Abstract
The development and complete differentiation of salivary glands is a complex process that involves a large number of co-ordinated events. Little is known about the molecular basis for salivary gland development. However, we have reported previously that integrins appear to play a role. Integrins are heterodimeric transmembrane receptors consisting of one α and one β subunit that play a pivotal role in the interaction of cells with the extracellular matrix. Such interactions regulate the organisation of cells of tissues and organs during development as well as cell proliferation and differentiation. Using immunohistochemistry and Western and Northern blot analysis, we mapped the localisation and expression of integrins β1, β3 and β4 in human salivary glands obtained from foetuses ranging from weeks 4–24 of gestation and compared it with adult salivary glands. Integrin β1 first appeared during the canalisation stage and during the differentiation stage. A message first appeared at week 6 of development. The expression of β4 integrin protein and message was observed only in the late stage of differentiation. Integrin β3 was not detected in the developing glands; however, integrins β1, β3 and β4 were all expressed in adult salivary gland tissues. The data suggest that integrins, particularly β1, have a role to play in salivary gland development and differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All salivary glands develop in a similar manner. Formation starts with the proliferation of a solid cord of cells from the epithelium of the stomatodeum into the underlying ectomesenchyme. This cord of cells extends deeply into the ectomesenchyme and branches extensively. These cells then canalise by degradation of the central cells to form the ductal system and the terminal secretory end pieces. The epithelial in-growths constitute the parenchyme of a salivary gland. The ectomesenchyme differentiates to form the connective tissue component of the gland that supports the parenchyma.
The parotid gland begins to develop at 4–6 weeks of embryonic life, the submandibular gland at 6 weeks, and the sublingual and minor salivary glands at 8–12 weeks. Branches from parasympathetic and sympathetic nerves migrate to the gland, and the collecting veins are formed. Salivary gland development consists of a series of ducts ending in terminal secretory end pieces grape-like in structure.
To date, little is known of the key regulators of human salivary gland development and function. In most systems, there is a requirement of co-ordination between cell proliferation, polarisation and differentiation. Temporal and spatial regulation of these events is likely to be important to salivary gland development and for proper tissue function. Integrins are the major adhesion receptors that connect cells to components of the extracellular matrix. Integrin-mediated adhesion can influence many different signal transduction cascades, support cell division and proliferation and modulate the expression of differentiation-related genes.
Interactions between cells and extracellular matrix are essential for tissue development and maintenance. These interactions are partially mediated by integrins, a family of heterodimeric transmembranic molecules comprised by two subunits—α (alpha) and β (beta) (Hynes 1992). These heterodimers are divided into subfamilies on the basis of their β subunit. Those of the β1 subfamily bind chiefly to components of the extracellular matrix, broadly represented by collagen, laminin and fibronectin; β4 to basement membrane proteins; and β3 acts as receptor for other proteins, such as vitronectin (Hynes 1987; Hynes 1992).
In addition to their anchorage properties, integrins have been implicated in the maintenance of intercellular contacts as well as being involved in other dynamic biological mechanisms, such as cell signalling and regulation of gene expression leading to proliferation and differentiation (Hynes 1992; Ruoslahti and Reed, 1994; Varner and Cheresh, 1996). Integrins have also been described in salivary gland neoplasms (Loducca et al., 2000; Araújo et al., 2001). Recently, a comprehensive review (Darribère et al., 2000) showed that integrins are fundamentally important in embryogenesis from fertilisation to processes of organogenesis and differentiation. Additionally, integrins are also implicated in other cellular physiological processes, such as cell migration, matrix assembly, apoptosis, etc.
The aim of this investigate was to study the expression and distribution of integrins β1, β3 and β4 in the developing and adult normal human salivary gland.
Materials and methods
Reagents
Primary antibodies for integrins β1, β3 and β4 were mouse monoclonals obtained from Chemicon (Temecula, CA, USA). The β1 antibody was clone 6S6, β3 was clone BB10 and β4 was clone ASC-3. All antibodies were used at a dilution of 1:100. Other routine chemicals were the best obtainable grade or as specified.
Tissue preparation
Fragments of the oral cavity from post-mortem human foetuses at 4–25 weeks of gestation were obtained from the Medical School of the University of São Paulo and in accordance with the authorisation of the Ethical Committee of this institution. The specimens were collected from different oral sites, including buccal mucosa, tongue, mandible and hard palate. Fully developed salivary gland specimens were retrieved from the Oral Pathology Department of the Dental School of the University of São Paulo. All specimens were fixed in buffered formalin and embedded in paraffin. Sections of the specimens collected were stained with hematoxylin and eosin to check for the presence of salivary glands and to study their morphology. Those with developing major or minor salivary glands were selected for the present immunohistochemical analysis.
Immunohistochemistry
Three micrometre serial sections of the specimens were re-hydrated and incubated in 3% aqueous hydrogen peroxide for 30 min to quench endogenous peroxidase activity. Incubation with 1% bovine serum albumin (BSA) and 5% fetal calf serum (FCS) in Tris-HCl pH 7.4 for 60 min at room temperature was performed to suppress non-specific binding of subsequent reagents. The sections were then incubated with the primary antibody and with the indirect dextran polymer detection system (En Vision—Dako Carpinteria, CA, USA). Staining was completed by a 3-min incubation with 3′3-diaminobenzidine tetrachloride (DAB). The specimens were then lightly counterstained with Mayer’s hematoxylin, dehydrated, and mounted with glass coverslip and xylene-based mountant. Negative controls were treated as above, but a solution of 1% BSA in Tris-HCl pH 7.4 replaced the primary antibody. Internal positive controls, such as basal layer of epithelium, were used.
Western blot analysis
Stage-specific salivary gland tissue was homogenised in a lysis buffer containing 50 mM NaCl, 25 mM Tris-Cl (pH 8.1) 0.5% Nonidet P40, 0.5% sodium deoxycholate, 1mM PMSF, 10 μg/ml leupeptin, 10 μg/ml pepstatin and 25 μg/ml aprotinin. The homogenate was centrifuged at 14,000 rpm, and the resulting supernatant was used for Western blot analysis. Next, 20 μg protein was heated to 99°C for 4 min, loaded into sample wells, resolved on a 10–20% tricine SDS-polyacrylamide gel (Novex, San Diego, CA, USA) and run at 120 V for 2 h. Transfer blotting was accomplished using the same apparatus, and proteins were transferred to a PDVF membrane (Immobilon, Millipore, UK) at 30 V for 4 h. Membranes were blocked overnight at 4°C in a solution of 5% dried milk in PBS containing 0.1% Tween 20. Membranes were then washed and incubated for 60 min at room temperature in 1:100 dilution of the appropriate β integrin monoclonal antibodies, washed three times in PBS and incubated in 1:200 goat anti-mouse biotinylated IgG (Vector Laboratories, Peterborough, UK) for 60 min at room temperature. Membranes were washed three times in PBS and the signal amplified/detected using enhanced chemiluminescence (ECL) following the manufacturers instructions (Amersham-Pharmacia-Biotech). All experiments were carried out in triplicate, and bar graphs represent the results obtained by pooling data together.
Northern blot analysis
Due to the limited availability of tissue, it was decided to perform Northern blot analysis on the salivary gland tissues for integrin β1 only. Total tissue RNA was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction using RNAzol solution (Biogenesis, Poole, UK) following the manufacturer’s instructions. The purity and concentration of RNA was measured spectrophotometrically at 260/280 nm. Probes for Northern blot were generated by PCR using the following oligonucleotide primers: GAPDH sense: 5′-accacagtccatgccatcac-3′, antisense: 5′-tccaccaccctgttgctgta-3′ and β1 integrin, sense: 5′-atctgcgagtgtggtgtctg-3′, antisense: 5′-ggggtaatttgtcccgactt-3′. The reaction cycles of PCR were 35 cycles denaturation for 30 s at 94°C, primer annealing for 1 min at 60°C and primer extension for 1 min at 72°C. The PCR products were excised from the agarose gel and purified.
Approximately 5 μg of total RNA was electrophoresed in a formaldehyde 1% agarose gel and transferred to Hybond-N nylon membrane (Amersham). After fixation by UV crosslinking, the membrane was hybridised (and sequentially stripped) overnight at 42°C with a 208-bp or 412-bp fragment [γ−32P]dCTP labelled integrin β1 or GAPDH probes, respectively. Washed blots were exposed to Kodak XAR-5 film. Bands underwent scanning densitometry, and the relative ratio of the net intensities of the β1 integrin and GAPDH bands from the same Northern blot reaction was determined to show β1 integrin mRNA expression with gestational age. All experiments were carried in triplicate, and bar graphs represent the results obtained by pooling data together.
Results
Immunostaining of human embryonic salivary glands
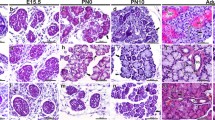
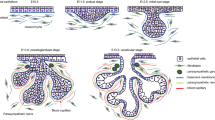
The specimens studied were of the major human salivary glands in various stages of development, that is bud, proliferation, canalisation, branching and cytodifferentiation. Salivary glands at the bud stage of development were negative for integrins β1, β3 and β4 (data not shown). During the canalisation stage, a few developing salivary gland ducts could be observed. Only β1 integrin was present in a few cells, showing a ring-like pattern seen in the apical pole of some luminal cells (Fig. 1a). In the stage of branching and initial cytodifferentiation, β1 integrin was present at the baso-lateral portion of developing acinic cells and the apical pole of luminal cells. A fine, dotted pattern was observed; β4 integrin was present around the membrane of scattered cells (Fig. 1b).
Developing salivary gland. a Ring-like pattern of β1 integrin expression in scattered cells during initial stage of ductal canalisation (original magnification ×640). b Strong expression of β1 integrin in a few cells during late canalisation stage (original magnification ×400). c Early acinar differentiation: expression of β4 integrin seen in cells surrounding future acinar structures (original magnification ×400). d Canalisation stage: strong expression of β4 integrin in cells surrounding a ductal system (original magnification ×400); e β1 integrin expression on a developing ductal system: luminal cells of developing excretory duct (distal portion) positive for β1 integrin. A few cells along the ductal system are positive for this integrin, and at the end of the branched system (end buds), scattered cells are strongly positive for this integrin subunit (original magnification × 200). f Focal expression of β1 integrin concentrated at the basal pole of developing acinic cells during initial stage of differentiation (original magnification ×400). g Advanced stage of acinic cell differentiation: marked presence of β4 integrin at the basal portion of acinar units (original magnification: ×200). h Mucous acinar units in fully developed glands showing β1 integrin expression along the basal pole of acinic cells (riginal magnification ×640).
In a further differentiation stage (advanced cytodifferentiation), β1 and β4 integrins could be observed in the salivary gland tissue studied. β4 integrin was present in early acinar differentiation (Fig. 1c) and in a few ductal cells of the ductal system (Fig. 1d). The ring-like pattern of β1 expression can still be observed in a few ductal cells (Fig. 1e). β4 was present in acinar structures in a fine dotted pattern and in the basal and baso-lateral portions of luminal cells of excretory duct (Fig. 1f, g). β3 integrin was not detected in the developing human salivary gland specimens included in our study but was present in the developing blood vessels (data not shown).
The specimens of normal fully developed salivary glands studied comprised minor glands comprised of mucous secretory units, myoepithelial cells and intercalated, striated and excretory ducts. Integrin β1 was intensely expressed on cells of the striated ducts, distributed all over the folded cell membrane or in clusters, concentrated for the most part in the apical pole of the luminal cells. Intercalated duct cells were positive for this integrin, which was distributed in a bipolar pattern along the cell membrane. Luminal cells of the excretory ducts were positive for integrin β1. However, this positivity became irregular, subtle and restricted to a few intercellular contacts as duct epithelium stratified and reached the surface. Mucous and serous acinic cells were positive for integrin β1 (Fig. 1h). Integrin β3 showed patterns of positivity and distribution similar to β1 in adult salivary glands, as described above.
Fig. 2a illustrates the expression of integrin β4 on serous salivary glands, with strong localisation on striated duct cells. Acinic cells were positive for integrin β4, which was distributed in a delicate, pulverised pattern especially concentrated at the baso-lateral portion of these cells. In developing acinar tissue, there was some integrin β4 positivity, as can be seen in Fig. 2b.
Fig. 3 illustrates more directly the patterns of integrin β1 expression in adult and developing salivary tissues. Figure 3a, b shows once again the distribution of β1 localisation in adult mucous gland, with integrin β 1 positivity around acinar structures and in striated duct. In developing salivary glands, the arborisation–canalisation stage of development, β1 immunostaining appears as shown in Fig. 3c, d. Staining for integrin β1 is seen around some ductal cells of the branching stage of human developing salivary gland. In Fig. 3e, f, canalised ductal structures show β1 integrin expression in ring-like patterns and around a few ductal cells,
a Presence of β1 integrins around mature mucous acinar structures (original magnification ×400). b Presence of integrin β1 around acinar structures and in striated duct of adult mucous salivary gland (original magnification ×100). c, d β1 integrin is present around a few ductal cells of branching stage of human developing salivary gland (original magnification ×200). e, f Canalised ductal structures showing β1 expression in ring-like pattern (e) and around a few ductal cells (f) (original magnification ×400 and ×200, respectively).
Western blot analysis
Western blotting was used to study the protein expression of β1, β3 and β4 integrins. Human embryonic parotid and submandibular gland tissue was obtained from gestational ages of 4–25 weeks. Adult tissues were used for comparison purposes. Fig. 4a (upper panel) illustrates the expression of β1 integrin in parotid tissue; the lower panel is a graphical representation of pooling densitometry data from three Western blot analyses. It can be seen that at a gestational age of 4 weeks, there was very little expression of integrin β1 protein that did not change up to the embryonic age of 12 weeks. At 17 weeks of gestation, there was a highly significant increase in integrin β1 protein expression. β1 expression then decreased dramatically at 25 weeks of gestation. Levels of integrin β1 in adult parotid tissue were higher than those seen at the embryonic age of 17 weeks.
Western blot for integrin β1 expression. Protein was extracted from salivary gland tissue taken from embryos at various ages of gestation and immunoblotted for β1 integrin. a Parotid gland: upper panel is a representative Western blot image of β1 integrin levels; lower panel, graph showing relative intensity of β1 protein expression after scanning densitometry. b Submandibular gland: upper panel is a representative Western blot image of β1 integrin levels; lower panel, graph showing relative intensity of β1 protein expression after scanning densitometry. Values are means ± SEM, n=3. All bars are *P<0.05, **P<0.01, ***P<0.001 compared with week 4 levels of β1 integrin (analysis of variance).
In submandibular tissue, there was a detectable presence of integrin β1 at 4 weeks of gestation (Fig. 4b). Expression appears to increase significantly at week 6 of gestation and then remains fairly constant thereafter.
In both parotid and submandibular tissues, integrin β4 expression followed a very similar pattern to β1 (data not shown). Integrin β3 expression was not detectable in any of the gestational tissues used (data not shown).
Northern blot analysis
Due to the limited amount availability of tissue, we were only able to carry out Northern blots for integrin β1 in the parotid and submandibular glands of the human foetuses of various gestational ages. Adult tissues were used for comparison purposes. Fig. 5 The upper panel ( a) illustrates the mRNA expression of integrin β1 and the housekeeping gene GAPDH in parotid tissue with gestational age. It can be seen that in tissues from weeks 4, 6 and 8, there is some (significant) expression of integrin β1. This increases dramatically at week 12 and continues at the same level of expression through the embryonic ages of weeks 17 and 25. Adult levels were similar to those observed at the gestational ages of weeks 17 and 25. The lower panel is a graphical representation of the data pooled from three embryos.
Northern blot for integrin β1 expression. Total RNA was extracted from embryos at various ages of gestation and immunoblotted for β1 integrin. a Parotid gland: upper panel is a representative Western blot image of β1 integrin levels. Lower panel, graph showing relative intensity of β1 protein expression after scanning densitometry and normalisation to GAPDH levels. b Submandibular gland: upper panel is a representative Western blot image of β1 integrin levels. Lower panel, graph showing relative intensity of β1 protein expression after scanning densitometry and normalisation to GAPDH levels. Values are means ± SEM, n=3. All bars are *P<0.05, **P<0.01, ***P<0.001 compared with week 4 levels of β1 integrin (analysis of variance).
The pattern of integrin β1 mRNA expression in the submandibular gland was different (Fig. 5b, upper panel). There was no detectable β1 in 4-week-old foetal tissue. Levels of integrin β1 were detectable in all ages of foetal tissue used, with the strongest expression in week-8 glands. Fully developed submandibular tissue from adults highly expressed integrin β1, which was comparable with week 8 foetal tissue. The lower panel is a graphical representation of pooled data obtained from three separate Northern blot analyses.
Discussion
The molecular mechanisms involved in salivary gland development have been described in some detail in the mouse and hamster (Menko et al., 2001; Fernandes et al., 1999). To date, there is little known of the expression of adhesive proteins during pre-natal and post-natal development of the human salivary gland. Just as in other glands and tissues, it is very likely that development is co-ordinated via several integrated events affecting proliferation, morphogenesis and cell-substratum interactions. These events are tightly regulated both temporally and spatially (see Cutler 1989 for review).
Differentiation of salivary glands begins around week 6 of foetal development when specific oral epithelium cells undergo organised, co-ordinated growth, which then produces secretory proteins specific to these glands. Development of salivary glands is based on epithelio-mesenchymal interactions, leading to morphogenesis and cytodifferentiation (Kashimata and Gresik 1996; Cutler 1990). These processes are partially linked but independently regulated, with regulation being dependent on the effects of growth factors supplied by the surrounding mesenchyme and cellular interactions with extracellular matrix (Wu and Santoro 1996). Integrins appear to be key molecules in these interactions.
During the initial stages of salivary gland morphogenesis—bud stage, β1, β3 and β4 integrins were not present. As the salivary glands developed with increased tissue differentiation, β1 and β4 subunits were expressed in an increasing number of cells. These results suggest that integrins may not be implicated in the initial phase of salivary gland development but are essential in the cytodifferentiation process. Expression of cytokeratins are also not detected in this stage, in which cell proliferation is the main event. Despite the lack of integrins in the salivary gland bud stage, these molecules are widely expressed in the immature mucosal epithelium, suggesting they have a role in the maintenance of tissue organisation prior to its fully mature status.
In early stages of salivary gland canalisation, integrin β1 was expressed in a peculiar, ring-like pattern in some cells of the developing ductal network. It has been reported that β1 integrin is required for maintenance of stem cells in epithelial developing tissues. They act as holding cells at the right place, and their loss or alteration ensures departure of the stem cell niche through differentiation or apoptosis (Watt and Hogan 2000). The presence of rare β1-positive cells during early phases of salivary gland differentiation (canalisation) may indicate the localisation of stem cells in this developing tissue. Additionally, extracellular matrix proteins can modulate expression and activation of β1 integrins, and local variation in the composition of basement membrane during the gland development might play a role in establishing and maintaining the distribution of these β1-positive cells (Watt and Hogan 2000). Integrin receptors and ligands have been shown to activate signalling pathways involving mitogen-activated protein kinases, tyrosine kinases or GTP-binding proteins. These are thought to affect the cellular cytoskeleton and cellular proliferative responses required during the several processes of organ development (Coraux et al. 2000)
In advanced canalisation stage of salivary gland histogenesis, integrin β1 was present in a greater number of cells (see Fig. 3). Some of these cells still showed the ring-like pattern of expression. Others expressed β1 integrin at the baso-lateral surface of the membrane. Integrin β4 was also seen during this phase, being present in the baso-lateral portion of some ductal cells (see Fig. 2). This lends strong evidence that integrins are implicated in differentiation and maintenance of salivary gland phenotype. The presence of integrin β1 at the baso-lateral portion of the basement membrane has been reported during the tubular morphogenesis of the trachea. This pattern of distribution is thought to be involved in the stable attachment of stationary epithelial cells to the matrix and in the maintenance of cell–cell interactions (Coraux et al. 2000). The role of β1 integrin has been investigated in mammary glands, and perturbation of its function is described to impair the development and differentiation of mammary gland secretory epithelium (Faraldo et al. 1998). Our findings showed stronger and wider presence of integrins as differentiation advanced. This is also reported in the development of human gastric mucosa, in which integrins β1 and β4 are present in foveolas gland units and in gastric epithelial cells (Chénard et al. 2000).
In late stages of salivary gland histogenesis, branching and cytodifferentiation of acinar cells and integrins β1 and β4 mimic the patterns seen in the normal adult salivary gland structures. β1 integrin showed expression in the apical pole of luminal cells as well as the baso-lateral portion of ductal cells. β4 integrin was prominently deposited in the basal pole and baso-lateral portion of salivary gland structures. This re-distribution phenomenon may account for the secretory functions of salivary glands, as it has also been reported in other secretory organs such as stomach, lungs and breast (Chénard et al. 2000). According to a study on tracheal tubular morphogenesis, the cellular localisation of β1 integrin may yet be related to cell migration and tubule formation (Coraux et al. 2000). Additionally, a previous report on salivary gland morphogenesis and differentiation suggests the role of different extracellular matrix proteins in the phases of gland development and their relationship with the regulation of gland secretory function (Cutler 1990).
Integrin β3 protein was not detected in the foetal and early developmental stages of salivary glands studied although positive reaction was found in foetal blood vessels. Its localisation was not totally surprising since the αvβ3 heterodimer is necessary for the survival of endothelial cells during angiogenesis, suggesting fundamental roles of this integrin in blood vessels formation (Brooks et al. 1996; Beauvais-Jouneau and Thierry 1997). β3 subunit is also important in earlier phases of embryogenesis, such as implantation and invasion at placentation (Darribère et al. 2000) but is probably not involved in the processes of salivary gland development and differentiation.
Finally, all integrins studied were present in adult normal salivary gland structures, showing variation of expression patterns, with β1 being the most abundant. Ductal cells expressed integrins β1, β3 and β4 mainly at the apical pole of luminal cells.
The presence of integrins in normal salivary gland structures may be associated with the exocytosis processes due to interactions with the cytoskeleton microfilament-associated proteins. Cytoskeleton re-organisation, establishment of cell polarity and signal transduction are implicated in secretion-granule intracellular transport and fusion with cell membrane and exocytosis (Segawa and Yamashina 1989; Muallem et al. 1995; Gumbiner 1996; Sheppard 1996; Segawa et al. 1998; Dogic et al. 1999). This multi-step process requires participation of microfilaments present in the luminal membrane of acinic and ductal cells (Segawa et al, 1989; Segawa et al, 1998). Interactions of these filaments with integrins may represent the key event for triggering the mechanism of secretion.
This study has shown that integrins are important molecules during salivary gland differentiation, being developmentally regulated. In the light of the present findings, the analysis of integrins and their ligands in other developmental human oral tissues is being performed in our laboratory.
References
Araújo VC, Loducca SVL, Sousa SOM, Williams DM, Araújo NS (2001) The cribriform features of adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma: cytokeratins and integrins expression. Ann Diag Pathol 5(6):330–334
Beauvais-Joneau A, Thierry JP (1997) Multiple roles for integrins during development. Biol Cell 89:5–11
Brooks PC, Strömbald S, Sanders LC, Steller-Stevenson WG, Quigley JP, Cheresh DA (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85:683–693
Chénard M, Barque JR, Chailler P, Trembaly E, Beaulieu JF, Ménard D (2000) Expression of integrin subunits correlates with differentiation of epithelial cell lineages in developing human salivary human gastric mucosa. Anat Embryol 202:223–233
Coraux C, Zahm JM, Puchelle E, Gaillard D (2000) Beta-1 integrins are involved in migration of human fetal tracheal epithelial cells and tubular morphogenesis. Am J Pathol 279:224–234
Cutler LS (1989) Functional differentiation of salivary glands. In: Forte J (ed) Handbook of physiology, salivary, pancreatic, gastric and hepatobiliary secretion vol 3. Am Physiological Soc Press, New York, pp 93–105
Cutler LS (1990) The role of extracellular matrix in the morphogenesis and differentiation of salivary glands. Adv Dental Res 4:27–33
Darribère T, Skalski M, Cousin H, Gaultier A, Montmory C, Alfandari D (2000) Integrins: regulator of embryogenesis. Biol Cell 92:5–25
Dogic D, Eckes B, Aumailley M (1999) Extracellular matrix, integrin and focal adhesions. Currr Topic Pathol 93:75–85
Faraldo MM, Deugnier MA, Lukashev M, Thierry JP, Glukhova MA (1998) Perturbation of β1-integrin function alters the development of murine mammary gland. EMBO J 17:2139–2147
Fernandes RP, Cotanche DA, Lennon-Hopkins K, Erkan F, Menko AS, Kukuruzinska MA (1999) Differential expression of proliferative, cytoskeletal, and adhesive proteins during postnatal development of the hamster submandibular gland. Histochem Cell Biol 111(2):153–162
Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357
Hynes RO (1987) Integrins: a family of cell surface receptors. Cell 48:549–554
Hynes RO (1992) Integrins: versatility, modulation, and signalling in cell adhesion. Cell 69:11–25
Jones JL, Walker RA (1999) Integrins: a role as cell signalling molecules. J Clin Pathol Mol Pathol 52:208–213
Kashimata M, Gresik EW (1996) Contemporary approaches to the study of salivary gland morphogenesis. Eur J Morphol 34:143–147
Loducca SVL, Raitz R, Araújo NS, Araújo VC (2000) Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: distinct architectural composition revealed by collagen IV, laminin and their integrin ligands α2β1 and α3β1. Histopathology 37:118–123
Menko AS, Kreidberg JA, Ryan TT, Van Bockstaele E, Kukuruzinska MA (2001) Loss of a3β1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev Dyn 220(4):337–349
Muallem S, Kwiatkowska K, Xu X, Yin HL (1995) Actin filament disassemby is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol 128:589–598
Ruoslahti E, Reed JC (1994) Anchorage dependence, integrins, and apoptosis. Cell 77:477–478
Segawa A, Yamashina S (1989) Roles of microfilaments in exocytosis: a new hypothesis. Cell Struct Funct 14:531–544
Segawa A, Loffredo F, Puxeddu R, Yamashina S, Testa-Riva F, Riva A (1989) Exocytosis in human salivary glands visualised by high resolution scanning electron microscopy. Cell Tissue Res 291:325–336
Segawa A, Riva A, Loffredo F, Congiu T, Yamashina S, Testa-Riva F (1998) Cytoskeletal regulation of human salivary secretion studied by high electron microscopy and confocal laser microscopy. Eur J Morphol 36:41–45
Sheppard D (1996) Epithelial integrins. Bioessays 18:655–660
Sunardhi-Widyaputra S, Van-Damme B (1994) Distribution of the VLA family of integrins in normal salivary gland and pleomorphic adenoma. Pathol Res Practice 190:600–608
Varner JA, Cheresh DA (1996) Integrins and cancer. Curr Opin Cell Biol 8:724–730
Watt FM, Hogan LM (2000) Out of Eden: stem cells and their niches. Science 287:1427–1430
Wu JE, Santoro AS (1996) Differential expression of integrin alpha subunits supports distinct roles during lung branching morphogenesis. Dev Dyn 206:169–118
Acknowledgements
Supported by FAPESP grants 02/02676-7 and 03/00450-4 and British Council Academic Link SPA/881/155
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lourenço, S.V., Kapas, S. Integrin expression in developing human salivary glands. Histochem Cell Biol 124, 391–399 (2005). https://doi.org/10.1007/s00418-005-0784-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0784-3