Abstract
The extracellular matrix (ECM) plays a prominent role in ovarian function by participating in processes such as cell migration, proliferation, growth, and development. Although some of these signaling processes have been characterized in the mouse, the relative quantity and distribution of ECM proteins within developing follicles of the ovary have not been characterized. This study uses immunohistochemistry and real-time PCR to characterize the ECM components type I collagen, type IV collagen, fibronectin, and laminin in the mouse ovary according to follicle stage and cellular compartment. Collagen I was present throughout the ovary, with higher concentrations in the ovarian surface epithelium and follicular compartments. Collagen IV was abundant in the theca cell compartment with low-level expression in the stroma and granulosa cells. The distribution of collagen was consistent throughout follicle maturation. Fibronectin staining in the stroma and theca cell compartment increased throughout follicle development, while staining in the granulosa cell compartment decreased. Heavy staining was also observed in the follicular fluid of antral follicles. Laminin was localized primarily to the theca cell compartment, with a defined ring at the exterior of the follicular granulosa cells marking the basement membrane. Low levels of laminin were also apparent in the stroma and granulosa cell compartment. Taken together, the ECM content of the mouse ovary changes during follicular development and reveals a distinct spatial and temporal pattern. This understanding of ECM composition and distribution can be used in the basic studies of ECM function during follicle development, and could aid in the development of in vitro systems for follicle growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The functional unit of the female gonad is the ovarian follicle, which contains three cell types: a centrally-located oocyte, one or more layers of granulosa cells and an outer layer of theca cells (Gomes et al. 1999). The extracellular matrix (ECM) provides structural support to the follicle, maintains cellular organization and connectivity, and provides biochemical signals that promote follicle development and maturation (Rodgers et al. 1999a, b). As in the other tissues, the ovarian ECM functions as a reservoir for growth factors and provides proliferation and differentiation signals to the granulosa cells (Carnegie 1990; Aharoni et al. 1997; Oktay et al. 2000). For example, the composition of the ECM affects steroid production by granulosa cells, a marker of differentiation (Furman et al. 1986; Aharoni et al. 1997). The ECM varies with follicle maturation and follicle stage, and may function as a dynamic regulator of follicle maturation. Folliculogenesis defines the process of explosive follicle growth from primordial to Graafian follicles that involves a complex series of cellular responses.
The follicular microenvironment changes substantially during folliculogenesis due to follicle movement through the ovary, growth of the follicle, and a varying extracellular milieu. The ECM may regulate folliculogenesis through the composition that varies with both follicle stage and cellular compartment. In the bovine, ECM composition varies during growth (Rodgers et al. 1998; van Wezel et al. 1998) and changes during atresia (Irving-Rodgers et al. 2002). The ECM in the ovary has been characterized for several species, such as bovine (Zhao and Luck 1995; Rodgers et al. 1998; McArthur et al. 2000; Rodgers et al. 2001), human (Yamada et al. 1999; Iwahashi et al. 2000), rat (Rajah et al. 1994; Frojdman et al. 1998), ovine (Huet et al. 2001; Le Bellego et al. 2002), and equine (Gentry et al. 1996), with type I collagen, type IV collagen, fibronectin, and laminin the most commonly examined ECM proteins (Bortolussi et al. 1989; Rajah et al. 1994; Figueiredo et al. 1995; Zhao and Luck 1995; Huet et al. 1997). The mouse has been a widely used model to examine folliculogenesis in vivo (Matzuk 2000) and in vitro (Cortvrindt and Smith 1998; Spears et al. 1998; O’Brien et al. 2003; Kreeger et al. 2005; Kreeger et al. 2006), yet the mouse ovarian ECM has not been well described relative to other species.
This report characterizes expression patterns of the primary ovarian ECM components based on follicle stages and cellular compartment in the mouse. As the ovary is continuously remodeled with follicle growth, development, and atresia, we characterize the distribution of type I collagen, type IV collagen, fibronectin, and laminin within the various follicles stages. Immunohistochemistry is used to determine the spatial and temporal distribution of ECM molecules within ovaries extracted from mice of different ages. The ages examined (8-, 10-, 12-, 16-, 23-, and 43-day-old mice) correlate to milestones used in systems for in vitro maturation of follicles. Additionally, mRNA levels for these ECM molecules are quantified using real-time PCR. This understanding of the ECM composition during follicular development contributes simultaneously to both basic research and translational applications, such as the design of culture systems that promote follicle development and oocyte maturation.
Materials and methods
Subject and sample preparation
C57B1/6 female and CBA male mice (Harlan, Indianapolis, IN, USA) were maintained in breeding colonies at Northwestern University (Evanston, IL, USA). The mice were housed in a temperature- and light-controlled environment of 12 h light/12 h dark photoperiod. Phyto-estrogen free food and water were provided ad libitum. All animals were treated in accordance with the National Institute of Health’s (NIH, Bethesda, MD, USA) Guide for the Care and Use of Laboratory Animals and the established IACUC protocol at Northwestern University.
Ovaries were dissected from 8-, 10-, 12-, 16-, 23-, and 43-day-old mice. A total of 12 ovaries per developmental stage were analyzed throughout this study. Ovaries from younger mice were kept in the bursa sac to keep surface epithelial cells intact. The samples were then fixed in 4% paraformaldehyde for 8–12 h and washed three times in 50% ethanol and three times in 70% ethanol at 4°C. The tissues were stored in 70% ethanol at 4°C for up to 1 week before processing. Samples were processed overnight and embedded in paraffin by the Pathology Core Laboratory (Northwestern University, Chicago, IL, USA). Sections (4 μm) were cut on a microtome and placed on Superfrost-Plus glass slides. Slides were allowed to dry overnight and stored at 4°C until used for immunohistochemistry. For each antibody, 10–15 sections from at least four ovaries, all from different mice, were examined. At least three follicles per developmental stage per ovary were examined at each time point.
Antibodies
Primary antibodies were rabbit polyclonal antibodies raised against mouse type I collagen (Research Diagnostics, Inc., Flanders, NJ, USA), rabbit polyclonal antibodies raised against mouse type IV collagen (Chemicon, Temecula, CA, USA), rabbit polyclonal antibodies raised against laminin from Englebreth Holm-Swarm mouse sarcoma (Sigma, St. Louis, MO, USA), and rabbit polyclonal antibodies raised against human fibronectin (Sigma) and were used at final concentrations of 25, 5, 6, and 7 μg/ml, respectively. Primary antibodies were diluted in 10% normal goat serum (Vector Laboratories, Burlingame, CA, USA) in 3% bovine serum albumin (BSA; Serologicals Corporation, Norcross, GA, USA) in tris-buffered saline (TBS). The secondary antibody used was biotinylated anti-rabbit IgG (H + L), made in goat (Vector Laboratories). Secondary antibodies were diluted 1:200 in 3% BSA–TBS. Antibody concentrations were optimized using tissue sections from 23-day-old mice using different combinations of primary and secondary antibody dilutions. Negative controls included primary antibodies pre-adsorbed with their corresponding antigen and incubation with serum instead of the primary antibody. Antigens and blocking peptides used included type I collagen (Sigma), type IV collagen from EHS (Sigma), laminin from EHS (Sigma), fibronectin from EHS (Sigma) at final concentration of 3,500, 1,990, 1,200, and 990 μg/ml, respectively.
Immunohistochemistry
Extracellular matrix proteins were immunolocalized using an immunoperoxidase method. First, slides were deparaffinized and rehydrated. To unmask antigenic sites, slides were heated in a 0.01 M solution of sodium citrate in a microwave oven (Panasonic NN-S666BA household microwave oven) for 2 min on the high power setting, followed by 7 min on the low power setting. After two washes in tris-buffered saline with tween (TBS-T) (15 min each), slides were incubated in 3% hydrogen peroxide in TBS to inhibit endogenous peroxidases (15 min). Endogenous biotins and biotin-binding substances were blocked using the Vectastain Avidin/Biotin Blocking Kit (Vector Laboratories). Tissue sections were then incubated with 10% serum in 3% BSA–TBS for 1 h at room temperature. Slides were then incubated with the appropriate primary antibody diluent overnight at 4°C. After three washes in TBS-T (5 min each), tissue sections were incubated in the corresponding secondary antibody diluent for 30 min at room temperature. Slides were washed three times in TBS-T and incubated with the Avidin-Biotin reagent from the Vectastain ABC Kit for 30 min (Vector Laboratories). Sections were washed five times with TBS-T (5 min each) and incubated with a working solution of 3,3′-diaminobenzidine from the Vector DAB Substrate Kit for Peroxidase (Burlingame, CA, USA) for 1.5 min. The reaction was stopped with H2O for 5 min. Slides were then counterstained with hematoxylin and dehydrated before being mounted in Cytoseal XYL Mounting Medium (Richard-Allan Scientific, Kalamazoo, MI, USA) and covered with glass cover slips.
Microscopy and photography
Slides were viewed and photographed using a Nikon Eclipse E600 microscope with a Diagnostic Instruments, Inc. 11.2 Color Mosaic camera and Spot Advanced 4.0.2 Software (Diagnostic Instruments, Inc.). Slides were also viewed using a Nikon Eclipse E200 microscope.
Staining intensity grading
Staining intensity was graded in a semi-quantitative fashion; 0, none; 1, weak; 2, moderately intense; 3, very intense. Measurement of staining intensity was performed by two investigators throughout all experiments.
Follicle classification
Follicles were classified according to commonly applied terms for the mouse. Primordial follicles consisted of a small oocyte surrounded by a layer of flattened (squamous) pregranulosa cells. Primary follicles contained a larger oocyte surrounded by a single layer of cuboidal granulosa cells. Preantral follicles contained an oocyte and were classified as two-layered secondary or multi-layered secondary depending on the number of granulosa cell layers present. These follicles were surrounded by theca cells. Antral follicles consisted of a large oocyte surrounded by cumulus cells, a fluid-filled antrum, granulosa cells, and theca cell layers. The ovarian surface epithelium (OSE) was identified as the flattened to cuboidal elongated cells lining the perimeter of the ovary.
RNA analysis
Total RNA from the extracted ovaries of all the ages was isolated using TRIzol reagent (Life Technologies Inc., Rockville, MD, USA). RNA concentration was determined by absorption at 260 nm on a spectrophotometer. RNA samples (5 μg) were then primed with random hexamers and reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) according to manufacturer’s instructions.
Real-time PCR analysis was performed using The ABI 7300 (Applied Biosystems, Foster City, CA, USA) on all ECM components. Real time PCR quantifies the PCR product on a cycle-by-cycle basis (Wang and Brown 1999; Bustin 2000). Specific primer/probe sets for the alpha I chain of type I collagen, alpha I chain of type IV collagen, fibronectin 1, alpha I chain of laminin, and the internal control human 18S ribosomal RNA were obtained from Applied Biosystems (Table 1). Final volume of the reaction was 25 μl. Amplification was performed using a 2 min 50°C decontamination stage and a 10 min 95°C enzyme activation stage followed by 40 cycles of 15 s 90°C melting stages and 1 min 60°C annealing stages.
Results
mRNA expression
Real-time PCR was used to quantify mRNA levels for the various ECM proteins for whole ovaries isolated from mice at different ages. As mice age, the distribution of follicles varies as the follicles are recruited and undergo atresia. The results for each experimental day were determined relative to the level of expression observed at the earliest time-point studied for each ECM molecule individually (Table 2). The level of mRNA for fibronectin increased about fourfold from day 10 to day 12, before returning to baseline. For the remaining ECM molecules examined, message levels varied by less than a factor of 1 throughout the period examined.
Type I collagen
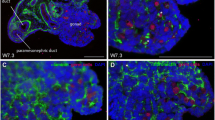
Type I collagen was immunolocalized to the stroma, follicular compartment, and OSE (outer cellular layer of the ovary). Homogeneous staining for type I collagen was found throughout the stroma for ovaries obtained at all ages. Primary, secondary, and preantral stage follicles had type I collagen in the granulosa and theca cell compartment of the follicle (Fig. 1a). Additionally, large antral follicles had staining in the antral fluid (Fig. 1b). No staining was observed in the granulosa cells of the primordial follicle. There was intense staining for type I collagen in the area surrounding the oocyte (Fig. 1b). Prior to day 16, staining was homogeneous between the follicle and stromal compartment; however, in ovaries of 16-, 23-, and 43-day old mice, the follicular compartments were more darkly stained than the stroma (Fig. 1a). High levels of type I collagen were observed in the OSE (Fig. 1c).
Immunolocalization of type I collagen in ovaries isolated at a Day 23 and b–d Day 43. a Photomicrograph staining of collagen type I in primary, secondary, and antral follicles (Original magnification: 40×). Photomicrograph of type I staining in and around b follicular fluid, oocytes, granulosa cells and stroma, (40×) c ovarian surface epithelium (OSE, 100×), and d control ovary (10×). Black asterisk follicular fluid, white arrowhead oocyte and surrounding area, black arrowhead stroma compartment, white arrow granulosa cell compartment, red arrow OSE, size bar 20 μm
Type IV collagen
Type IV collagen immunolocalized to the theca cell compartment and stroma for all follicle stages and ovaries from different aged animals. Consistent low level staining was observed in the stroma for ovaries isolated at different ages. Primordial follicles did not react with type IV collagen. Staining occurred most intensely around the theca compartment, initially appearing in primary follicles and increasing in intensity with more mature follicle stages (Fig. 2a). The staining formed a dark region outlining the basement membrane and defined the barrier between the follicle and the stroma (Fig. 2b). Type IV collagen abundance was greater in ovaries collected from older animals (16-, 23- and 43-day old) (Fig. 2a, b) than in the younger ovaries (8-, 10-, and 12-day old) (Fig. 2c), which is expected as mature follicles have greater collagen IV content and ovaries from older animals have more mature follicles. Granulosa cells stained positive for type IV collagen to a much lesser degree than the theca. Interestingly, the pubertal ovary (day 43) showed more immunoreactivity in the granulosa cells than all prepubertal ovaries (Fig. 2d). Inconsistent immunoreactivity was observed in the OSE (Fig. 2d).
Immunolocalization of type IV collagen in ovaries. a Antral, secondary, and primary follicles in a Day 16 ovary (40×). b Thecal layer and granulosa cell compartments around antral follicles (40×). c Primary and secondary follicles of Day 10 ovary (40×). Note that the oocyte in lower right corner is atretic. d OSE and secondary follicles in Day 43 ovary (40×). e Control ovary (10×). Black arrow theca compartment, black arrowhead stroma compartment staining, red arrow OSE staining, size bar 20 μm
Fibronectin
Fibronectin immunolocalized to the stroma, follicles, and OSE, though the consistency and intensity of staining was less than with other ECM components. The stroma of younger ovaries (8-, 10-, and 12-day old) did not stain as intensely as the older ovaries (16-, 23-, and 43-day old) (Fig. 3a, b). Additionally, staining was inconsistent with irregular patches in the stroma and the granulosa cells in primary and secondary follicles (Fig. 3a, b) of younger ovaries; however, this pattern in the granulosa cells was also seen in young follicles of older ovaries (Fig. 3c). In tissue samples from 16-, 23-, and 43-day-old mice, fibronectin became more uniform throughout the ovary with slightly darker staining outside the granulosa cell compartment in the basement membrane of later stage secondary and antral follicles (Fig. 3b). Fibronectin darkly stained the follicular fluid (Fig. 3d) and was detected in OSE cells (Fig 3e).
Immunolocalization of fibronectin in the ovary. a Primary and secondary follicles within surrounding stroma of Day 16 ovary. b Stroma surrounding primary and secondary follicles of Day 16 ovary (40×). c Secondary and antral follicles of Day 43 ovary (40×). d Antral follicle with follicular fluid of Day 43 ovary (40×). e OSE of Day 23 ovary. f Control ovary (10×). White arrow granulosa cell compartment staining, black arrowhead stroma compartment staining, black asterisk follicular fluid staining, red arrow OSE staining, size bar 20 μm
Laminin
Laminin immunolocalized to the stroma, follicular compartment and was observed adjacent to the OSE. The stroma of older ovaries (12-, 16-, 23-, and 43-day old) was darker (Fig. 4a) compared to younger ovaries (8-, 10-day old) (Fig. 4b). Laminin was immunoreactive in the granulosa cells and theca cells of primary, secondary and antral follicles with darker staining in the antral follicles (Fig. 4a–d). There was staining throughout the theca cells with linear staining encircling each follicle, dividing the granulosa cell compartment from theca cells in larger follicles (Fig. 4a–d). This pattern was also apparent in primordial follicles dividing the granulosa cell compartment from the stroma (Fig. 4b). This staining appeared to outline the follicular basement membrane. The OSE, itself, was not immunoreactive but a basal lamina-like barrier between the OSE and stroma was stained positive by laminin (Fig. 4d).
Immunolocalization of EHS laminin. a Antral follicle from Day 16 ovary (40×). b Primordial and primary follicles of Day 8 ovary (40×). c Secondary and antral follicles of Day 23 ovary (40×). d OSE cells and stroma of Day 23 ovary (40×). e Control ovary (10×). Black arrow theca cell compartment staining, white arrow granulosa cell compartment staining, white arrowhead ring-like staining, red arrow OSE staining, size bar 20 μm
Discussion
This report characterizes the mouse ovarian ECM protein expression, composition, abundance, and distribution, with a particular emphasis on the distribution within the various follicles stages of development (summarized in Fig. 5; Table 3). The expression of mRNA for the selected ECM molecules is consistent throughout the ovaries during development, with the exception of fibronectin, which shows an increase at day 10 of development.
Schematic of individual ECM distribution for various follicle classes. Each follicle is drawn in relative proportions. In the mouse ovary, primordial follicles are approximately 20 μm in diameter, and antral follicles range from 350–450 μm in diameter. Each ECM is depicted individually within the different follicle classes. a Type I collagen, b Type IV collagen, c Fibronectin and d Laminin
The ECM composition (type I collagen, type IV collagen, fibronectin, and laminin) is dynamic during the early stages of folliculogenesis. Oocyte morphology in younger ovaries differed from those oocytes found in older ovaries, tending to be less round and uniform in shape. Type I collagen was detected in all compartments of all follicles, whereas type IV collagen, fibronectin, and laminin were localized to specific areas. The primordial follicle has a ring of laminin defining the basement membrane, and no other ECM component investigated was found in these follicles. Primary follicles acquire type I collagen in the granulosa cell compartment and maintain the laminin-composed basement membrane. As the follicles develop to two-layered and multi-layered secondary follicles, type IV collagen and fibronectin are expressed. In the two-layered secondary follicles, type I collagen was localized to the granulosa and theca cells while fibronectin stained the granulosa cells in an irregular pattern, and both laminin and type IV collagen localized to the basement membrane and theca cell layers. In the multi-layered secondary follicles, fibronectin staining was detected in the granulosa cells, stroma and the theca cells. In antral follicles, fibronectin was found in the follicular fluid and lightly stained the theca cells, while type IV collagen and laminin stained the basement membrane and theca layers. These staining patterns observed in each ECM molecule may correlate to their function in follicle development.
Type I collagen was the most ubiquitously expressed ECM molecule within the mouse ovary, localized in all compartments, and most noticeably darkly staining the OSE. Reports with other animal models have not indicated type I collagen localization to the OSE, yet freshly isolated rat OSE and an immortal OSE line was shown to produce Type I collagen (Auersperg et al. 1991). Type I collagen has been widely used with in vitro cultures of granulosa cells and follicles (Carnegie et al. 1988; Bussenot et al. 1993; Huet et al. 2001). Type I collagen gels affect the shape and steroidogenesis of granulosa cells. Ovine granulosa cells cultured on type I collagen had low proliferation and maintained estradiol secretion (Huet et al. 2001). In a three-dimensional model that employs alginate hydrogels to structurally support the follicle and maintain cell–cell connections, mouse two-layer secondary follicles increased in size when type I collagen was blended with alginate relative to gels without collagen (Kreeger et al. 2006). However, culture of multi-layered secondary follicles in alginate hydrogels produced no significant effect on follicle growth, yet increased progesterone production and decreased both inhibin A and estradiol levels.
Fibronectin staining varied significantly between early and late stage follicles, consistent with reports for other species. In the mouse, primary and secondary follicles had irregular patches among the granulosa cells closest to the oocyte, whereas later stage follicles had intense fibronectin staining in the stroma, the exterior of the granulosa cell compartment, and follicular fluid, and slight staining in the theca cells. The pattern of fibronectin staining in the earlier stage follicles of the mouse is similar to that of early stage follicles of the rat, both with immunoreactivity in the granulosa cells (Akkoyunlu et al. 2003). In equine, fibronectin staining correlated with the size of the follicle and estradiol levels, whereas in the human this pattern correlated with follicle size and progesterone levels. Fibronectin could be acting differently during the early and late stages of folliculogenesis. Interestingly, the follicular fluid was intensely immunoreactive for fibronectin, consistent with observations from rat (Akkoyunlu et al. 2003), equine (Gentry et al. 1996) and human (Tsuiki et al. 1988). This observation suggests that fibronectin may be soluble or loosely associated with the ECM, thus likely serving a signaling function rather than a structural role.
Laminin and type IV collagen consistently stained the theca compartment, with laminin localizing to a ring-like structure immediately surrounding the granulosa cell in all stage follicles and type IV collagen reactive in the theca compartment as it developed. Collagen IV presented in the theca layer of the follicle, with more intense staining detected in later stage follicles and older ovaries. These observations are consistent with staining in the rat (Bortolussi et al. 1989; Frojdman et al. 1998) and bovine (Zhao and Luck 1995; Rodgers et al. 1998; Irving-Rodgers et al. 2002). Laminin also stains the basal lamina in the pig and rabbit (Lee et al. 1996) and type IV collagen was found in the theca of human ovaries (Iwahashi et al. 2000). The ring-like band of laminin observed around the basement membrane of the follicle was also observed between the OSE and the stroma. A basement membrane separating the OSE and the ovarian stroma has been known to exist, with little characterization of the ECM composition (Auersperg et al. 1991, 2001). Laminin clearly defined the barrier between the OSE and stroma, whereas type I collagen stained the OSE cell compartment for all age ovaries.
Knowledge of this dynamic ECM composition combined with studies of ECM function may aid the development of in vitro culture systems for the maturation of ovarian follicles. In vitro culture systems are being developed to enable egg banking by ovary cryopresevation, which could preserve fertility when traditional in vitro fertilization is not possible, such as in cases of reproductive disorders (e.g., PCOS) or chemotherapy-induced infertility. A study incorporating various ECM components into a three-dimensional follicle culture system demonstrated that follicle size, steroidogenesis, and oocyte maturity was dependent upon the ECM composition (Kreeger et al. 2006). Additionally, the functions of the ECM were dependent upon the stage at which the follicles were placed into the in vitro system. Incorporating whole collagen or laminin into whole ovarian cultures increased follicle densities (Oktay et al. 2000), with collagen and laminin having opposite effects on the growth of multi-layered secondary follicles in the presence of activin A. Finally, matrigel, which contains a mixture of ECM proteins derived from mouse tumor cells, increased the follicle survival by 10–15 days in ovarian culture tissue that had been cryopreserved (Hovatta et al. 1997). These culture systems have demonstrated a role for ECM in follicle growth and oocyte maturation, though studies have been limited, in part, due to incomplete knowledge of the ECM composition. Characterization of the ECM in larger follicles has been more common than with smaller follicles. However, characterization of these smaller, more immature follicles is necessary, as these follicles are the most abundant in younger ovaries and are most appropriate for cryopreservation (Smitz and Cortvrindt 2002). This study suggests that the composition of ECM molecules subtly varies with stage of follicle development as well as with age of the animal from which the follicle was derived. Thus, conditions for in vitro culture systems may require specialized ECM environments that vary based on the stage of both follicular and ovarian development.
The ECM is a dynamic regulator of follicle development whose expression varies with follicle stage and cellular compartment. It has been shown to affect follicle growth, survival, steroidogenesis, basement membrane formation, and oocyte maturation, and interactions between specific ECM components and cellular compartments can affect cell lineage decisions and, subsequently, its function (Rodgers et al. 1999). This report characterized the distribution of four ECM components (Type I and Type IV collagen, fibronectin, laminin) that are widely used for studies in ovarian function and follicle development. Knowledge of the ECM composition and distribution during folliculogenesis could be used to create the appropriate signaling environment for folliculogenesis, act as a marker for standard development, and aid in the development of stage-specific in vitro culture conditions, which may ultimately be translated into systems to facilitate preservation of fertility by ovary cryopreservation.
References
Aharoni D, Meiri I, Atzmon R, Vlodavsky I, Amsterdam A (1997) Differential effect of components of the extracellular matrix on differentiation and apoptosis. Curr Biol 7:43–51
Akkoyunlu G, Demir R, Ustunel I (2003) Distribution patterns of TGF-alpha, laminin and fibronectin and their relationship with folliculogenesis in rat ovary. Acta Histochem 105:295–301
Auersperg N, Maclaren I AKruk PA (1991) Ovarian surface epithelium:autonomous production of connective tissue-type extracellular matrix. Biol Reprod 44:717–724
Auersperg N, Wong AS, Choi KC, Kang S KLeung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22:255–288
Bortolussi M, Zanchetta R, Doliana R, Castellani I, Bressan GM, Lauria A (1989) Changes in the organization of the extracellular matrix in ovarian follicles during the preovulatory phase and atresia. An immunofluorescence study. Basic Appl Histochem 33:31–38
Bussenot I, Ferre G, Azoulay-Barjonet C, Murgo C, Vieitez G, Parinaud J (1993) Culture of human preovulatory granulosa cells: effect of extracellular matrix on steroidogenesis. Biol Cell 77:181–186
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–193
Carnegie JA (1990) Secretion of fibronectin by rat granulosa cells occurs primarily during early follicular development. J Reprod Fertil 89:579–589
Carnegie JA, Byard R, Dardick I, Tsang BK (1988) Culture of granulosa cells in collagen gels: the influence of cell shape on steroidogenesis. Biol Reprod 38:881–890
Cortvrindt R, Smitz J (1998) Early preantral mouse follicle in vitro maturation: oocyte growth, meiotic maturation and granulosa-cell proliferation. Theriogenology 49:845–859
Figueiredo J, Hulshof SCJ, Thiry M, Van den Hurk R, Bevers MM, Nusgens B, Beckers JF (1995) Extracellular matrix proteins and basement membrane: their identification in bovine ovaries and significance for the attachment of cultured preantral follicles. Theriogenology 43:845–858
Frojdman K, Pelliniemi LJ, Virtanen I (1998) Differential distribution of type IV collagen chains in the developing rat testis and ovary. Differentiation 63:125–130
Furman A, Rotmensch S, Kohen F, Mashiach S, Amsterdam A (1986) Regulation of rat granulosa cell differentiation by extracellular matrix produced by bovine corneal endothelial cells. Endocrinology 118:1878–1885
Gentry PA, Zareie M, Liptrap RM (1996) Fibronectin concentrations correlate with ovarian follicular size and estradiol values in equine follicular fluid. Anim Reprod Sci 45:91–102
Gomes JE, Correia SC, Gouveia-Oliveira A, Cidadao AJ, Plancha CE (1999) Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev 54:163–172
Hovatta O, Silye R, Abir R, Krausz T, Winston RM (1997) Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod 12:1032–1036
Huet C, Monget P, Pisselet C, Monniaux D (1997) Changes in extracellular matrix components and steroidogenic enzymes during growth and atresia of antral ovarian follicles in the sheep. Biol Reprod 56:1025–1034
Huet C, Pisselet C, Mandon-Pepin B, Monget P, Monniaux D (2001) Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J Endocrinol 169:347–360
Irving-Rodgers HF, Mussard ML, Kinder JE, Rodgers RJ (2002) Composition and morphology of the follicular basal lamina during atresia of bovine antral follicles. Reproduction 123:97–106
Iwahashi M, Muragaki Y, Ooshima A, Nakano R (2000) Type VI collagen expression during growth of human ovarian follicles. Fertil Steril 74:343–347
Kreeger PK, Fernandes NN, Woodruff TK, Shea LD (2005) Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod 73:942–950
Kreeger PK, Deck JW, Woodruff TK, Shea LD (2006) The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 27:714–723
Le Bellego F, Pisselet C, Huet C, Monget P, Monniaux D (2002) Laminin-alpha6beta1 integrin interaction enhances survival and proliferation and modulates steroidogenesis of ovine granulosa cells. J Endocrinol 172:45–59
Lee VH, Britt JH, Dunbar BS (1996) Localization of laminin proteins during early follicular development in pig and rabbit ovaries. J Reprod Fertil 108:115–122
Matzuk MM (2000) Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol 163:61–66
McArthur ME, Irving-Rodgers HF, Byers S, Rodgers RJ (2000) Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol Reprod 63:913–924
O’Brien MJ, Pendola JK, Eppig JJ (2003) A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 68:1682–1686
Oktay K, Karlikaya G, Akman O, Ojakian GK, Oktay M (2000) Interaction of extracellular matrix and activin-A in the initiation of follicle growth in the mouse ovary. Biol Reprod 63:457–461
Rajah R, Sundaram GS (1994) Protein distribution and gene expression of collagen type IV in the neonatal rat ovary during follicle formation. Cell Mol Biol (Noisy-le-grand) 40:769–780
Rodgers HF, Irvine CM, van Wezel IL, Lavranos TC, Luck MR, Sado Y, Ninomiya Y, Rodgers RJ (1998) Distribution of the alpha1 to alpha6 chains of type IV collagen in bovine follicles. Biol Reprod 59:1334–1341
Rodgers RJ, Lavranos TC, van Wezel IL, Irving-Rodgers HF (1999a) Development of the ovarian follicular epithelium. Mol Cell Endocrinol 151:171–179
Rodgers RJ, van Wezel IL, Irving-Rodgers HF, Lavranos TC, Irvine CM, Krupa M (1999b) Roles of extracellular matrix in follicular development. J Reprod Fertil Suppl 54:343–352
Rodgers RJ, Irving-Rodgers HF, van Wezel IL, Krupa M, Lavranos TC (2001) Dynamics of the membrana granulosa during expansion of the ovarian follicular antrum. Mol Cell Endocrinol 171:41–48
Smitz JE, Cortvrindt RG (2002) The earliest stages of folliculogenesis in vitro. Reproduction 123:185–202
Spears N, Murray AA, Allison V, Boland NI, Gosden RG (1998) Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil 113:19–26
Tsuiki A, Preyer J, Hung TT (1988) Fibronectin and glycosaminoglycans in human preovulatory follicular fluid and their correlation to follicular maturation. Hum Reprod 3:425–429
Wang T, Brown MJ (1999) mRNA quantification by real time TaqMan polymerase chain reaction:validation and comparison with RNase protection. Anal Biochem 269:198–201
van Wezel IL, Rodgers HF, Rodgers RJ (1998) Differential localization of laminin chains in bovine follicles. J Reprod Fertil 112:267–278
Yamada S, Fujiwara H, Honda T, Higuchi T, Nakayama T, Inoue T, Maeda M, Fujii S (1999) Human granulosa cells express integrin alpha2 and collagen type IV: possible involvement of collagen type IV in granulosa cell luteinization. Mol Hum Reprod 5:607–617
Zhao Y, Luck MR (1995) Gene expression and protein distribution of collagen, fibronectin and laminin in bovine follicles and corpora lutea. J Reprod Fertil 104:115–123
Acknowledgements
This research was supported by the NICHD/NIH through cooperative agreement U54 HD041857 as part of the Specialized Cooperative Centers Program in Reproductive Research and the NIH Biotechnology Training Grant for C.B.B. We would like to thank the Pathology Core Laboratory (Northwestern University, Chicago, IL, USA), especially Andrew Lisowski, for the paraffin embedding and sectioning of all ovarian tissue. A special thanks to Sarah Bristol and Joanna Burdette for their technical assistance and support with both the immunohistochemistry and real-time PCR. We would also like to thank Erin West for her review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berkholtz, C.B., Lai, B.E., Woodruff, T.K. et al. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol 126, 583–592 (2006). https://doi.org/10.1007/s00418-006-0194-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-006-0194-1