth of the human cranium and brain (Stedman et al. 2004). Opposing thoughts have been published (McCollum et al. 2006) and what is the hen and the egg in the evolution of the brain has still not been settled.
The next step in the evolution of human MyHC genes was the presence of two genes: MYH14 located on chromosome 20 and MYH15 on chromosome 3. The human MYH14 gene is encoding for MyHC slow tonic, an isoform present in muscle spindle fibres, extra ocular muscles, laryngeal muscles and in primary generation muscle fibres during development (Rossi et al. 2010). The MYH15 is also expressed in mice extra ocular muscles but appears to have been silenced in humans (Rossi et al. 2010). Thereafter, two highly conserved gene clusters present in all mammals appeared, although located on different chromosomes (Berg et al. 2001; Desjardins et al. 2002). One cluster, in humans located in chromosome 14, is composed of two tandemly arrayed genes, MYH6 and MYH7. They code for MyHC-α cardiac and for MyHC-β cardiac, the latter being equivalent to MyHC-I or MyHC slow-twitch expressed in skeletal muscle (Mahdavi et al. 1984; Weiss et al. 1999). In jaw-closing muscles of some animals like rabbit, MyHC-α cardiac is the predominant isoform whereas in others like cows, jaw muscles almost exclusively express MyHC-β cardiac/MyHC-I (Kang et al. 1994). The two genes MYH6 and MYH7 have a very high degree of identity (93.2 % ,Mahdavi et al. 1984; Weiss et al. 1999). They are also closely linked from a functional point of view and show in the heart an intricate regulation by thyroid hormones, functional load as well as in developmental aspects (Arnostova et al. 2011; Hoh 2002; Swynghedauw 1986). In the human masseter, both these genes are expressed with a huge predominance for MyHC-I, although MyHC-α cardiac is present in masseter fibres (Bredman et al. 1991; Pedrosa-Domellöf et al. 1992). This finding can be explained in terms of a common progenitor for both heart myogenesis and jaw-closing muscles (Harel et al. 2009; Sambasivan et al. 2011).
The cluster of fast MyHC genes, in humans, located in chromosome 17, has developed through gene duplications and these genes are highly conserved (Weiss et al. 1999). The most ancient gene MYH13 encodes for a MyHC referred to as MyHC-extra ocular since it was first found in extra ocular muscles, but also expressed in laryngeal muscles (Hoh 2005). Other paired genes developed further into MYH3 coding for MyHC-embryonic and a gene which gave raise to MYH8 and MYH4, coding for MyHC-fetal/perinatal and MyHC-IIb, respectively, on the one hand, and to MYH1 and MYH2 coding for MyHC-IIx and MyHC-IIa, respectively, on the other hand. This gene scheme gives some perspective on the differential expression of MyHCs in masseter and limb and trunk muscles in humans. While adult limb muscles have kept the expression of slow MyHC-β cardiac/MyHC-I and fast MyHC-IIa and MyHC-IIx, the typical fast MyHC found in rodents, MyHC-IIb, is silenced (Rossi et al. 2010). The MyHC-embryonic and MyHC-fetal appear during development and upon repair of muscle fibres, which recapitulates the developmental pattern of MyHC expression. Compared with extrafusal fibres, intrafusal, muscle spindle, fibres are even more diverse in expression of MyHC-isoforms, probably reflecting differences in pathway of differentiation (Walro and Kucera 1999). The present work extends our previous study on the human masseter during childhood and we propose that the special MyHC expression in the masseter reflects both specific developmental and evolutionary aspects of the jaw-closing muscles. During childhood, new specialized functional demands appear in parallel with craniofacial changes, teeth eruption and improvement of jaw functions for mastication and speech.
In conclusion, our study provides evidence that the young masseter muscle has a unique MyHC expression, including unconventional MyHC isoforms as well as hitherto unrecognized spliced variants of both MyHC-fetal and MyHC-IIx. Comparison between present results and previously reported data for adult and elderly masseter suggests marked plasticity in MyHC expression during life span toward less-slow and more-fast and fetal MyHC isoforms. Comparison of MyHC expression in young versus adult and elderly muscles revealed differences between masseter and biceps in MyHC plasticity and therefore in alterations of contractile properties during life. Differences in MyHC expression between masseter and biceps are proposed to reflect diverse evolutionary and developmental origins and accord with the masseter and biceps being separate allotypes of muscle.
References
Arnostova P, Jedelsky PL, Soukup T, Zurmanova J (2011) Electrophoretic mobility of cardiac myosin heavy chain isoforms revisited: application of MALDI TOF/TOF analysis. J Biomed Biotechnol 2011:634253
Berg JS, Powell BC, Cheney RE (2001) A millennial myosin census. Mol Biol Cell 12:780–794
Bredman JJ, Wessels A, Weijs WA, Korfage JA, Soffers CA, Mooman AF (1991) Demonstration of “cardiac-specific” myosin heavy chain in masticatory muscles of human and rabbit. Histochem J 23:160–170
Butler-Browne G, Eriksson P-O, Laurent C, Thornell L-E (1988) Adult human masseter muscle fibres express myosin isozymes characteristic of development. Muscle Nerve 11:610–620
Cho M, Webster SG, Blau HM (1993) Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J Cell Biol 121:795–810
Desjardins PR, Burkman JM, Shrager JB, Allmond LA, Stedman HH (2002) Evolutionary implications of three novel members of the human sarcomeric myosin heavy chain gene family. Mol Biol Evol 19:375–393
Dubowitz V (2007) Muscle biopsy: a practical approach, vol 3. Bailliére Tindall, London, pp 47–74
Ecob-Prince M, Hill M, Brown W (1989) Immunocytochemical demonstration of myosin heavy chain expression in human muscle. J Neurol Sci 91:71–78
Engel A, Franzini-Armstrong C (2004) Myology: basic and Clinical, New York
Eriksson P-O (1982) Muscle-fibre composition of the human mandibular locomotor system: enzyme-histochemical and morphological characteristics of functionally different parts. Swed Dent J Suppl 12(Suppl):1–44
Eriksson P-O, Thornell L-E (1983) Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol 28:781–795
Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E (2009) Distinct origins and genetic programs of head muscle satellite cells. Dev Cell 16:822–832
Harzer W, Maricic N, Gedrange T, Lewis M, Hunt N (2010) Molecular diagnosis in orthodontics, facial, and orthognathic surgery: implications for treatment progress and relapse. Semin Orthod 16:118–127
Herron TJ, McDonald KS (2002) Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res 90:1150–1152
Hoh JF (2002) Superfast or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J Exp Biol 205:2203–2210
Hoh JF (2005) Laryngeal muscle fibre types. Acta Physiol Scand 183:133–149
Hoh JF, Hughes S, Kang LHD, Rughani A, Qin H (1993) The biology of cat jaw-closing muscle cells. J Comput-Assist Microsc 5:65–70
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158:183–199
Kang L, Hughes S, Pettigrew J, Hoh J (1994) Jaw-specific myosin heavy chain gene expression in sheep, dog monkey, flying fox and microbat jaw-closing muscles. Basic Appl Myol 4:381–392
Karsch-Mizrachi I, Travis M, Blau H, Leinwand LA (1989) Expression and DNA sequence analysis of a human embryonic skeletal muscle myosin heavy chain gene. Nucleic Acids Res 17:6167–6179
Kjellgren D, Thornell L-E, Andersen J, Pedrosa-Domellöf F (2003) Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 44:1419–1425
Léger JJ (1985) Institut National de la Santé et de la Recherche Médical, Unité 249, Montpellier, France
Liu J-X, Eriksson P-O, Thornell L-E, Pedrosa-Domellöf F (2002) Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem 50:171–183
Lucas CA, Hoh JF (2003) Distribution of developmental myosin heavy chains in adult rabbit extraocular muscle: identification of a novel embryonic isoform absent in fetal limb. Invest Ophthalmol Vis Sci 44:2450–2456
Mahdavi V, Chambers AP, Nadal-Ginard B (1984) Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci USA 81:2626–2630
McCollum MA, Sherwood CC, Vinyard CJ, Lovejoy CO, Schachat F (2006) Of muscle-bound crania and human brain evolution: the story behind the MYH16 headlines. J Hum Evol 50:232–236
Monemi M, Eriksson P-O, Eriksson A, Thornell L-E (1998) Adverse changes in fibre type composition of the human masseter versus biceps brachii muscle during aging. J Neurol Sci 154:35–48
Monemi M, Eriksson P-O, Kadi F, Butler-Browne GS, Thornell L-E (1999) Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil 20:351–361
Noden DM, Francis-West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235:1194–1218
Österlund C, Thornell L-E, Eriksson P-O (2011) Differences in fibre type composition between human masseter and biceps muscles in young and adults reveal unique masseter fibre type growth pattern. Anat Rec (Hoboken) 294:1158–1169
Oukhai K, Maricic N, Schneider M, Harzer W, Tausche E (2011) Developmental myosin heavy chain mRNA in masseter after orthognathic surgery: a preliminary study. J Craniomaxillofac Surg 39:401–406
Pedrosa-Domellöf F, Eriksson P-O, Butler-Browne G, Thornell L-E (1992) Expression of alpha-cardiac myosin heavy chain in mammalian skeletal muscle. Experientia 48:491–494
Pette D, Staron RS (2000) Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50:500–509
Qin H, Hsu MK, Morris BJ, Hoh JF (2002) A distinct subclass of mammalian striated myosins: structure and molecular evolution of “superfast” or masticatory myosin heavy chain. J Mol Evol 55:544–552
Ringqvist M (1973) Histochemical enzyme profiles of fibres in human masseter muscles with special regard to fibres with intermediate myofibrillar ATPase reaction. J Neurol Sci 18:133–141
Rossi AC, Mammucari C, Argentini C, Reggiani C, Schiaffino S (2010) Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol 588:353–364
Rowlerson A, Pope B, Murray R, Weeds A (1981) A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil 2:415–438
Sambasivan R, Kuratani S, Tajbakhsh S (2011) An eye on the head: the development and evolution of craniofacial muscles. Development 138:2401–2415
Sawchak JA, Leung B, Shafiq SA (1985) Characterization of a monoclonal antibody to myosin specific for mammalian and human type II muscle fibers. J Neurol Sci 69:247–254
Schiaffino S (2010) Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 199:451–463
Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10:197–205
Schmidt-Nielson K (1984) Scaling: why animal size is so important. Cambridge University Press, Cambridge
Sewry CA, Uziyel Y, Torelli S, Buchanan S, Sorokin L, Cohen J, Watt DJ (1998) Differential labelling of laminin alpha 2 in muscle and neural tissue of dy/dy mice: are there isoforms of the laminin alpha 2 chain? Neuropathol Appl Neurobiol 24:66–72
Silberstein L, Webster SG, Travis M, Blau HM (1986) Developmental progression of myosin gene expression in cultured muscle cells. Cell 46:1075–1081
Smerdu V, Soukup T (2008) Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 52:179–190
Soussi-Yanicostas N, Barbet J, Laurent-Winter C, Barton P, Butler-Browne GS (1990) Transition of myosin isozymes during development of human masseter muscle: persistence of developmental isoforms during postnatal stage. Development 108:239–249
Stål P, Eriksson P-O, Schiaffino S, Butler-Browne GS, Thornell L-E (1994) Differences in myosin composition between human orofacial, masticatory and limb muscles: enzyme-, immunohist- and biochemical-studies. J Muscle Res Cell Motil 15:517–534
Stedman HH, Kozyak BW, Nelson A, Thesier DM, Su LT, Low DW, Bridges CR, Shrager JB, Minugh-Purvis N, Mitchell MA (2004) Myosin gene mutation correlates with anatomical changes in the human lineage. Nature 428:415–418
Sternberger LA (1979) The unlabeled antibody (PAP) method, introduction. J Histochem Cytochem 27:1657
Swynghedauw B (1986) Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev 66:710–771
Talmadge RJ, Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75:2337–2340
Thornell L-E, Billeter R, Eriksson P-O, Ringqvist M (1984) Heterogenous distribution of myosin in human masticatory muscle fibres as shown by immunocytochemistry. Archs oral Biol 29:1–5
Thornell L-E, Grove B, Pedrosa F, Butler-Browne G, Dhoot G, Fischman D (1989) Expression of slow tonic myosin in muscle spindle fibres early in mammalian development. In: Stockdale F, Kedes I (eds) Molecular biology of muscle development. Alan R Liss, New York, pp 471–480
Walro JM, Kucera J (1999) Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci 22:180–184
Weiss A, Schiaffino S, Leinwand LA (1999) Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functioanl diversity. J Mol Biol 290:61–75
Acknowledgments
The authors wish to thank Mrs. Inga Johansson for excellent technical assistance and associate professor Albert Crenshaw for English revision and valuable comments. This work was supported by grants from the Department of Odontology, Umeå University, Västerbotten County Council and the Swedish Dental Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Österlund, C., Lindström, M., Thornell, LE. et al. Remarkable heterogeneity in myosin heavy-chain composition of the human young masseter compared with young biceps brachii. Histochem Cell Biol 138, 669–682 (2012). https://doi.org/10.1007/s00418-012-0985-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-0985-5