Abstract
Peroxisomes are intimately involved in the metabolism of reactive oxygen species, in the synthesis of ether lipids and of polyunsaturated fatty acids as well as in the β-oxidation of bioactive and toxic lipid derivatives. Therefore, the metabolic pathways of this organelle might play an important role in pulmonary biology by protection of inner pulmonary surface epithelia against oxidative stress, induced by the high oxygen levels in the air and/or by regulation of the lipid homeostasis in pulmonary epithelia and the pulmonary surfactant film. In this article, original results on the distribution of peroxisomal marker proteins, involved in the biogenesis, ROS- and lipid-metabolism of this organelle in the bronchiolar epithelium and the alveolar region of the adult human lung in comparison to newborn and adult murine lungs are presented. In addition, we investigated the expression of the PEX11β-mRNA, encoding a protein involved in peroxisomal division. Our study revealed significant differences in the abundance and distribution of peroxisomal proteins in distinct cell types of the lung and different developmental stages and led to the discovery of species-specific differences in the peroxisomal compartment in pulmonary epithelia between mouse and man. Finally, the structure and general biology of pulmonary airways—with special emphasis on Clara cells—are reviewed and discussed in relation to peroxisomal metabolism and proliferation. Future prospects of peroxisomes and Pex11 proteins for pulmonary cell biology are highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peroxisomes are ubiquitous organelles, present in virtually all eukaryotic cells except for erythrocytes and spermatozoa. Their metabolic functions and enzyme composition varies between distinct cell types, tissues and organ systems. In addition, peroxisomes are very flexible organelles, adjusting their number and enzyme composition to metabolic needs and to cellular demands. Peroxisomal functions have been comprehensively investigated in liver and kidney, in which these organelles are intimately involved in the metabolism of reactive oxygen species (ROS) and lipids. In contrast, only very little is known about peroxisomal metabolism in the lung. Since the lung is the target of various forms of reactive oxygen and nitrogen species (ROS and RNS), due to exposition to high concentrations of oxygen on its large inner surface (Rahman and MacNee 2000), peroxisomes might perform important protective functions in this organ.
Pulmonary airways are exposed to high concentrations of environmental oxidants, eventually leading to oxidation of proteins, DNA and lipids and therefore causing direct lung injury. The first line of defence against the oxidants are non-enzymatic antioxidants, such as glutathione, vitamin C and β-carotene as well as ether lipids (plasmalogens) and polyunsaturated fatty acids (PUFA) in the plasma membranes of airway epithelial cells or in the surfactant film, covering the alveolar region. Interestingly, crucial steps in the synthesis of these lipids occur in peroxisomes. Enzymatic antioxidant enzymes are responsible for the second line of defence against ROS, such as superoxide dismutases, catalase, glutathione peroxidases and peroxiredoxins. They are degrading various types of ROS and are localized in different pulmonary cell types as well as distinct intracellular subcompartments (Karnati and Baumgart-Vogt 2008; Immenschuh and Baumgart-Vogt 2005). If the fragile balance between the ROS production and the defensive capacity of the antioxidant system is severely disturbed, pathological alterations may occur in the affected tissue, leading to lung injury or diseases. In this respect, it is noteworthy that oxidative stress alters the transcriptional activity of various genes, including the ones encoding proteins of signalling pathways of the inflammatory response. Increased levels of ROS have been implicated in various airway diseases, such as asthma, chronic obstructive pulmonary disease and pulmonary fibrosis (Rahman and MacNee 2000).
Peroxisomes might protect the pulmonary airway epithelium by three different mechanisms: (1) their high and adaptable content in various antioxidative enzymes, (2) their involvement in the synthesis of plasmalogens and PUFA, as well as (3) their capacity to degrade a variety of the toxic and bioactive lipid derivatives via their β-oxidation systems (for a review see Karnati and Baumgart-Vogt 2008). In addition to the peroxisomal marker enzyme catalase, several other antioxidant enzymes have been described in this organelle, such as Cu, Zn-superoxide dismutase (SOD1), glutathione peroxidase and peroxiredoxins I, V and VI (Immenschuh and Baumgart-Vogt 2005). Our previous comprehensive paper on peroxisomes in the lung has mainly focussed on the alveolar region, in which we have revealed the overall distribution and the heterogeneity of this organelle in various cell types of the alveolus and discussed its possible role in pulmonary lipid metabolism (Karnati and Baumgart-Vogt 2008). However, in this article only little information was given on peroxisomes in pulmonary airway epithelia and to date, nothing is known on the overall distribution of peroxisomal proteins in bronchiolar epithelial cells—such as Clara cells—of the human lung.
Peroxisomal proteins in bronchiolar epithelial cells
In the 1970s, peroxisomes were revealed with certainty solely in alveolar epithelial cells II (AECII) and Clara cells in electron microscopic studies by using catalase cytochemistry with the alkaline DAB method (Petrik 1971; Schneeberger 1972). No information was available from the literature on the distribution of peroxisomal proteins in other cell types of the lung, until our group described with a variety of morphological techniques the localization and distribution of different peroxisomal proteins—catalase, Pex13p, Pex14p, ABCD3, ACOX1 and thiolase—in various cell types of the alveolar region and conducting airways, including Clara cells and ciliated cells of bronchioles in the adult mouse lung (Karnati and Baumgart-Vogt 2008). Murine Clara cells possess larger peroxisomes, which are more abundant and contain stronger catalase activity in comparison to ciliated cells of the bronchiolar epithelium (Karnati and Baumgart-Vogt 2008). In addition, peroxisomes in murine Clara cells exhibit a strong heterogeneity in their enzyme content and are labelled with distinct intensities for the above mentioned peroxisomal markers (Karnati and Baumgart-Vogt 2008). Despite the limited information on peroxisomes in mouse lungs, no information is available on these organelles in the bronchiolar epithelium of the human lung. Therefore, the aim of this study is to give some more insights on peroxisomes in distinct pulmonary epithelial cells in newborn and adult murine as well as human lungs, to review the biology and to highlight the prospects of peroxisomes for the function of the epithelial cells of the bronchiolar epithelium.
Materials and methods
Most materials and methods were similar to the described details in one of our previous publications (Karnati and Baumgart-Vogt 2008). Shortly, human lung tissue was obtained from the University of Giessen Lung Center (UGLC). Non-transplanted areas of three human donor lungs, conserved for transplantation, fixed by immersion in 4% paraformaldehyde (PFA)-PBS and embedded into paraffin, were used. Adult mouse lungs were fixed by perfusion fixation as described (Karnati and Baumgart-Vogt 2008). In addition, the lungs of newborn or E18.5 mice were perfused via the heart with 4% PFA-PBS, pH 7.4. The paraffin-embedded left lung was cut and sections were further processed for application of immunohistochemical (IHC)-, immunofluorescence (IF)- and in situ hybridization (ISH)-procedures. The different labelling procedures were carried out according to Karnati and Baumgart-Vogt (2008) (for IHC and IF) and Grabenbauer et al. (2001) (for ISH). The digoxigenin-labelled cRNA-probe for the ISH-preparations was generated from a plasmid containing the PEX11β-cDNA, described in detail by Schrader et al. (1998). An mRNA (sense) probe for PEX11β was used in parallel for corresponding negative controls.
Results
Peroxisomes are abundant in the bronchiolar epithelium of the human lung
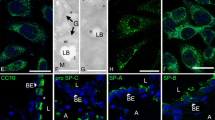
With the optimized immunofluorescence technique for the localization of peroxisomes in the lung, we were able to visualize these organelles in a punctuate staining pattern in ciliated cells and nonciliated Clara cells of the bronchiolar epithelium of the human airways (Fig. 1a–e). In contrast to our results obtained in the adult mouse lung (see Fig. 2h, i), peroxisomes were of similar size and numerical abundance in both cell types in the human bronchiolar epithelium (Fig. 1a) and were mainly localized at the apical poles of ciliated cells and nonciliated Clara cells. Furthermore, with the anti-Pex14p antibody, we were able to stain peroxisomes in alveolar macrophages very prominently, which covered the surface of the bronchioles in some regions in the human donor lungs. In stainings for Pex14p, peroxisomes were labelled with similar intensity in Clara cells and ciliated cells (Fig. 1a). In contrast, Clara cells in human bronchioli seemed to be labelled slightly less intensively for catalase than neighbouring ciliated cells (Fig. 1b). Peroxisomes were also strongly labelled with an antibody against ABCD3, a lipid transporter of the peroxisomal membrane, in both cell types. With this antibody, Clara cells could be visualized that contained large clusters of tubular peroxisomes (Fig. 1c). In addition, peroxisomes of the bronchiolar epithelium also contained acyl-CoA oxidase I (data not shown), the rate-limiting enzyme of the β-oxidation pathway 1 in the peroxisomal matrix. With the rabbit antibody directed against mouse peroxisomal 3-keto-acyl-CoA thiolase, only peroxisomes in Clara cells could be visualized with certainty. The high levels of ABCD3 and β-oxidation enzymes in human Clara cells suggest an active peroxisomal lipid metabolism in this cell type. In the alveolar region of human lungs, peroxisomes were mainly present in high abundance in AECII and alveolar macrophages, which were intensively stained for Pex14p and catalase (Fig. 1f, g). ABCD3 and peroxisomal thiolase were also found in AECII of human lungs (Karnati and Baumgart-Vogt 2008). Parallel sections of corresponding negative controls revealed the specificity of our immunofluorescence protocol. These preparations were always devoid of reaction product and only exhibited autofluorescent staining of residual bodies (lysosomes), erythrocytes or components of the extracellular matrix (Fig. 1e, h).
Localization of peroxisomal proteins in the adult human lung. Double-IF preparations for Pex14p (a), catalase (CAT) (b), ABCD3 (c), and thiolase (d), combined with CC10-labelling, depicting the distribution of peroxisomal proteins in distinct cell types of the bronchiolar epithelium in the human lung. Stainings for the peroxisomal proteins Pex14p (f) and catalase (CAT) (g) in distinct cell types of the alveolar region. Note that AECII and alveolar macrophages are strongly labelled for peroxisomal proteins. Appropriate negative controls with anti-rabbit IgG-Alexa488 and anti-goat IgG-Alexa594 (e, h). Nuclei were counterstained with TOTO-3-iodide. Bars represent a–c: 50 μm; f–h: 50 μm; d, e: 25 μm
Distribution of PEX11β-mRNA and of peroxisomal proteins in the murine lung. In situ hybridization (ISH) for the PEX11β-mRNA in newborn lung and liver tissues (a) and the corresponding mRNA (sense) control (inset in a), showing the high specificity of the ISH-reaction. Overviews of the immunohistochemical detection of catalase immunoreactivity (b) and an immunofluorescence (IF) preparation for Pex14p-labelling (c) in the left lung of E18.5 mice. Higher magnification views of IF-preparations for Pex14p and catalase localization (d, e). Double-IF for Pex14p, combined with pro SP-C (f) or CC10 (h) as well as for catalase, combined with pro SP-C (g) or α-tubulin (i) in the adult mouse lung, depicting the distribution and abundance of different peroxisomal proteins in distinct pulmonary cell types. Bars represent c: 50 μm; d, e: 25 μm; f–i: 20 μm
Peroxisomal enzymes are already present at high numerical abundance at birth
Immunohistochemical analysis of catalase distribution and protein abundance on E18.5 mouse lung tissue revealed the highest protein levels for this enzyme in the distal conducting airways and AECII (Fig. 2b). In addition, also in immunofluorescence preparations, a high numerical abundance of peroxisomes and protein levels of the peroxisomal markers, Pex14p and catalase in these cell types were noted (Fig. 2c–e). In comparison to adult lungs (Fig. 2f–i), peroxisomes were still more generally abundant in different cell types of the distal airways and the undifferentiated alveolar regions of the E18.5 lungs (Fig. 2b–e). In addition, peroxisomes in most cells of the yet undifferentiated alveolar region showed strong staining for catalase, most probably labelling AECII in the early phase in the process of their transdifferentiation into alveolar epithelial cells I (AECI). In contrast to E18.5 lungs, peroxisomes in the alveolar region of adult murine lungs were mainly present in high abundance in AECII and were more prominently stained for catalase than AECI (Fig. 2f, g). Stainings for Pex14p and catalase were also strong in large peroxisomes of Clara cells in bronchiolar epithelia of adult animals (Fig. 2h, i).
Furthermore, we wanted to visualize the distribution of Pex11βp, a protein involved in the regulation of peroxisome division, proliferation and the control of numerical abundance of the organelle in different cell types. This protein is deeply embedded into the peroxisomal membrane and good antibodies are not available world-wide, hampering its morphological visualization on the organelles. Therefore, we decided to localize the PEX11β mRNA in the newborn lung by using a large digoxigenin-labelled PEX11β-cRNA as a probe with the ISH-technique established previously in our group for the localization of mRNAs encoding for other peroxisomal proteins (Grabenbauer et al. 2001). Our results showed that the PEX11β mRNA is expressed in the lung at higher level than in the liver in newborn animals (Fig. 2a), even though peroxisomes are most abundant in hepatocytes, suggesting that the turnover of the organelles might be higher in the lung. The strongest expression of the PEX11β mRNA was observed in the distal airway epithelium and AECII of the alveolar epithelium (Fig. 2a). Control incubations of parallel sections with corresponding mRNA (sense)-probes for the ISH procedure were consistently negative, confirming the specificity of the method (see inset in Fig. 2a).
Discussion
The results of the present article revealed that peroxisomes are highly abundant in the bronchiolar epithelium of pulmonary airways and that species-specific differences in the peroxisomal compartment in distinct pulmonary epithelial cells exist between man and mice.
Major cell types of pulmonary epithelia
Lung epithelia are differentiated into two distinct major portions—the epithelia of the conducting airways and the epithelium of the respiratory region—both having sets of specialized cells, serving different functions in the respiratory system (Gail and Lenfant 1983). The functional integrity of the airway and alveolar epithelia is essential for the regular process of respiration in the lung (Plopper and Pinkerton 1992). Various cell types are present in the distinct lung epithelia, the major cell types of which—from proximal to distal regions—are: ciliated cells, intermediate and basal cells of the respiratory epithelium, mucous (goblet) cells, serous cells, nonciliated Clara cells and ciliated cells of the bronchiolar epithelium and AECI and AECII of the alveolar region (Sorokin 1988). Pulmonary epithelia are considered to be unique (1) for their exposure to high levels of environmental oxidants and (2) also for their unusual high concentrations of antioxidants and antioxidative enzymes (Halliwell and Gutteridge 1999).
Clara cell proteins and their involvement in ROS metabolism of the lung
Based upon morphology and histochemistry, Clara cells, the nonciliated cells of bronchioli, were first described by Kölliker (1881) and characterized in more detail by Clara (1937). Clara cells are nonmucous, nonserous, nonciliated, columnar to cuboidal secretory cells in the bronchiolar region of the pulmonary airways. The distribution and abundance of Clara cells in the airway epithelium is distinct among different species (e.g. mouse vs. human). In mouse bronchioli, Clara cells comprise 80% of the bronchiolar cells, whereas in the human lung, this cell type accounts only between 10 and 20% of the bronchiolar cells and ciliated cells predominate (Plopper et al. 1983). In contrast to this numerical difference, Clara cells of all species showed to be one of the most oxidant-resistant airway cell types. Their physiological role in pulmonary biology has not yet been entirely clarified, however, parts of their major functions include: (1) the secretion of the bronchioalveolar fluid as well as part of its constituent proteins and (2) the metabolism of xenobiotics, associated with cytochrome P450-dependent oxygenases. Moreover, precursor cells with Clara cell markers at the bronchioalveolar junction, expressing also low levels of the surfactant protein C (SP-C), are discussed as progenitor cells for the regeneration of the bronchiolar epithelium in the normal regeneration cycle and also during lung injury (Boyd 1977; Kim et al. 2005). Previous literature on this aspect showed that Clara cells actively regenerated the bronchiolar epithelium in an experimental animal model, using oxidant-induced damage to ciliated cells in rodent lungs (Evans et al. 1976). The major proteins secreted by Clara cells into the airway lumen and the extracellular lining fluid are the Clara cell 10-kDa protein (CC10) and the surfactant proteins A, B and D (SP-A, SP-B, SP-D). A series of investigations were carried out to elucidate the role of CC10 in airway biology, however, the exact functions of this protein remain elusive. Based on biochemical and biological properties of CC10 it was suggested that it (1) binds progestins or other lipophilic compounds, (2) binds calcium, (3) inhibits the secretory phospholipase A2 and (4) decreases phagocyte chemotaxis (Singh and Katyal 2000). In addition, the involvement of CC10 in the protection against oxidative stress has been extensively investigated in recent years. Studies using chronic ozone exposure revealed that Clara cells compensate the ozone-induced oxidative stress by increasing their number and CC10 secretion, as well as increasing the activity of antioxidant enzymes. These results were further substantiated by investigations of Mango et al., exposing CC10-deficient mice to an ozone challenge (Mango et al. 1998). Upon ozone exposure, these CC10-KO-mice showed elevated levels of oxidative stress, suggesting that the CC10 protein plays a vital role in regulating the ROS homeostasis of the airway epithelium. Furthermore, CC10-deficient mice are very sensitive to hyperoxia and exhibit alterations in inflammatory cytokine levels (Johnston et al. 1997). The high numerical abundance of peroxisomes in murine Clara cells and AECII and the prominent levels of peroxisomal enzymes in these cell types suggest that these organelles play a pivotal role in protecting the bronchiolar epithelium and the alveolar wall against high oxygen concentration and oxidative imbalance. In the human respiratory epithelium of bronchi (Karnati and Baumgart-Vogt 2008) and the bronchiolar epithelium (this article), the high numerical abundance of peroxisomes in ciliated cells may also contribute to the protection of the surface epithelia against ROS. Furthermore, peroxisomes might strongly influence the lipid metabolism in pulmonary epithelia, since they also contain high levels of lipid transporters and β-oxidation enzymes in these cell types (for a review on peroxisomal lipid metabolism in the lung see Karnati and Baumgart-Vogt 2008).
Alterations of “peroxisomal” antioxidant enzymes in airway epithelial cells
As mentioned in the “Introduction”, besides catalase, peroxisomes contain a variety of antioxidant enzymes, such as SOD1, glutathione peroxidase and peroxiredoxins I, V and VI. In addition, it is well known that oxidative stress also induces alterations in the peroxisomal compartment, such as tubulation of the organelles (Schrader and Fahimi 2006). Antibodies against SOD1, an enzyme with dual subcellular localization in the cytoplasm and the peroxisome, labelled the bronchiolar epithelium in healthy control subjects. Interestingly, the activity of this enzyme was decreased in asthmatic airway epithelia (Rahman et al. 2006). Furthermore, catalase and glutathione peroxidase activities also decreased in patients with asthma (Rahman et al. 2006). Therefore, it is most likely, that the peroxisomal compartment is affected in the airway epithelium of these patients. An altered peroxisomal lipid metabolism, such as a reduced ether lipid synthesis or a reduced peroxisomal β-oxidation of eicosanoids, important lipid mediators of inflammation, might perpetuate the inflammatory reaction. In this respect, it is noteworthy that activation of PPARs, the nuclear receptors regulating genes encoding peroxisomal proteins, ameliorates the inflammatory reaction in pulmonary airways (Paola and Cuzzocrea 2007). In addition, PPARγ interferes also with the regulation of genes encoding for antioxidant proteins. Furthermore, some peroxisome proliferators, such as clofibrate or nafenopin, increase the number of lamellar bodies in parallel to a significant increase in the number of peroxisomes in AECII of the rodent lung and it was suggested that surfactant synthesis is influenced in the lung by treatment with this compounds (Fringes et al. 1988; Fringes and Reith 1988). In addition, both compounds activate PPARα, which induces the gene transcription of the PEX11α gene, encoding a protein involved in peroxisome proliferation in the liver (Schrader et al. 1998). All of our results suggest a functional importance of the dynamic organelle “peroxisome” in airway protection and pulmonary cell biology.
Regulation of peroxisome proliferation by the proteins of the Pex11p family
The half life of the peroxisomes is only 3 days, wherefore peroxisomes are constantly formed or replaced by newly built peroxisomes, a process which is termed “peroxisomal biogenesis”. Peroxisomes are replicated by fission of pre-existing ones, regulated by proteins of the Pex11 family and DLP1/VpS1p (Delille et al. 2009). PEX11 proteins are components of the peroxisomal membrane in a wide variety of species including yeast, protozoan parasites and mammals. The mammalian family of Pex11 proteins contains three different isoforms: Pex11α, Pex11β, and Pex11γ (Li et al. 2002a; b). PEX11 deletion studies in yeast or fibroblasts revealed a significant reduction in numerical peroxisome abundance, whereas overexpression of PEX11β caused a pronounced increase in their abundance (Li and Gould 2002; Schrader et al. 1998). Overexpression of PEX11-cDNAs even led to a hyperproliferation of peroxisomes in distinct cell types. For more details on other proteins involved in peroxisome division and proliferation see Delille et al. (2009).
The vital importance of the Pex11βp for the survival of the organism was revealed by the generation of PEX11β deficient mice (Li et al. 2002b). Despite the presence of a reduced number of peroxisomes in all tissues, PEX11β KO animals are severely growth retarded and die shortly after birth, a phenotype similar to patients with Zellweger syndrome (Baumgart et al. 2003; Gärtner 2003). Interestingly, however, plasmalogens and very‐long‐chain fatty acid levels were normal in PEX11β KO-mice, suggesting that the defective peroxisome biogenesis rather than the disruption of peroxisomal metabolism led to the phenotype of these KO animals (Li and Gould 2002).
Future prospects for peroxisomes and Pex11β on pulmonary biology
The presence of high levels of the PEX11β-mRNA in distal airways in the newborn lung suggests that peroxisome proliferation and division is an important process in the developing airways and during alveolarization. Indeed, PEX11β-knockout mice exhibit less developed distal airways and reduced alveolarization in comparison to control animals (unpublished observation of E. Baumgart-Vogt). A detailed report on the pathological alterations of pulmonary airways and the alveolar region in these knockout animals will be published in a separate article in the near future. Delay and alterations in pulmonary development, however, certainly contribute to the early death of the PEX11β animals shortly after birth. Therefore, studies with lung tissue of PEX11β (-/-) animals for the elucidation of the molecular alterations due to peroxisome deficiency will provide more insights on the function of this organelle in pulmonary biology.
References
Baumgart E, Fahimi HD, Steininger H, Grabenbauer M (2003) A review of morphological techniques for detection of peroxisomal (and mitochondrial) proteins and their corresponding mRNAs during ontogenesis in mice: application to the PEX5-knockout mouse with Zellweger syndrome. Microsc Res Tech 61:121–138
Boyd MR (1977) Evidence for the Clara cell as a site of cytochrome P450-dependent mixed-function oxidase activity in lung. Nature 269:713–715
Clara M (1937) Zur Histobiologie des Bronchiaepithels. Z Mikrosk Anat Forsch 41:321–347
Delille HK, Alves R, Schrader M (2009) Biogenesis of peroxisomes and mitochondria: linked by division. Histochem Cell Biol. doi:10.2007/s00418‐009‐0561‐9
Evans MJ, Johnson LV, Stephens RJ, Freeman G (1976) Cell renewal in the lungs of rats exposed to low levels of ozone. Exp Mol Pathol 24:70–83
Fringes B, Reith A (1988) Two hypolipidemic peroxisome proliferators increase the number of lamellar bodies in alveolar cells type II of the rat lung. Exp Mol Pathol 48:262–271
Fringes B, Gorgas K, Reith A (1988) Clofibrate increases the number of peroxisomes and of lamellar bodies in alveolar cells type II of the rat lung. Eur J Cell Biol 46:136–143
Gail DB, Lenfant CJ (1983) Cells of the lung: biology and clinical implications. Am Rev Respir Dis 127:366–387
Gärtner J (2003) Is there a phenotype/genotype correlation in peroxisome biogenesis disorders (PBDs)? Adv Exp Med Biol 544:59–65
Grabenbauer M, Fahimi HD, Baumgart E (2001) Detection of peroxisomal proteins and their mRNAs in serial sections of fetal and newborn mouse organs. J Histochem Cytochem 49:155–164
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, Oxford
Immenschuh S, Baumgart-Vogt E (2005) Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal 7:768–777
Johnston CJ, Mango GW, Finkelstein JN, Stripp BR (1997) Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol 17:147–155
Karnati S, Baumgart-Vogt E (2008) Peroxisomes in mouse and human lung: their involvement in pulmonary lipid metabolism. Histochem Cell Biol 130:719–740
Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823–835
Kölliker A (1881) Zurkeniniss des Baues der Lunge des Menschen. Verh Phys Med Ges 16:1–24
Li X, Gould SJ (2002) PEX11 promotes peroxisome division independently of peroxisome metabolism. J Cell Biol 156:643–651
Li X, Baumgart E, Dong GX, Morrell JC, Jimenez-Sanchez G, Valle D, Smith KD, Gould SJ (2002a) PEX11alpha is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor alpha-mediated peroxisome proliferation. Mol Cell Biol 22:8226–8240
Li X, Baumgart E, Morrell JC, Jimenez-Sanchez G, Valle D, Gould SJ (2002b) PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol Cell Biol 22:4358–4365
Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR (1998) Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol 275:L348–L356
Paola RD, Cuzzocrea S (2007) Peroxisome proliferator-activated receptors and acute lung injury. PPAR Res 2007:63745
Petrik P (1971) Fine structural identification of peroxisomes in mouse and rat bronchiolar and alveolar epithelium. J Histochem Cytochem 19:339–348
Plopper CG, Pinkerton KE (1992) Overview of diversity in the respiratory system of mammals. In: Parent RA (ed) Comparative biology of the normal lung I. CRC Press, Boca Raton, FL, pp 3–5
Plopper CG, Mariassy AT, Wilson DW, Alley JL, Nishio SJ, Nettesheim P (1983) Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res 5:281–294
Rahman I, MacNee W (2000) Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16:534–554
Rahman I, Biswas SK, Kode A (2006) Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 533:222–239
Schneeberger EE (1972) A comparative cytochemical study of microbodies (peroxisomes) in great alveolar cells of rodents, rabbit and monkey. J Histochem Cytochem 20:180–191
Schrader M, Fahimi HD (2006) Growth and division of peroxisomes. Int Rev Cytol 255:237–290
Schrader M, Reuber BE, Morrell JC, Jimenez-Sanchez G, Obie C, Stroh TA, Valle D, Schroer TA, Gould SJ (1998) Expression of PEX11beta mediates peroxisome proliferation in the absence of extracellular stimuli. J Biol Chem 273:29607–29614
Singh G, Katyal SL (2000) Clara cell proteins. Ann N Y Acad Sci 923:43–58
Sorokin SP (1988) The respiratory system: cell and tissue biology. In: Weiss L (ed) Chapter 25, Sixth edn. Urban and Schwarzenberg, Munich, pp 751–814. ISBN: 753‐541‐72176‐72176
Acknowledgments
The technical assistance of Andrea Klein, Elke Richter and Magdalena Gottwald is gratefully acknowledged. In addition, we thank Profs. D.I. Crane, S.J. Gould, P.P. Van Veldhoven and A. Völkl for providing of antibodies (for addresses see Karnati and Baumgart-Vogt 2008). We are indebted also to Dr. M. Schrader (University of Aveiro, Portugal) for the generous gift of the plasmid with the murine PEX11β-cDNA. This study was supported by funds of the PhD-programme and LOM-funds of the Medical Faculty of the Justus Liebig University, Giessen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karnati, S., Baumgart-Vogt, E. Peroxisomes in airway epithelia and future prospects of these organelles for pulmonary cell biology. Histochem Cell Biol 131, 447–454 (2009). https://doi.org/10.1007/s00418-009-0566-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0566-4