Abstract
Although ischemia reperfusion (I/R) induces apoptotic damage of mammalian small intestine, the molecular mechanism is largely unknown. We investigated the appearance of apoptosis at various time-points (0–24 h) of reperfusion after 1-h ischemia and the expression of various apoptosis-related proteins, such as Bcl-2, Bax, Fas, Fas ligand (FasL), activated caspase-3, and cytochrome c, immunohistochemically in rat small intestine. As assessed by TUNEL and electron microscopy, apoptotic cells were increased at 3 h of reperfusion in all intestinal parts (villous epithelium, crypt epithelium, and stroma of intestine). Moreover, the TUNEL-positive cells in the stroma were later identified as T cells. The expression of Fas and FasL as well as activated caspase-3 was markedly increased at 3 h of reperfusion in the stroma. In the villous epithelium, a transient decrease in Bcl-2 expression was found while in the crypt epithelium, Fas expression was induced. Finally, intraperitoneal injection of leupeptin (an SH-protease inhibitor) after I/R resulted in a significant inhibition of the induction of apoptosis in the stroma and crypt epithelium. Our results indicate that the triggering molecules of apoptosis in the I/R rat small intestine may vary depending on cell type and that the use of a broad-spectrum protease inhibitor may reduce intestinal damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal tissue dysfunction induced by ischemia reperfusion (I/R) is a common event in the clinical settings of sock, sepsis, vascular surgery, and strangulation ileus (Hassoun et al. 2002). Moreover, the graft damage caused by I/R is a serious complication in small intestinal transplantation (Itoh et al. 2000). Recently, several reports have indicated that ischemia or I/R induces apoptosis in various organs (Gottlieb et al. 1994; Koji et al. 2001; Nogae et al. 1998). Actually, in the case of the small intestine, which is the organ most sensitive to ischemic insult (Granger et al. 1995), apoptosis has been known as the principal mode of cell death after I/R injury (Genesca et al. 2002). Furthermore, the role of apoptosis in the physiological turnover of epithelial cells for maintaining a balance between cell production and cell loss in the small intestine is well documented (Potten et al. 1994, 1997).
Apoptosis can be discriminated from necrosis by peculiar morphological features, including cell shrinkage, chromatin condensation to the nuclear periphery, and fragmentation of the cell into apoptotic bodies (Kerr et al. 1972). On the other hand, necrosis is manifested by severe cell swelling or cell rupture, denaturation of nuclear chromatin, coagulation of cytoplasmic proteins, breakdown of cell organelles, and a significant inflammatory response (Cotran et al. 1994). More importantly, apoptosis is induced through the activation of a particular set of genes. At least two major molecular cascades are involved in apoptotic cell death: the Fas/Fas ligand (FasL) and Bcl-2/Bax systems (Green 1998). The interaction between Fas and FasL activates caspase-3, a downstream effector, leads to apoptotic cell death (Nagata et al. 1995; Wolf et al. 1999). The other pathway involves the release of cytochrome c from the mitochondria, which interacts with Apaf-1 (Kuida et al. 1998) and subsequently activates caspase-3 via proteolytic processing (Liu et al. 1996; Zou et al. 1997). Members of the Bcl-2 family play crucial roles in this pathway (Zou et al. 1997) where an elevated level of Bcl-2 favors extended survival of cells, and an increasing level of Bax expression accelerates cell death (Baba et al. 1999; Oltvai et al. 1993). Although the molecular basis of apoptosis is complex, its regulatory pathways converge on a common cell death effector called the caspase family (Eskes et al. 1998), and the activation of caspases, especially caspase-3, is now considered a key biochemical hallmark of apoptosis (Cohen 1997). Despite these advances in the understanding of molecular mechanisms to induce apoptosis, our knowledge of small intestinal cell apoptosis induced by I/R remains limited.
In the present study, we first analyzed the kinetics of apoptotic cell death of rat small intestinal cells after I/R by TUNEL and electron microscopy (EM). Second, to gain further insights into the molecular pathway, we performed immunohistochemistry for various apoptosis-related proteins such as Bcl-2, Bax, Fas, FasL, and cytochrome c, as well as activated caspase-3. Finally, to confirm the involvement of caspases in the induction of cell death in the I/R small intestine, we investigated the effect of leupeptin, a cysteine protease inhibitor, on the induction of small intestinal cell apoptosis after I/R.
Materials and methods
Chemicals and biochemicals
Nembutal was purchased from Dainippon Pharmaceutical Co. (Osaka, Japan), leupeptin was obtained from the Peptide Institute (Osaka, Japan), paraformaldehyde (PFA) was purchased from Merck (Darmstadt, Germany), glutaraldehyde and Epon 812 resin were obtained from Taab laboratories (Aldermastton, Berkshire, UK), 3,3′-diaminobenzidine-4 HCl (DAB) was purchased from Dojin Laboratories (Kumamoto, Japan), and 4-chloro-1-naphthol was purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan). Proteinase K, 3-aminopropyltriethoxysilane, bovine serum albumin (BAS, minimum 98%, electrophoresis), and Brij 35 were purchased from Sigma Chemical Co. (St Louis, MO, USA), and biotin-16-dUTP and terminal deoxynucleotidyl transferase (TdT) were from Roche Diagnostics (Mannheim, Germany). All other reagents used in this study were from Wako Pure Chemicals (Osaka, Japan) and were of analytical grade.
Antibodies
Anti-Fas (P4; 1:800) serum (Hakuno et al. 1996; Koji et al. 2001) was prepared by immunization of rabbits against synthetic oligopeptides corresponding to the intracellular domain (P4; amino acids 292–306) of mouse Fas (Watanabe-Fukunaga et al. 1992). Anti-FasL (P5; 1:100) serum (Hakuno et al. 1996; Koji et al. 1994) was generated with a synthetic peptide corresponding to the intracellular domain (P5; amino acids 41–55) of rat FasL (Suda et al. 1993). Rabbit polyclonal anti-Bcl-2 (N-19; 2 μg/ml) (Sun et al. 2002), rabbit polyclonal anti-Bax (P-19; 0.125 μg/ml) (Sun et al. 2002), rabbit polyclonal anticytochrome c (H-104; 5 μg/ml) (Damavandi et al. 2002), and goat polyclonal anti-CD3 (M-20; 1 μg/ml) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antihuman/mouse caspase-3 active (1 μg/ml) was from Techne Corporation (Minneapolis, MN, USA), mouse monoclonal anti-ED1 (2.5 μg/ml) was from Serotec Corporation (Oxford, UK), horseradish peroxidase (HRP)-conjugated goat antirabbit IgG F (ab′)2 (1:200) was from MBL (Nagoya, Japan), HRP-conjugated rabbit anti goat IgG F (ab′)2 (1:200) was from Wako Pure Chemicals, HRP antibiotin (1:100) was from Vector Laboratories (Burlingame, CA, USA), normal rabbit IgG, normal goat IgG, and normal mouse IgG were purchased from Sigma, and normal rabbit serum was purchased from DAKO (Glostrup, Denmark).
Animals
Male Wistar rats (7–8 week) weighing 200–300 g were purchased from Ohtsubo Experimental Animal Co. (Isahaya, Japan), and each experimental group consisted of five rats. The animals were fasted for 12 h before the experiments but had free access to water. Animal care and experimental procedure were performed in accordance with the Guidelines for Animal Experimentation of Nagasaki University with the approval of the Institutional Animal Care and Use Committee.
Ischemia reperfusion of the rat small intestine
The rats were anesthetized with an intraperitoneal injection of Nembutal (40 mg/kg body weight), and the abdomen was opened. The superior mesenteric artery (SMA) was occluded with an atraumatic microvascular clamp for a period of 60 min. At the end of the ischemic period, the clamp was removed (time 0), and the intestine was allowed to be reperfused for 0, 3, 6, 12, and 24 h. To block any collateral blood supply, we used the procedure developed by Megison et al. (1990). At various time-points of reperfusion after 1-h ischemia, the segments of the jejunum, ileum, and ascending colon were removed.
In the sham-operated group, laparotomy was performed, and the SMA was exposed but not occluded. Vascular occlusions and sham operations were always performed at the same time of the day (between 0900 and 1100 h).
Treatment with leupeptin
Leupeptin, which was dissolved in 0.9% NaCl at a concentration of 10 mg/ml, was administered intraperitoneally at a dose of 10 mg/kg or 50 mg/kg body weight at 1 h before ischemia in the 1-h ischemia-alone group or 1 h after reperfusion in the 3 h of reperfusion group. Control animals were treated with an equivalent volume of 0.9% NaCl instead of leupeptin.
Tissue preparation
We used the jejunum in this study because it is the part of the intestine most sensitive to ischemia after occlusion of the SMA. Tissue samples were fixed in 4% PFA in phosphate-buffered saline (PBS) at room temperature (RT) overnight and embedded in paraffin using standard procedures. The tissues were cut into 5-μm-thick sections and mounted onto 3-aminopropyltriethoxysilane-coated slides, and adjacent sections were stained with hematoxylin and eosin for histological analysis. One piece of each jejunum was fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4, and then processed routinely and embedded in Epon 812 for EM.
Electron microscopy
Epon-embedded specimens of jejunum were cut into semithin and ultrathin sections. The semithin sections were stained with toluidine blue, and the ultrathin sections were stained with uranium acetate and lead nitrate. The ultrathin sections were observed under a JEOL 1200 EX electron microscope at accelerated voltage of 60 KV, as described previously (Hashimoto et al. 1995).
TUNEL staining
The TUNEL assay was carried out according to the method of Gavrieli et al. (1992) with a slight modification, as described previously (Wang et al.1998). Briefly, the paraffin-embedded sections were deparaffinized with toluene and rehydrated in serially graded ethanol solutions. After washing with PBS, the sections were treated with 1.0 μg/ml proteinase K in PBS for 15 min at 37°C. The sections were then incubated with 1× TdT buffer (25 mM Tris-HCl buffer, pH 6.6, containing 0.2 M potassium cacodylate and 0.25 mg/ml BSA) alone for 30 min at RT. After incubation, the slides were reacted with 100 U/ml TdT dissolved in TdT buffer supplemented with 1.5 mM CoCl2, 5 μM biotin-16-dUTP, 20 μM dATP, and 0.1 mM dithiothreitol for 90 min at 37°C. The reaction was terminated by washing with 50 mM Tris-HCl buffer (pH 7.4), and endogenous peroxidase activity was inhibited by immersing the slides in 0.3% H2O2 in methanol for 15 min at RT followed by washing with PBS. After incubation with 500 μg/ml normal goat IgG in 5% BSA in PBS for 60 min at RT, the sections were incubated with HRP goat antibiotin antibody (1:100) diluted with 5% BSA in PBS overnight at RT. After washing with 0.075 Brij 35 in PBS, the HRP sites were visualized by DAB, H2O2, CoCl2, and NiSO4(NH4)2SO4 according to the method of Adams (1981). As a negative control, some sections were subjected to reaction without TdT.
Immunohistochemistry
Immunohistochemical staining was performed by the indirect enzyme-labeled antibody method, as described previously (Koji et al. 1994; Damavandi et al. 2002; Kawano et al. 2004). Briefly, jejunal paraffin-embedded sections were deparaffinized with toluene and dehydrated with serially graded ethanol solutions. For Bax staining, the sections were autoclaved in a 0.01 M citrate buffer (pH 6.0) for 10 min at 120°C. After inactivation of endogenous peroxidase activity with 0.3% H2O2 in methanol for 15 min at RT, the sections were preincubated with 500 μg/ml normal goat IgG and 1% BSA in PBS for 1 h at RT. In the case of CD3 staining, normal rabbit serum was used as a blocking solution. The sections were then reacted with the first antibodies for 2 h (Fas, FasL, ED1, and CD3) or overnight (Bax, Bcl-2, cytochrome c, and activated caspase-3) at RT. After incubation, the slides were washed three times with 0.075% Brij 35 in PBS. The sections were then incubated with HRP-conjugated goat antirabbit IgG (1:200) for 1 h at RT, for CD3, which was incubated with HRP-conjugated rabbit antigoat IgG (1:200), and washed three times with 0.075% Brij 35 in PBS. The sites of HRP were visualized with H2O2 and DAB. Except for Bax, the sections were counterstained with methyl green. As a negative control, normal rabbit IgG, normal rabbit serum, normal goat IgG, or normal mouse IgG was used instead of the first antibody in each run. Serial sections were used to compare the expression of these apoptosis-related proteins with TUNEL-positive cells at the individual cell level. Double staining for TUNEL and activated caspase-3 or TUNEL and CD3 were performed, as described previously (Nakane 1968).
Statistical analysis
More than 2,000 cells in all intestinal parts (villous epithelium, crypt epithelium, and stroma of intestine) were counted, and the number of TUNEL-positive cells was evaluated as mean ± SEM (%). In the villous epithelium, the sloughing part of epithelial cells was excluded. Data for different groups were compared for statistical difference using Student’s t test. A P value of <0.05 denoted the presence of a significant difference. All analyses were preformed with a statistical software package (Statview, version 4.51; Abacus Concepts, Berkeley, CA, USA).
Results
Histological changes in the jejunum at various time-points after reperfusion
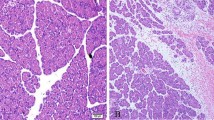
As shown in Fig. 1, histological changes were observed in the jejunum after 1-h ischemia, including obvious epithelial lifting along the length of the villi with a few denuded villous tips and disintegration of the lamina propria (Fig. 1b). At 3 h and 6 h of reperfusion after 1-h ischemia, the changes were markedly attenuated, as indicated by the absence of epithelial sloughing and villous denudation (Fig. 1c–d). Most of the damage affecting the villi and lamina propria was restored by 24 h of reperfusion (Fig. 1e).
TUNEL staining in rat small intestine after I/R
In the sham-operated groups, a few TUNEL-positive cells were observed in the epithelium of the villous tips as well as the lower regions of crypts (Fig. 2a). In the I/R groups, the number of TUNEL-positive cells in the villous epithelium increased significantly at 3 h and 6 h of reperfusion (Figs. 2c–d, 3a) and returned almost to the level of the normal and sham-operated groups by 24 h of reperfusion (Fig. 2e). It should be noted that since the sloughing parts of the villous epithelium included many apoptotic cells, the number of TUNEL-positive epithelial cells could be underestimated. Similarly, the number of TUNEL-positive cells in the lamina propria and crypt epithelium increased significantly at 3 h and 6 h of reperfusion (Figs. 2c–d, 3b–c), respectively.
TUNEL-positive cells in the ischemia-reperfusion (I/R) rat small intestine. a Sham operated, b 1-h ischemia, c 3 h of reperfusion, d 6 h of reperfusion, and e 24 h of reperfusion. Arrows indicate TUNEL-positive cells in all intestinal parts (villous epithelium, stroma, and crypt epithelium). Scale bar: 50 μm.
Quantitative analysis of TUNEL-positive cells at various time points of reperfusion after 1-h ischemia in all parts (a villous epithelium, b lamina propria, c crypt epithelium) of the rat small intestine. Five rats were studied in each group. More than 2,000 cells/slide were counted. The number of TUNEL-positive cells was evaluated as mean ± SEM (%). *P<0.01.
Electron microscopic analysis of the rat small intestine after I/R
To confirm the presence of apoptosis, we performed EM examination of the villous epithelium at various time points (0–24 h) of reperfusion and the lamina propria at 3 h of reperfusion after 1-h ischemia, respectively. In the villous epithelium, EM observation revealed that typical apoptotic epithelial cells were found at 0, 3, 6, and 12 h of reperfusion (Fig. 4b–e), and in the lamina propria, apoptotic lymphocytes (Fig. 5a) were also abundant at 3-h reperfusion. Moreover, EM observation revealed that the specimens were essentially free from necrotic cells in the villous epithelium and in the lamina propria. In addition, examination of typical macrophages often revealed phagocytosis of many apoptotic bodies in the lamina propria (Fig. 5b). In accordance with these EM findings, TUNEL-positive cells were simultaneous positive to the antisurface marker of pan-T cells, indicating that the apoptotic cells were T cells (Fig. 5c) but not ED1 (data not shown).
Electron micrographs of apoptotic cells in the villous epithelium of rat small intestine after ischemia reperfusion (I/R). a Sham operated, b 1-h ischemia, c 3 h of reperfusion, d 6 h of reperfusion, e 12 h of reperfusion, and f 24 h of reperfusion. Arrows indicate positive cells. Original magnification ×2,000 (a–f).
Electron micrographs (EM) of apoptotic cells in the lamina propria of rat small intestine in the ischemia-reperfusion (I/R) group with 3 h of reperfusion. a EM showing a large number of typical apoptotic cells, b EM showing that examination of typical macrophages often revealed phagocytosis of many apoptotic bodies, and c double staining for TUNEL and CD3. Arrows indicate positive cells. Original magnification ×2,000 (a), ×4,000 (b). Scale bar: 20 μm.
Immunohistochemical detection of apoptosis-related proteins in rat small intestine after I/R
Bcl-2 and Bax
As shown in Fig. 6, in the sham-operated groups, Bcl-2 staining was also observed in the cytoplasm of villous epithelial cells and in the lamina propria (Fig. 6a) but was almost negligible in the crypts (data not shown). When the I/R rat small intestines were analyzed, the epithelial staining for Bcl-2 was markedly decreased after 1-h ischemia (Fig. 6b) and at 3 h of reperfusion (Fig. 6c), and then reappeared around 24 h of reperfusion (Fig. 6d). In contrast, Bax staining was observed in the cytoplasm of villous (Fig. 6e) and crypt (Fig. 6f) epithelial cells without changes throughout the I/R experiment (data not shown).
Immunohistochemical staining of Bcl-2 (a–d) and Bax (e–f) in the ischemia-reperfusion (I/R) rat small intestine. a, e, f Sham operated, b 1-h ischemia, c 3 h of reperfusion, and d 24 h of reperfusion. The staining for Bcl-2 in epithelial cells was markedly decreased at 1-h ischemia alone and 3 h of reperfusion (b–c). Arrows indicate positive cells. Scale bar: 50 μm.
Fas and FasL
In the sham-operated groups, Fas was not detected in any part (Fig. 7a). In the I/R groups, however, Fas was found in the cytoplasm of the stromal cells and epithelial cells of crypts at 1-h ischemia alone (Fig. 7b) and was markedly increased in the stroma at 3 h of reperfusion after 1-h ischemia (Fig. 7c). On the other hand, FasL was observed in the epithelial cells of villi and crypts in the sham-operated groups (Fig. 7d). In the I/R groups, FasL-positive cells were also found at 1-h ischemia alone (Fig. 7e) and were markedly increased in the stroma at 3 h of reperfusion (Fig. 7f). However, FasL staining was essentially unchanged in the epithelial cells of crypts throughout the I/R experiment (data not shown).
Immunohistochemical staining of Fas (a–c) and FasL (d–f) in the ischemia-reperfusion (I/R) rat small intestine. a, d Sham operated; b, e 1-h ischemia; c, f 3 h of reperfusion. Fas- and FasL-positive cells were markedly increased at 3 h of reperfusion in the stromal cells (c, f). Insets indicate FasL-positive cells in the crypt cells in sham-operated (d) and Fas- (c) and FasL- (f) positive cells at 3 h of reperfusion in the stroma. Arrows indicate positive cells. Scale bars: 50 μm and 10 μm (insets in c, d, f).
Cytochrome c and activated caspase-3
To gain some insights into the role of death cascades in the induction of intestinal apoptosis, we examined the expression of cytochrome c and activated caspase-3 by immunohistochemistry using the same sets of paraffin-embedded tissues. We observed an intense staining for cytochrome c in the cytoplasm of villous epithelial cells in the sham-operated groups while it was almost negligible in the lamina propria and crypts (Fig. 8a). In the I/R groups, cytochrome c was also found in the cytoplasm of villous epithelial cells after 1-h ischemia (Fig. 8b). Unexpectedly, however, the staining for cytochrome c was markedly decreased at 3 h of reperfusion (Fig. 8c) and then reappeared at around 24 h of reperfusion (Fig. 8d). For activated caspase-3, we found a weak signal in the cytoplasm of some villous epithelial and stromal cells in the sham-operated groups (Fig. 8e). In the I/R groups, the staining for activated caspase-3 was markedly increased at 3 h of reperfusion only in the stroma (Fig. 8g). Moreover, as shown in Fig 8h, TUNEL-positive cells were also positive for activated caspase-3, demonstrating the involvement of the Fas/FasL-dependent cascade via activated caspase-3 in stromal-cell apoptosis.
Immunohistochemical staining of cytochrome c (a–d) and activated caspase-3 (e–g) in the ischemia-reperfusion (I/R) rat small intestine. a, e Sham operated; b, f 1-h ischemia; c, g 3 h of reperfusion, and d 24 h of reperfusion. The staining for cytochrome c of epithelial cells was markedly decreased at 3 h of reperfusion (c) and reappeared at around 24 h of reperfusion (d). The activated caspase-3 expression was markedly increased at 3 h of reperfusion in the stroma (g). Double staining for TUNEL and activated caspase-3 at 3 h of reperfusion after 1-h ischemia in the stroma (h). Inset indicates positive cells in the stroma. Arrows indicate positive cells. Scale bars: a–g 50 μm; h 20 μm; g 10 μm (inset).
Effect of leupeptin on the induction of apoptosis by I/R in rat small intestine
To examine the possible involvement of activated caspase-3 and/or other caspases in the induction of apoptosis, we examined the effect of leupeptin on the number of TUNEL-positive cells 1 h before ischemia in the 1-h ischemia-alone group or 1 h after reperfusion in the 3 h of reperfusion group. Injection with leupeptin significantly reduced the number of TUNEL-positive cells dose dependently in the lamina propria at 3 h of reperfusion (Fig. 9b) compared with that of the control group (Fig. 9a) but not in the 1-h ischemia-alone group (data not shown). In accordance with these light microscopic findings, EM observation revealed that the number of apoptotic cells was markedly reduced in the leupeptin-treated group (Fig. 9d) compared with that of the control group (Fig. 9c). Quantitative analysis revealed that the number of TUNEL-positive cells in the leupeptin-treated group was significantly lower than that of the control in the lamina propria and the crypts (Fig. 10). Interestingly, injection of leupeptin also decreased the number of activated caspase-3-positive cells in the lamina propria at 3 h of reperfusion (data not shown).
Effect of leupeptin on the induction of apoptosis by ischemia reperfusion (I/R) in the stroma and crypts of rat small intestine. a, c 3 h of reperfusion; b, d 3 h of reperfusion with leupeptin treatment (50 mg/kg). Arrows indicate positive cells. Original magnification ×2,000 (c–d). Scale bar: 50 μm.
Discussion
In the present study, we first investigated the time-course changes in the induction of apoptosis by I/R in rat small intestine using TUNEL and EM and found that the number of TUNEL-positive cells was significantly increased after reperfusion, reaching a maximum at 3 h of reperfusion in the lamina propria and the epithelia of villi and crypts. Moreover, in parallel with the induction of small intestinal cell apoptosis by I/R, differential expression of apoptosis-related proteins was evident depending on intestinal parts of the lamina propria, the epithelia of villi, and crypts. Finally, we found that injection of leupeptin significantly decreased the induction of intestinal cell apoptosis while the degree of inhibition varied depending on the segment of the small intestine. These results emphasize the need to understand the molecular mechanism underlying the induction of apoptosis in each tissue component for a better management of I/R damage at the organ level.
In the present study, coexpression of Fas and FasL was induced in the lamina propria by I/R. The Fas system was originally characterized as a key mechanism for inducing apoptosis in immune cells, but later it transpired to be very common in various tissues such as liver, ovary, kidney, and testis, especially under I/R conditions. In the case of the small intestine, French et al. (1996) demonstrated the expression of Fas and FasL mRNA in mouse small intestine. Until now, however, the involvement of the Fas system in the regulation of small intestinal cell apoptosis has been unknown. Two intracellular signaling pathways for Fas-mediated apoptosis are currently known: type I cell death in which activation of procaspase-8 directly leads to the activation of caspase-3, and type II cell death in which the death signal of caspase-8 activation is delivered to the downstream machinery through the cleavage of Bid, the release of cytochrome c, and the subsequent activation of caspase-3. Therefore, the type I cell death pathway may be triggered for induction of apoptosis in the lamina propria of the small intestine by I/R. In the villous epithelium, however, the release of cytochrome c accompanied by a transient loss of Bcl-2 expression seemed to be associated with the induction of apoptosis, and so it is more likely that the type II death pathway is involved, at least in part, in the apoptotic process. More interestingly, it might be possible to assume that the death pathway of the villous epithelial cells may not include the activation of caspases because of no increase in the staining of activated caspase-3 and of no inhibition of apoptosis by the leupeptin treatment. In this context, it should be noted that the biochemical study on the induction of intestinal cell apoptosis in a similar I/R rat intestine model detected the release of cytochrome c from mitochondria but no changes in the expression of activated caspase-3 (Wu et al. 2002). Moreover, our results also indicate that Fas expressed by I/R in the crypts may contribute to the induction of epithelial cell apoptosis in a different manner from type I and II cell death because of the lack of expression of cytochrome c and activated caspase-3.
It is interesting to identify the cell types that undergo apoptosis after I/R in the lamina propria since this layer contains many types of cells. Actually, our double-staining study revealed that TUNEL-positive cells were mostly positive for CD3. However, EM examination clearly indicated that there were many intact macrophages that phagocytosed apoptotic bodies. Therefore, we concluded that the cells actively engaged in apoptosis were principally T cells. Considering that activated T cells, which usually express Fas and FasL, have been implicated in the routine induction of intestinal apoptosis (Lin et al. 1998), this may suggest the involvement of activated T cells through the production of a variety of cytokines in protection of intestinal cells against I/R injury or in the immediate reconstruction of intestinal structure after reperfusion.
Consistent with the finding by Krajewski et al. (1994), we detected Bcl-2 and Bax in the epithelial cells of villi and crypts, respectively. However, I/R resulted in a transient decrease in the expression of Bcl-2 at 3–6 h after reperfusion in the villous epithelium. In contrast, Bax expression did not change throughout the experiment in the intestine. Bcl-2 is thought to act as an antiapoptotic molecule at least partly by inhibiting the translocation of Bax (Nomura et al. 1999) as well as the release of cytochrome c from the mitochondria. The ratio of Bcl-2 to Bax has also been reported to be essential in determining whether a cell survives or dies (Baba et al. 1999). Indeed, Coopersmith et al. (1999) recently reported that Bcl-2 inhibits the induction of apoptosis by I/R in the intestinal epithelium of Bcl-2 overexpressing mice and indicated that enhanced expression of Bcl-2 produces a twofold reduction in the number of TUNEL-positive cells that appear following transient SMA occlusion in the villous epithelium. Therefore, it might be expected that down-regulation of Bcl-2 expression may lead to the induction of apoptosis in villous epithelial cells.
Finally, injection of an SH-protease inhibitor 1 h after reperfusion effectively inhibited apoptosis in the I/R rat small intestine at 3 h of reperfusion. This result seems to indicate that the apoptotic cascades, including various caspases, play crucial roles in the induction of apoptosis in the I/R intestine although the key step in the cascade was not determined in the present study. In addition, the degree of inhibition of apoptosis by the protease inhibitor varied according to the tissue component, providing additional evidence for differences in the pathway leading to apoptosis among lamina propria, villous epithelium, and crypt epithelium.
In conclusion, the present results indicate that apoptosis was a major mode of cell death in the I/R rat small intestine. However, the triggering mechanisms of apoptosis may vary significantly among the lamina propria, villous epithelium, and cryptic epithelium. Further studies are necessary to define the role of protease inhibitors with a broad spectrum of action, such as leupeptin, in the management of intestinal injury after I/R.
References
Adams JC (1981) Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29:775
Baba N, Koji T, Itoh M, Mizuno A (1999) Reciprocal changes in the expression of Bcl-2 and Bax in hypoglossal nucleus after axotomy in adult rats: possible involvement in the induction of neuronal cell death. Brain Res 827:122–129
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Coopersmith CM, O’Donnell D, Gordon JI (1999) Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am J Physiol 276:G677–G686
Cotran RS, Kumar V, Robbins SL (1994) Cellular injury and cellular death. In: Cotran RS, Kumar V, Robbins SL, Schoen FJ (eds) Pathologic basis of disease, 5th edn. Saunders, Philadelphia, pp 1–33
Damavandi E, Hishikawa Y, Izumi S, Shin M, Koji T (2002) Involvement of Bax redistribution in the induction of germ cell apoptosis in neonatal mouse testes. Acta Histochem Cytochem 35:449–459
Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC (1998) Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol 143:217–224
French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J (1996) Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol 133:335–343
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Genesca M, Sola A, Miquel R, Pi F, Xaus C, Alfaro V, Hotter G (2002) Role of changes in tissular nucleotides on the development of apoptosis during ischemia/reperfusion in rat small bowel. Am J Pathol 161:1839–1847
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL (1994) Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94:1621–1628
Granger DN, Korthuis RJ (1995) Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol 57:311–332
Green DR (1998) Apoptotic pathways: the roads to ruin. Cell 94:695–698
Hakuno N, Koji T, Yano T, Kobayashi N, Tsutsumi O, Taketani Y, Nakane PK (1996) Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 137:1938–1948
Hashimoto S, Koji T, Niu J, Kanematsu T, Nakane PK (1995) Differential staining of DNA strand breaks in dying cells by non-radioactive in situ nick translation. Arch Histol Cytol 58:161–170
Hassoun HT, Zou L, Moore FA, Kozar RA, Weisbrodt NW, Kone BC (2002) Alpha-melanocyte-stimulating hormone protects against mesenteric ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 282:G1059–G1068
Itoh H, Yagi M, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K (2000) Activation of immediate early gene, c-fos, and c-jun in the rat small intestine after ischemia/reperfusion. Transplantation 69:598–604
Kawano N, Koji T, Hishikawa Y, Murase K, Murata I, Kohno S (2004) Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem Cell Biol 121:399–405
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Koji T, Kobayashi N, Nakanishi Y, Yoshii A, Hashimoto S, Shibata Y, Anjiki N, Yamamoto R, Aoki A, Ueda T, Kanazawa S, Nakane PK (1994) Immunohistochemical localization of Fas antigen in paraffin sections with rabbit antibodies against human synthetic Fas peptides. Acta Histochem Cytochem 27:459–463
Koji T, Hishikawa Y, Ando H, Nakanishi Y, Kobayashi N (2001) Expression of Fas and Fas ligand in normal and ischemia-reperfusion testes: involvement of the Fas system in the induction of germ cell apoptosis in the damaged mouse testis. Biol Reprod 64:946–954
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–337
Lin T, Brunner T, Tietz B, Madsen J, Bonfoco E, Reaves M, Huflejt M, Green DR (1998) Fas ligand-mediated killing by intestinal intraepithelial lymphocytes. Participation in intestinal graft-versus-host disease. J Clin Invest 101:570–577
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Megison SM, Horton JW, Chao H, Walker PB (1990) A new model for intestinal ischemia in the rat. J Surg Res 49:168–173
Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456
Nakane PK (1968) Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem 16:557–560
Nogae S, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, Nakane PK, Nakanishi Y, Koji T (1998) Induction of apoptosis in ischemia-reperfusion model of mouse kidney: possible involvement of Fas. J Am Soc Nephrol 9:620–631
Nomura M, Shimizu S, Ito T, Narita M, Matsuda H, Tsujimoto Y (1999) Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res 59:5542–5548
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Potten CS, Merritt A, Hickman J, Hall P, Faranda A (1994) Characterization of radiation-induced apoptosis in the small intestine and its biological implications. Int J Radiat Biol 65:71–78
Potten CS, Wilson JW, Booth C (1997) Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells 15:82–93
Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178
Sun F, Akazawa S, Sugahara K, Kamihira S, Kawasaki E, Eguchi K, Koji T (2002) Apoptosis in normal rat embryo tissues during early organogenesis: the possible involvement of Bax and Bcl-2. Arch Histol Cytol 65:145–157
Wang RA, Nakane PK, Koji T (1998) Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol Reprod 58:1250–1256
Watanabe-Fukunaga R, Brannan CI, Itoh N, Yonehara S, Copeland NG, Jenkins NA, Nagata S (1992) The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol 148:1274–1279
Wolf BB, Green DR (1999) Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem 274:20049–20052
Wu B, Iwakiri R, Tsunada S, Utsumi H, Kojima M, Fujise T, Ootani A, Fujimoto K (2002) iNOS enhances rat intestinal apoptosis after ischemia-reperfusion. Free Radic Biol Med 33:649–658
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (no. 15390058 to T.K.). The authors thank Mr. Takashi Suematsu for this excellent ME techniques assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, S., Hishikawa, Y. & Koji, T. Induction of cell death in rat small intestine by ischemia reperfusion: differential roles of Fas/Fas ligand and Bcl-2/Bax systems depending upon cell types. Histochem Cell Biol 123, 249–261 (2005). https://doi.org/10.1007/s00418-005-0765-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0765-6