Abstract
During early pregnancy in the rat, focal adhesions disassemble in uterine luminal epithelial cells at the time of implantation to facilitate their removal so that the implanting blastocyst can invade into the underlying endometrial decidual cells. This study investigated the effect of ovarian hormones on the distribution and protein expression of two focal adhesion proteins, talin and paxillin, in rat uterine luminal and glandular epithelial cells under various hormone regimes. Talin and paxillin showed a major distributional change between different hormone regimes. Talin and paxillin were highly concentrated along the basal cell surface of uterine luminal epithelial cells in response to oestrogen treatment. However, this prominent staining of talin and paxillin was absent and also a corresponding reduction of paxillin expression was demonstrated in response to progesterone alone or progesterone in combination with oestrogen, which is also observed at the time of implantation. In contrast, the distribution of talin and paxillin in uterine glandular epithelial cells was localised on the basal cell surface and remained unchanged in all hormone regimes. Thus, not all focal adhesions are hormonally dependent in the rat uterus; however, the dynamics of focal adhesion in uterine luminal epithelial cells is tightly regulated by ovarian hormones. In particular, focal adhesion disassembly in uterine luminal epithelial cells, a key component to establish successful implantation, is predominantly under the influence of progesterone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal adhesions are specialised regions of the plasma membrane found in sites where cells attach to the cell-matrix and cell-substratum (Beckerle and Yeh 1990; Critchley 2000). They provide a structural link between the extracellular matrix and the actin cytoskeleton intracellularly via heterodimeric cell surface receptors known as integrins (Burridge and Chrzanowska-Wodnicka 1996; Burridge et al. 1990; Carragher and Frame 2004; Critchley 2000; Lo 2006; Turner 2000). Integrin clustering initiates the assembly of focal adhesions, where multiple cytoskeletal and signalling proteins are recruited to the site of focal adhesions, which mediate a wide variety of biological processes including cell attachment, migration, proliferation, differentiation and gene expression (Lo 2006; Turner 2000).
Previous studies have found numerous proteins involved in focal adhesions (Gilmore and Burridge 1996; Lo 2006; Turner 2000), in particular talin and paxillin are well-established focal adhesion proteins used to visualise focal adhesions in immunofluorescence microscopy studies (Burridge et al. 1988; Critchley 2000; Geiger et al. 1984a, b).
Talin is a high molecular weight protein (225–235 kDa) providing an actin–membrane linkage by binding directly to the actin stress fibres and the cytoplasmic domain of integrin β subunits (Beckerle and Yeh 1990; Critchley 2000; Kaufmann et al. 1991). Paxillin (68 kDa) is a molecular adaptor protein involved in recruiting multiple cytoskeletal and signalling proteins to focal adhesions and, in turn, provides docking sites for these proteins (Brown and Turner 2004; Schaller 2001; Turner 1994, 2000).
Focal adhesions are found in a variety of tissues and cells (Burridge et al. 1988; Carragher et al. 1999; Fuchs et al. 1997; Macphee and Lye 2000). A previous study has demonstrated that focal adhesion proteins talin and paxillin in rat uterine epithelial cells during early pregnancy undergoes extensive alteration in distribution and expression (Kaneko et al. 2008). The dynamics of focal adhesions play a pivotal role at the time of implantation where focal adhesion proteins talin and paxillin showed a significant loss of basal staining along the uterine luminal epithelial cells, leading to the disassembly of focal adhesions (Kaneko et al. 2008). Focal adhesion disassembly at the time of implantation is probably critical in allowing the uterine luminal epithelial cells to become less adherent to the underlying basal lamina. This facilitates their removal, enabling the trophoblastic cells to breach and invade into the underlying endometrial decidual cells (Enders and Schlafke 1967; Finn and Porter 1975; Welsh 1993; Wynn 1977).
To date, however, the hormonal regulation of focal adhesions and their principal components, talin and paxillin in the rat uterus, have not been established. Thus, the present study investigates the effect of ovarian hormones on the focal adhesion proteins talin and paxillin in uterine luminal and glandular epithelial cells in ovariectomised rats.
Materials and methods
Animals
Female virgin Wistar rats (10–12 weeks of age) were used in the study. They were housed in plastic cages at 21°C under 12 h light/dark and were provided with water and food ad libitum. All experimental procedures were approved by the University of Sydney Ethics Committee.
Ovariectomy
Rats were bilaterally ovariectomised under deep anaesthetic with an intraperitoneal injection of xylazine (4 mg/kg; TROY laboratories Pty. Ltd., Smithfield, NSW, Australia) and ketamine (75 mg/kg; Parrell Laboratories (AUST) Pty. Ltd., Alexandria, NSW, Australia) and allowed to recover for 3 weeks. Animals were randomly allocated to the hormonal regimes. Progesterone (Sigma, St Louis, MO, USA) and 17-β-oestradiol (Sigma) were dissolved in benzyl alcohol (Sigma) and peanut oil in 1:4 v/v and injected subcutaneously in the back of the neck as previously described (Murphy and Rogers 1981). Group 1 (control) was injected with 0.1 ml of carrier (benzyl alcohol and peanut oil) alone. Group 2 (PPP) received 5 mg progesterone dissolved in 0.1 ml of carrier for three consecutive days. Group 3 (EEE) received 0.5 μg 17-β-oestradiol in 0.1 ml of carrier for 3 consecutive days. Group 4 (PPPE) received 0.1 ml of 5 mg progesterone for 2 consecutive days and 0.1 ml of 5 mg progesterone as well as 0.1 ml of 0.5 μg 17-β-oestradiol on the opposite side of the neck on the third day. Group 4 (PPPE) was the minimal requirement in obtaining a receptive uterus (Psychoyos 1973; Murphy and Rogers 1981). Injections were given in the morning and animals were killed 24 h after the last injection. Animals were killed with an intraperitoneal injection of sodium pentobarbitone (Nembutal; Merial Australia, Paramatta, NSW, Australia). Uterine horns were excised and randomly allocated for either immunofluorescence microscopy or Western blotting analysis.
Immunofluorescence microscopy
Uterine horns from ovariectomised rats of each treatment group were excised and embedded in OCT compound (Tissue-Tek; SakuraFiretek, Torrance, CA, USA), immersed in supercooled isopentane (BDH Laboratory supplies, Poole, England), snap-frozen in liquid nitrogen and stored at −80°C until use. Frozen uterine tissues were sliced at 7 μm thickness using a Leica LM 3050 cryostat (Leica, Heerbrugg, Switzerland). Tissue sections were air dried on gelatine-chrome alum-coated glass slides at room temperature and fixed in 4% formaldehyde for 10 min followed by phosphate-buffered saline (PBS) wash for 10 min. Sections were blocked in 1% bovine serum albumin (BSA; Sigma) in PBS for 30 min. All primary and secondary antibodies were diluted with this blocking solution. Sections were incubated with primary antibody of mouse monoclonal anti-paxillin antibody (140 μg mL−1; Sigma) or mouse monoclonal anti-talin antibody (8.3 μg mL−1; Sigma) for 2 h. After being washed in PBS 3 × 5 min, sections were incubated with fluorescein isothiocyanate (FITC)-conjugated AffiniPure Goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at a concentration of 3 μg mL−1 for 30 min in the dark followed by 3 × 5 min PBS wash. Subsequently sections were stained with 0.001 mg mL−1 Hoechst (BisBenzimide H; Sigma) for 2 min to counterstain the nuclei. Sections were washed in PBS and mounted with vectashield (Vector, Burlingame, CA, USA) and coverslipped. The sections were then examined under Zeiss Deconvolution microscope (Carl Zeiss Inc., Jena, Germany). Immunofluorescence micrographs were taken using a Zeiss AxioCamHR digital monochrome CCD camera (Carl Zeiss Inc., Jena, Germany) and Zeiss AxioVision version 4.0 image-acquisition software. Negative controls were carried out with the experimental runs. Non-immune controls were performed where sections were incubated with mouse IgG purified immunoglobulin (1 μg mL−1; Sigma) in place of the primary antibody. In addition, negative controls omitting the primary antibody were also performed (data not shown).
Isolation of uterine luminal epithelial cells
Uterine luminal epithelial cells were isolated from each uterine horn as previously described (Kaneko et al. 2008). The uterine horn was opened longitudinally and surface luminal epithelial cells were removed using sterile surgical blades (Livingstone, International, Rosebery, NSW, Australia) and immediately placed into a lysis buffer (50 mM Tris–HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 0.5% Deoxycholic acid, 1% Igepal and protease inhibitor cocktail; Sigma Mammalian Cell lysis kit, Sigma). The purity of uterine epithelial cells was confirmed by immersing the remaining uterine tissue in 10% neutral buffered formalin, followed by embedding in paraffin wax utilising a tissue processor (Sakura Tissue-Tek, Sakura Finetek, Torrance, California, USA). Paraffin blocks were sectioned and stained with haematoxylin and eosin to show that uterine epithelial cells were isolated adequately from other uterine tissue (data not shown).
Western blotting analysis
Isolated uterine luminal epithelial cells from ovariectomised rats from each treatment group were immediately placed in lysis buffer and passed through a 23 G needle using a 1-ml syringe (Livingstone, International, Rosebery, NSW, Australia) and briefly centrifuged at 8,000g at 4°C. The supernatant was carefully removed and frozen immediately in liquid nitrogen and then stored at −80°C until use.
Protein concentrations were determined using the BCA protein assay (Micro BCA™ Protein assay kit; Quantum Scientific, Murarrie, Qld, Australia) and POLARstar microplate reader (BMG LabTech, Durham, NC, USA) according to the manufacturer’s instructions. As much as 20 μg of total protein and sample buffer (8% glycerol, 50 mM Tris–HCl, pH 6.8, 1.6% SDS, 0.024% Bromophenol blue, 4% β-mercaptoethanol) were added to prepare the protein samples. Protein samples were boiled at 95°C for 5 min and resolved on 7.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene dilfluoride (PVDF) membrane (Immunobilon™ transfer membrane; Millipore, Bedford, MA, USA) by standard procedures. Nonspecific binding sites were blocked by incubating the membrane with 5% skim milk powder in TBST (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature with constant agitation followed by primary antibody incubation with mouse monoclonal anti-paxillin antibody (70 μg mL−1; Sigma) or mouse monoclonal anti-talin antibody (0.83 μg mL−1; Sigma) overnight at 4°C. All antibodies were diluted with 1% skim milk powder in TBST. The membranes were washed 3 × 10 min in TBST and subsequently incubated for 1 h with sheep anti-mouse IgG horseradish peroxidase-linked secondary antibody (1:5,000; Amersham, GE Health, Buckinghamshire, UK) at room temperature with constant agitation. The proteins were detected by enhanced chemiluminescence (ECL Plus Western Blotting Detection System; Amersham, GE Healthcare) and unsaturated images were captured using the Alpha Innotech Digital Imaging System (Alpha Innotech, San Leandro, CA, USA). To confirm equal protein loading, membranes were rinsed in TBST and stripped by heating at 50°C for 30 min in stripping buffer (62.5 mM Tris–HCl, pH 6.7, 2% SDS and 100 mM β-mercaptoethanol) and reprobed with mouse monoclonal anti-β-actin antibody (0.4 μg mL−1; Sigma).
Statistical analysis
The intensity of the band detected from the Western blotting analysis was quantified using the two-dimensional spot density from the Alpha Innotech Digital System (Alpha Innotech). The integrated density value (IDV) was calculated using the AlphaEaseFC software. Statistical analysis was performed by one-way ANOVA for comparison of hormone treatment groups followed by Tukey’s honest significant difference (HSD) test. The mean ± SD was calculated and p < 0.05 was considered to be significant.
Results
Immunofluorescence microscopy
Talin
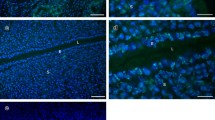
Immunofluorescence staining for talin demonstrated a distributional change in uterine luminal epithelial cells between different hormone treatment groups. In the group treated only with oestrogen, the uterine lumen was widely open and lined by tall columnar uterine luminal epithelial cells. An intense staining along the basal region of the uterine luminal epithelial cells was observed in ovariectomised animals treated with oestrogen alone (Fig. 1a, b). However, there was a marked reduction in basal staining along the uterine luminal epithelial cells in ovariectomised animals treated with progesterone alone (Fig. 1c) and progesterone in combination with oestrogen (Fig. 1d) compared to the oestrogen only group. The uterine lumen was completely closed down where the opposing uterine epithelial cells were in close cellular contact in both progesterone and progesterone plus oestrogen groups. Ovariectomised animals treated with vehicle alone showed little staining of talin along the basal region of the uterine luminal epithelial cells (Fig. 1e).
Immunofluorescence micrographs of rat uterine luminal epithelial cells (le) stained with talin, and nuclei counterstained with Hoechst from ovariectomised rats treated with oestrogen alone (a, b), progesterone alone (c), progesterone in combination with oestrogen (d) and vehicle alone (e). Oestrogen alone treatment (a, b) showed a prominent basal band along the basal luminal epithelial cell surface (arrow). Progesterone alone (c) and progesterone in combination with oestrogen groups (d) demonstrated a markedly reduced basal staining (arrows). Vehicle alone group (e) also showed little staining along the basal cell surface (arrow). Representative non-immune control of the oestrogen only-treated group (f) showed no specific immunofluorescence staining. Scale bar 20 μm. L uterine lumen, S stromal cells
Non-immune control sections from all hormone-treated groups showed no specific immunofluorescence staining. Representative non-immune control sections from the oestrogen only group are shown in Fig. 1f.
Immunofluorescence staining for talin in uterine glandular epithelial cells showed intense staining along the basal region in all treatment groups, which remained unchanged regardless of hormonal treatment (Fig. 2a, oestrogen alone, b progesterone alone, c progesterone in combination with oestrogen, d vehicle alone). Talin staining was also observed within the stroma cells in a punctate pattern only in response to progesterone alone (Fig. 2b) and progesterone in combination with oestrogen (Fig. 2c). Non-immune control sections from all hormone-treated groups showed no specific immunofluorescence staining. Representative non-immune control sections are shown for oestrogen only (Fig. 2e) and progesterone plus oestrogen (Fig. 2f).
Immunofluorescence micrographs of rat uterine glandular epithelial cells (ge) stained with talin, and nuclei counterstained with Hoechst from ovariectomised rats treated with oestrogen alone (a), progesterone alone (b) and progesterone plus oestrogen (c) and vehicle alone (d). In all treatment groups, talin formed a distinctive band along the basal cell surface of glandular epithelial cells (arrow). Talin staining in a punctate pattern (asterisk) was seen in stromal cells in response to progesterone alone (b) and progesterone plus oestrogen (c). Representative non-immune control of the oestrogen only (e) and progesterone plus oestrogen (f) showed no specific immunofluorescence staining. Scale bar 20 μm. S stromal cells
Paxillin
Immunofluorescence staining for paxillin in uterine luminal epithelial cells also showed changes in distribution between different hormone treatment groups. In ovariectomised animals treated with oestrogen alone, paxillin was highly concentrated along the basal region of the uterine luminal epithelial cells (Fig. 3a, b). Progesterone alone (Fig. 3c) and progesterone plus oestrogen groups (Fig. 3d) demonstrated similar staining and varied considerably from the oestrogen alone group, as the intense staining along the basal cell surface was absent relative to the staining observed in the ovariectomised animals treated with oestrogen alone. Ovariectomised animals treated with vehicle alone showed little staining of paxillin along the basal cell surface of uterine luminal epithelial cells (Fig. 3e). Non-immune control sections from all hormone-treated groups showed no specific immunofluorescence staining. A representative non-immune control section from the oestrogen only group is shown in Fig. 3f.
Immunofluorescence micrographs of rat uterine luminal epithelial cells (le) stained with paxillin, and nuclei counterstained with Hoechst from ovariectomised rats treated with oestrogen alone (a, b), progesterone alone (c) progesterone in combination with oestrogen (d) and vehicle alone (e). Paxillin was highly concentrated along the basal cell surface (arrow) in the oestrogen only-treated group (a, b); however, the intense staining was lost (arrow) in the progesterone only (c) and progesterone plus oestrogen groups (d). Similar staining pattern was seen in the vehicle alone group (e). Representative non-immune control of the oestrogen only-treated group (f) showed no specific immunofluorescence staining. Scale bar 20 μm. L uterine lumen, S stromal cells
Immunofluorescence staining for paxillin in uterine glandular epithelial cells exhibited concentrated staining along the basal cell surface and remained unchanged in all hormone treatment groups (Fig. 4a, oestrogen alone, B progesterone alone, c progesterone in combination with oestrogen, d vehicle alone). Non-immune control sections from all hormone-treated groups showed no specific immunofluorescence staining. Representative non-immune control sections are shown for oestrogen only (Fig. 4e) and progesterone plus oestrogen (Fig. 4f).
Immunofluorescence micrographs of rat uterine glandular epithelial cells (ge) stained with paxillin, and nuclei counterstained with Hoechst from ovariectomised rats treated with oestrogen alone (a), progesterone alone (b) progesterone plus oestrogen (c) and vehicle alone (d). Paxillin was concentrated basally along the glandular epithelial cells (arrow) in all treatment groups. Representative non-immune control of the oestrogen only (e) and progesterone plus oestrogen (f) showed no specific immunofluorescence staining. Scale bar 20 μm. S stromal cells
Western blotting analysis
Talin
Talin expression from isolated uterine luminal epithelial cells was detected in all treatment groups as a 225-kDa intact talin and a 190-kDa calpain 2-mediated protein cleaved fragment of talin (Fig. 5a). The amount of talin was not significantly different between any treatment groups (Fig. 5b). β-actin was used as a loading control to ensure that equal amount of protein was loaded for all treatment groups.
a Western blotting demonstrates talin expression from isolated uterine luminal epithelial cells from ovariectomised rats treated with vehicle alone (oil), oestrogen alone (E), progesterone alone (P) and progesterone plus oestrogen (P + E). The 225-kDa intact talin and 190-kDa protease cleaved fragment were detected in all treatment groups. β-actin was used as a loading control. b Densitometric analysis of Western blotting was performed followed by statistical analysis using one-way ANOVA. The amount of talin was not statistically different between treatment groups. Each bar is the mean ± SD of n = 3
Paxillin
Paxillin expression from isolated uterine luminal epithelial cells was detected from all treatment groups as a 68-kDa band (Fig. 6a). The amount of protein from progesterone alone (P) and progesterone plus oestrogen groups (P + E) was significantly reduced compared to the oestrogen alone group (E) (Fig. 6b). Oestrogen alone was the only treatment group, which detected the 46-kDa paxillin δ band. β-actin was used as a loading control.
a Western blotting of isolated uterine luminal epithelial cells, immunoblotted with paxillin from ovariectomised rats treated with vehicle alone (oil), oestrogen alone (E), progesterone alone (P) and progesterone plus oestrogen (P + E). The 68-kDa band was detected in all treatment groups. The 46-kDa band paxillin δ was only detected in the oestrogen only treatment. β-actin was used as a loading control. b Densitometric analysis of Western blotting was performed and statistical significance was evaluated by one-way ANOVA followed by Tukey’s honest significant difference (HSD) test. Progesterone alone or in combination with oestrogen showed a significant reduction in the paxillin expression compared to the group treated with oestrogen alone. Asterisks indicate statistical significant difference of IDV values compared with to the group treated with oestrogen alone (E) (* p < 0.05, ** p < 0.01, ns no significant difference; HSD test). Each bar is the mean ± SD of n = 3
Discussion
This is the first study that examined the effect of ovarian hormones on the principal focal adhesion proteins, talin and paxillin, in rat uterine luminal and glandular epithelial cells. Talin and paxillin showed considerable variation in their distribution in uterine luminal epithelial cells under different hormonal regimes. Talin and paxillin exhibited a prominent basal staining in uterine luminal epithelial cells in ovariectomised animals treated with oestrogen only. However, there was much less staining in the basal region seen in ovariectomised animals treated with progesterone only and progesterone in combination with oestrogen.
The immunofluorescence results were consistent with the protein expression for paxillin in which the amount of 68 kDa of paxillin was significantly reduced in progesterone only and progesterone in combination with oestrogen groups compared to the oestrogen only treatment. The 46-kDa band paxillin δ was only detected in the oestrogen only treatment. Paxillin δ (46 kDa) is an internal translation product of paxillin α (68 kDa), which is restricted to epithelial cell types and functions to inhibit cell migration (Sorenson and Sheibani 1999; Tumbarello et al. 2005). Thus, the absence of paxillin δ and the reduction of the 68-kDa paxillin seen in progesterone alone or in combination with oestrogen in the present study probably allows uterine luminal epithelial cells to become more labile and less adherent to their underlying basal lamina. The disappearance of paxillin δ was also documented at the time of implantation in rat uterine luminal epithelial cells during normal pregnancy (Kaneko et al. 2008) where progesterone is the dominant ovarian hormone (Ljungkvist 1972).
Despite the variation in the distribution of talin amongst different hormone treatments, the protein expression of talin did not change in any of the hormonal conditions. This was consistent with studies during early pregnancy where the protein expression was not significantly different between day 1 of pregnancy and at time of implantation (Kaneko et al. 2008). The reason for this discrepancy between the immunofluorescence and Western blotting analysis of talin is not clear. However, it may be that disassembly of focal adhesions results in talin redistributing into the cytoplasm where there is insufficient concentration of the molecule for immunofluorescence staining. Hence, the overall amount of talin did not change between different hormone-treated groups. Previous studies on human platelets have shown a dramatic shift in distribution of talin in response to platelet activation (Beckerle et al. 1989; Beckerle and Yeh 1990). In activated adhesion-competent platelets, talin was localised in the plasma membrane; however, in inactive non-adhesive platelets, talin was distributed throughout the cytoplasm (Beckerle et al. 1989; Beckerle and Yeh 1990). This further suggests that cytoplasmic talin in uterine epithelial cells may be in an inactive non-adhesive state and may not be sufficient to be detected by immunofluorescence microscopy resulting in the lack of cytoplasmic talin staining reported here.
Previous ultrastructural studies on the basal plasma membrane of rat uterine luminal epithelial cells under different hormonal regimes have found that electron-dense structures along the basal plasma membrane were only observed under oestrogen only treatment (Shion and Murphy 1995), Progesterone alone and progesterone in combination with oestrogen groups showed a highly tortuous outline of the basal plasma membrane with the disappearance of these electron-dense structures (Shion and Murphy 1995). The electron-dense structures are now referred to as focal adhesions (Murphy 2000). Taken together, these observations show that oestrogen plays a major role in maintaining the firm adhesion between the uterine luminal epithelial cells and the underlying basal lamina by the formation of focal adhesions. In contrast, progesterone is the hormone responsible for the loss of focal adhesion proteins from the basal plasma membrane, so leading to focal adhesion disassembly. The dynamics of focal adhesion in uterine epithelial cells is thus clearly dependent on ovarian hormones.
The distributional change of the focal adhesion proteins, paxillin and talin, in the present study was observed globally throughout the uterine horn. The changes seen in the apical plasma membrane and the lateral plasma membrane during early pregnancy, collectively known as the plasma membrane transformation, also occurs globally throughout the uterine horn regardless of the presence of a blastocyst or not and are tightly regulated by ovarian hormones (Murphy 1993; Murphy 2001, 2004). Thus, the global alteration taking place under hormonal control, including focal adhesion disassembly, plays an important role during early pregnancy, so that the blastocyst has the potential to successfully implant along the entire uterine horn.
Interestingly, talin and paxillin remained localised on the basal cell surface of uterine glandular epithelial cells regardless of hormonal treatment. This shows that not all focal adhesions are hormonally dependent in the uterus. The finding from the present study reinforces the fact that it is the uterine luminal epithelial cells, which come in direct contact with the blastocyst at the time of implantation and are the cells that are most tightly regulated under hormonal control. In particular, ovarian hormones control the disassembly of focal adhesions in the uterine luminal epithelial cells enabling these cells to be removed so that the invading blastocyst can penetrate into the underlying endometrial decidual cells at the time of implantation. Our findings provide further evidence that uterine luminal epithelial cells have a major role in implantation and uterine receptivity.
In the stroma, the increase in the punctate pattern of staining was only seen for talin in response to progesterone alone or in combination with oestrogen. This is consistent with the increase in actin filaments during decidualisation (Christensen et al. 1995) and increase in talin distributed within the decidual cells after implantation (Kaneko et al. 2008). Since talin directly binds to actin filaments (Beckerle and Yeh 1990; Critchley 2000), it may be responsible for the formation of firm adhesions between decidual cells to regulate the invasive nature of the trophoblastic cells as they enter the stroma.
In summary, ovarian hormones have a major effect on the distribution of the focal adhesion proteins, talin and paxillin, in rat uterine luminal epithelial cells, but not in glandular epithelial cells. The marked loss of staining of talin and paxillin along the basal cell surface of uterine luminal epithelial cells leading to focal adhesion disassembly was predominantly under the influence of progesterone. Thus, focal adhesion disassembly during uterine receptivity is another key component tightly regulated by ovarian hormones.
References
Beckerle MC, Yeh RK (1990) Talin: role at sites of cell–substratum adhesion. Cell Motil Cytoskeleton 16:7–13
Beckerle MC, Miller DE, Bertagnolli ME, Locke SJ (1989) Activation-dependent redistribution of the adhesion plaque protein, talin, in intact human platelets. J Cell Biol 109:3333–3346
Brown MC, Turner CE (2004) Paxillin: adapting to change. Physiol Rev 84:1315–1339
Burridge K, Chrzanowska-Wodnicka M (1996) Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12:463–518
Burridge K, Fath T, Kelly G, Nuckolls G, Turner C (1988) Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol 4:487–525
Burridge K, Nuckolls G, Otey C, Pavalko F, Simon K, Turner C (1990) Actin–membrane interaction in focal adhesions. Cell Differ Dev 32:337–342
Carragher NO, Frame MC (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol 14:241–249
Carragher NO, Levkau B, Ross R, Raines EW (1999) Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125FAK, paxillin, and talin. J Cell Biol 147:619–629
Christensen S, Verhage H, Nowak G, De Lanerolle P, Fleming S, Bell S (1995) Smooth muscle myosin II and alpha smooth muscle actin expression in the baboon (Papio anubis) uterus is associated with glandular secretory activity and stromal cell transformation. Biol Reprod 53:598–608
Critchley DR (2000) Focal adhesions: the cytoskeletal connection. Curr Opin Cell Biol 12:133–139
Enders AC, Schlafke S (1967) A morphological analysis of the early implantation stages in the rat. Am J Anat 120:185–226
Finn CA, Porter DG (1975) The uterus. Elek Science, London
Fuchs E, Dowling J, Segre J, Lo SH, Yu QC (1997) Integrators of epidermal growth and differentiation: distinct functions for beta1 and beta4 integrins. Curr Opin Genet Dev 7:672–682
Geiger B, Avnur Z, Kreis TE, Schles-singer J (1984a) The dynamics of cytoskeletal organization in areas of cell contact. J Muscle Res Cell Motil 5:195–234
Geiger B, Avnur Z, Rinnerthaler G, Hinssen H, Small VJ (1984b) Microfilament-organising centers in areas of cell contact: cytoskeletal interactions during cell attachment and locomotion. J Cell Biol 99:83–91
Gilmore AP, Burridge K (1996) Molecular mechanisms for focal adhesion assembly through regulation of protein–protein interactions. Structure 4:647–651
Kaneko Y, Lindsay L, Murphy CR (2008) Focal adhesions disassemble during early pregnancy in rat uterine epithelial cells. Reprod Fertil Dev 20:892–899
Kaufmann S, Piekenbrock T, Goldmann WH, Bärmann M, Isenberg G (1991) Talin binds to actin and promotes filament nucleation. FEBS Lett 284:187–191
Ljungkvist I (1972) Attachment reaction of rat uterine luminal epithelium. IV. The cellular changes in the attachment reaction and its hormonal regulation. Fertil Steril 23:847–865
Lo SH (2006) Focal adhesions: what’s new inside? Dev Biol 294:280–291
Macphee DJ, Lye SJ (2000) Focal adhesion signaling in the rat myometrium is abruptly terminated with the onset of labor. Endocrinology 141:274–283
Murphy CR (1993) The plasma membrane of uterine epithelial cells: structure and histochemistry. Prog Histochem Cytochem 27:1–66
Murphy CR (2000) Junctional barrier complexes undergo major alterations during the plasma membrane transformation of uterine epithelial cells. Hum Reprod 15:182–188
Murphy CR (2001) The plasma membrane transformation: a key concept in uterine receptivity. Reprod Med Rev 9:197–208
Murphy CR (2004) Uterine receptivity and the plasma membrane transformation. Cell Res 14:259–267
Murphy CR, Rogers AW (1981) Effects of ovarian hormones on cell membranes in the rat uterus. III. The surface carbohydrates at the apex of the luminal epithelium. Cell Biophys 3:305–320
Psychoyos A (1973) Hormonal control of ovoimplantation. Vitam Horm 31:201–256
Schaller MD (2001) Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459–6472
Shion YL, Murphy CR (1995) The basal plasma membrane and lamina densa of uterine epithelial cells are both altered during early pregnancy and by ovarian hormones in the rat. Eur J Morphol 33:257–264
Sorenson CM, Sheibani N (1999) Focal adhesion kinase, paxillin, and Bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn 215:371–382
Tumbarello DA, Brown MC, Hetey SE, Turner CE (2005) Regulation of paxillin family members during epithelial–mesenchymal transformation: a putative role for paxillin delta. J Cell Sci 118:4849–4863
Turner CE (1994) Paxillin: a cytoskeletal target for tyrosine kinases. Bioessays 16:47–52
Turner CE (2000) Paxillin and focal adhesion signalling. Nat Cell Biol 2:231–236
Welsh AO (1993) Uterine cell death during implantation and early placentation. Microsc Res Techn 25:223–245
Wynn RM (1977) Biology of the uterus. Plenum press, London
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaneko, Y., Lecce, L. & Murphy, C.R. Ovarian hormones regulate expression of the focal adhesion proteins, talin and paxillin, in rat uterine luminal but not glandular epithelial cells. Histochem Cell Biol 132, 613–622 (2009). https://doi.org/10.1007/s00418-009-0641-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0641-x