Abstract
Tritrichomonas foetus is an amitochondriate parasite that possesses hydrogenosomes, unusual anerobic energy-producing organelles. In these organisms the “mitochondrial cell death machinery” is supposed to be absent, and the mechanisms that lead to cell demise remain to be elucidated. The presence of a cell death program in trichomonads has already been reported, suggesting the existence of a caspase-like execution pathway in such organisms. Here we demonstrate the alterations provoked by the fungicide griseofulvin and raise the possibility that other cell death pathways may exist in T. foetus. Dramatic changes in trichomonads morphology are presented after griseofulvin treatment, such as intense plasma membrane and nuclear envelope blebbing, nucleus fragmentation, and an abnormal number of oversized vacuoles. One important finding was the exposition of phosphatidylserine (PS) in the outer leaflet of the plasma membrane in cells after drug treatment, and also the presence of a high amount of misshapen flagella and tubulin precipitates as vacuolar contents, suggesting an autophagic process of abnormal cellular elements. Interestingly, immunoreactivity for activated caspase-3 was not detected during griseofulvin treatment, a finding distinct from the observed when this cell was treated with H2O2. The possibility of the existence of different pathways to cell death in trichomonads is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tritrichomonas foetus is a protist parasite that causes a sexually transmitted bovine disease named trichomonosis. This parasite lacks mitochondria but possesses hydrogenosomes, an unusual anerobic energy-producing organelle.
It is well established that mitochondria plays a pivotal role during cell death of many different cell types, including unicellular organisms (Arnoult et al. 2001, 2002; Lee et al. 2002; Sen et al. 2004). This organelle constitutes the primary site of action of members of the Bcl-2 protein family, which control the mitochondrial membrane permeabilization, and release of some mitochondrial proteins to cytosol. These molecules act in the amplification of the apoptotic signal, and in effectuation of cell death (for review see Ravagnan et al. 2002; van Loo et al. 2002). In amitochondriate organisms such as trichomonads, such death machinery is supposed to be absent, and the mechanisms that lead to cell demise remain to be elucidated.
In previous reports, Chose et al. (2002) and Mariante et al. (2003) have shown the presence of an execution program in the amitochondriate T. vaginalis and T. foetus, respectively. In both works it was suggested that there is the existence of a caspase-like pathway for cell death in trichomonads.
In the present study we raised the possibility that another different execution pathway may exist in T. foetus submitted to distinct stressing conditions. Unlike hydrogen peroxide (Mariante et al. 2003), griseofulvin seems to lead cells to death in a fashion apparently independent of caspase-3, one of the key executioners of caspase-mediated cell death programs in several cell types (for review see Cohen 1997; Chang and Yang 2000; Stennicke and Salvesen 2000). The significance of alternative routes of cell demise in such organisms is discussed.
Materials and methods
Parasites
T. foetus, K strain, was isolated by Dr. H. Guida (EMBRAPA, Rio de Janeiro, Brazil) from the urogenital tract of a bull, and cultivated at 37°C in TYM Diamond’s medium (Diamond 1957) supplemented with 10% fetal calf serum.
Drug treatment
Cells were treated with 50 μg/ml griseofulvin at different time intervals (from 3 to 48 h). Cell motility was checked by visual inspection by light microscopy, and the number of motile cells (those cells presenting normal movement) was calculated using a Neubauer hemocytometer. In order to verify the reversibility of griseofulvin effects, cells were grown in presence of drug and then transferred to fresh medium without the drug. Controls were performed with cells grown in the absence of the griseofulvin or in presence of 0.5% DMSO, the diluting agent of griseofulvin.
Fluorescence microscopy
DAPI
Cells were harvested by centrifugation and fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2). Afterwards, cells were rinsed with phosphate-buffered saline (PBS) and allowed to adhere to poly-l-lysine-coated glass coverslips. In order to investigate morphological alterations of the nucleus, cells were stained with 5 μg/ml DAPI (Molecular Probes, USA) for 15 min and examined in an Axiophot II Zeiss microscope equipped with UV epifluorescence. Images were acquired with a Hamamatsu chilled CCD camera C5985 and processed using Adobe Photoshop (Adobe, USA).
Acridine orange
In order to verify the presence of autophagic vacuoles, living cells were incubated for 15 min in the dark with 20 μg/ml acridine orange (Sigma, USA) in TYM medium. Next, cells were collected by centrifugation, washed with PBS and observed as described earlier.
Didansylcadaverine (DDC)
Following griseofulvin treatment, cells were incubated with 50 μg/ml DDC (Sigma, USA) in TYM medium at 37°C for 2 h. Cells were then covered with glycerol and analyzed in an Axiophot II Zeiss microscope as described earlier.
3-Methyladenine (3-MA)
In order to verify if the incorporation of DDC was via autophagy, cells were pre-incubated for 3 h with 10 mM 3-MA (Sigma, USA), an autophagic inhibitor, before induction of cell death.
Conventional scanning electron microscopy (SEM)
Cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2), rinsed with PBS, and allowed to adhere to poly-l-lysine-coated glass coverslips. Parasites were post-fixed in 1% OsO4 in 0.1 M sodium cacodylate buffer (pH 7.2), dehydrated in ethanol, and critical-point-dried using CO2. The samples were coated with gold-palladium and observed in a JEOL 5800 scanning electron microscope.
High-resolution field emission scanning electron microscopy (FESEM)
In order to observe the effects of griseofulvin in parasite’s internal structures, drug-treated cells were adhered to poly-l-lysine-coated glass coverslips and then treated with a permeabilization buffer (2 mM glycerol, 0.1 M pipes, 1 mM PMSF, 1 mM MgSO4, 2 mM EGTA, 0.1% Nonidet P-40, pH 6.8) (Gordon et al. 2001) for 1–10 min. Afterwards, cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2), post-fixed with 1% OsO4 in 0.1 M sodium cacodylate buffer (pH 7.2), dehydrated in ethanol, critical-point-dried using CO2, and sputter-coated with carbon. Samples were examined in a Jeol JSM-6340F FESEM.
Transmission electron microscopy (TEM)
Cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2), washed with PBS, and post-fixed with 1% OsO4 in 0.1 M sodium cacodylate buffer plus 5 mM CaCl2 and 0.8% potassium ferricyanide. Afterwards, parasites were washed with PBS, dehydrated in acetone, and embedded in Epon. Ultra-thin sections were stained with uranyl acetate and lead citrate, and observed in a Jeol 1210 electron microscope.
Immunocytochemistry
Caspase-3
The CM1 antibody was a gift from Dr. Anu Srinivasan (Idun Pharmaceuticals, USA), and a detailed description was provided by Namura et al. (1998). Briefly, the antiserum was raised against the C-terminal peptide-spanning amino acids 163±175 of the p20 cleavage product of human or mouse caspase-3, which has been demonstrated, by Western blots, to preferentially recognize the activated form of caspase-3 (Namura et al. 1998). Cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) and were allowed to adhere to coverslips previously coated with poly-l-lysine. Fixed cells were incubated with 3% H2O2 in PBS for 30 min to inactivate endogenous peroxidase activity, followed by PBS washes. Afterwards, cells were incubated with a buffer containing 2% bovine serum albumin, 0.2% non-fat milk powder, 2% normal goat serum, and 0.8% Triton X-100 in PBS for 1 h at room temperature in order to quench and block unspecific sites. After blocking, parasites were incubated overnight at 4°C with the CM1 primary antibody diluted (1:5,000) in blocking buffer. The immunocytochemical reaction was developed with HRP-ABC kit (Vectastain, Vector Laboratories, USA). Cells were analyzed in an Axiophot II Zeiss microscope.
Tubulin
Cells were fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 8.0), and allowed to adhere to poly-l-lysine-coated glass coverslips. Parasites were then permeabilized with 3% Nonidet P-40 for 40 min and acetone at −20°C for 15 min. Fixed cells were quenched using 50 mM ammonium chloride solution and 3% bovine serum albumin in PBS (BSA/PBS). Next, they were incubated overnight, at 4°C, with the monoclonal anti-tubulin TAT-1 antibody, which is provided by Dr. Keith Gull (Oxford, UK), followed by 1 h incubation with a fluorescein-conjugated anti-mouse antibody diluted (1:100) in BSA/PBS. Parasites were also stained with 5 μg/ml DAPI to visualize the nucleus. Finally, cells were washed and examined as described earlier for fluorescence microscopy.
Flow cytometry
In order to verify phosphatidylserine (PS) exposition on the outer plasma membrane annexin V (Molecular Probes, USA) was used. Parasites were washed twice with cold PBS and annexin-binding buffer (ABB, 10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2), and 105 cells were resuspended in 100 μl ABB containing 1 μl annexin V FITC-conjugated. Cells were then incubated for 15 min in the dark at room temperature. Afterwards, 200 μl of ABB were added to cells, which were kept on ice until analysis. This was conducted for 30,000 cells using a FACSCalibur flow cytometer (BD Biosciences, San José, CA, USA) using the WinMDI software (http://facs.scripps.edu/, Joseph Trotter). Cell viability was analyzed using 1 μg/ml propidium iodide prior analysis.
Results
Cell growth and motility
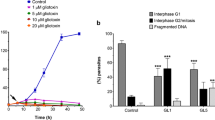
Initially, the cytotoxic effects of drug were investigated by treating parasites with 50 μg/ml griseofulvin at different time intervals (Fig. 1). Within the first few hours it was possible to verify the inhibitory effects provoked by the drug. Untreated cells, or cells treated with 0.5% DMSO, displayed a normal growth (Fig. 1a), and presented no changes in motility (Fig. 1b). Treatment with griseofulvin led to culture growth inhibition (Fig. 1a), and loss of motility in about 50% of the population within the first 3 h (Fig. 1b). Interestingly, almost 100% of the cells have lost the motility after 9 h of drug treatment (Fig. 1b). These drug effects were found completely irreversible after 12-h treatment (Fig. 6c). The cells’ appearance was quite different from any known morphology of trichomonads since cells showed their cytoplasm and glycogen with intense extraction, and an unhealthy aspect (Fig. 6c). The incubation time of 24 h was chosen for structural studies.
Griseofulvin effects on T. foetus growth (a) and motility (b). The drug was added with 50 griseofulvin μg/ml in different time intervals. Legend: CT control with no drug treatment; DMSO control with 0.5% dimethyl sulfoxide, the fungicide diluting agent; GF 50 μg/ml griseofulvin. Note the inhibitory effects of the compound within the first few hours of treatment
Cell morphology
A typical untreated trichomonas displays a tear-drop shape, one nucleus, three anterior flagella, a recurrent flagellum forming the undulating membrane, several roughly spherical hydrogenosomes, the microtubular axostyle-pelta complex, a costa, some endoplasmic reticulum profiles, a prominent Golgi complex, lysosomes localized at the cells’ posterior region, and some small-sized vacuoles (Figs. 2a, 3a, 5a, 7a, 8a, 9a, 10a).
Differential interference contrast (DIC) and fluorescence microscopy of T. foetus stained with 5 μg/ml DAPI. a Control cell, with no drug treatment. b, c Tritrichomonas foetus cells treated with 50 μg/ml griseofulvin for 24 h. Note the membrane blebbing (arrowheads), apoptotic body formation (black arrows), axostyle-pelta complex (asterisks), and multiple nuclei or nuclear fragmentation (white arrows). Peripheral masses of heterochromatin are also seen (c). Bar 10 μm
SEM of T. foetus under griseofulvin treatment. a A typical untreated cell displaying its elongated shape, three anterior flagella (AF), a recurrent flagellum (RF), and axostyle tip (A). b–e After 50 μg/ml griseofulvin treatment for 24 h the cells displayed abnormal size and shape, internalized flagella, plasma membrane projections (arrows) and apoptotic body formation (arrowheads). Bars 5 μm
After griseofulvin treatment striking alterations were observed: cells became rounded with intense membrane blebbing; the axostyle-pelta complex was seen fragmented when longer incubation times were used (Fig. 2b–c); giant cells with multiple nuclei and/or nucleus fragmentation were frequent (Figs. 2b–c, 7c). Some of these multinucleated cells also exhibited peripheral masses of heterochromatin (Fig. 2c). The nuclear apoptotic condensation pattern was confirmed using dyes as Hoechst 33342 and propidium iodide (not shown). Structures resembling apoptotic bodies were found associated with the cell’s surface (Fig. 2b–c).
In order to better analyze these changes after griseofulvin treatment, TEM and SEM were used. As seen by SEM, parasites clearly exhibited a different morphology (Fig. 3b–e). Besides the apoptotic features as membrane blebbing and apoptotic bodies, treated parasites also displayed flagella internalization and abnormal cell shapes (Fig. 3b–e). These observations were confirmed by TEM (Fig. 5b–e). Interestingly, when plasma membrane was removed by detergent treatment and analyzed by high-resolution FESEM, blebbing was found in the nuclear envelope (Fig. 4). In addition, intense cytoplasmic vacuolization (Figs. 5b–e, 6), a feature usually associated with autophagic cell death, was evident. Other alterations included axostyle-pelta complex fragmentation and abnormal hydrogenosomes (Fig. 5d–e).
TEM of T. foetus after griseofulvin treatment. a An untreated cell displaying its typical structures. Three anterior flagella (F), recurrent flagellum (RF), Golgi complex (G), costa (C), axostyle (A), pelta (P), nucleus (N) and hydrogenosomes (H) are seen. b–e Cell observed after 50 μg/ml griseofulvin treatment for 24 h present intense membrane blebbing (arrows), and cytoplasmic vacuolization (V). Nucleus membrane projections could also be noticed (b arrowhead). Hydrogenosomes presenting high electrondense contents are seen, and membranous profiles are observed within the hydrogenosome matrix in some cells (d asterisk). Endoplasmic reticulum profiles are also seen surrounding the vacuoles (d arrowheads). The axostyle-pelta complex became completely fragmented after drug treatment (e). Bars 2 μm (a), 3 μm (b–c), 1.5 μm (d–e).
Autophagic vacuoles
Drug-treated cells observed by TEM exhibited vacuoles surrounded by membranous profiles similar to endoplasmic reticulum, a feature commonly found during autophagic processes. Interestingly, these vacuoles displayed different contents, including membrane profiles, and remnants of disorganized flagellar axonemes (Fig. 6). In addition, they contained deposits of unknown periodic structures, which were hypothesized to be precipitated tubulin (Fig. 6b). Positive reaction for tubulin was found in these vacuoles by immunofluorescence microscopy (Fig. 7). Untreated cells presented a weak labeling in flagella, and an intense staining in the axostyle-pelta complex (Fig. 7a), whereas griseofulvin-treated cells displayed an intense labeling in internal structures, possibly corresponding to internalized flagella and/or the axostyle-pelta complex (Fig. 7b), and in vacuoles of similar sizes observed by TEM (Fig. 7b–c). When the medium containing the drug was removed and replaced by fresh medium without drug, the cells did not recover and died (Fig. 6c).
High magnification of vacuoles under 50 μg/ml griseofulvin treatment for 24 h. Membranous profiles are found within most of the vacuoles (a, b). Several compartments were seen fulfilled with abnormal flagella axonemes (b, arrowheads), and microtubule-like deposits (b asterisks). In c the drug was removed and the cells were maintained in fresh medium without drug for 24 h. The cells present glycogen and cytoplasm extraction, and this culture was not recovered. H hydrogenosome; N nucleus. Bars 700 nm (a), 800 nm (b), 1 μm (c)
DIC and immunofluorescence microscopy of T. foetus stained with the monoclonal anti-tubulin TAT-1 antibody. a Control cell showing intense labeling at Ax-Pe, and F. b, c Cells treated with 50 μg/ml griseofulvin for 24 h display a different labeling pattern, possibly corresponding to fragmented axostyle-pelta complex and/or flagella (arrows), and cytoplasmic vacuoles (arrowheads). Note the presence of more than one nucleus stained with 5 μg/ml DAPI. Bar 10 μm
Autophagic vacuoles were detected with acridine orange (Fig. 8). In control cells, an intense labeling was found in structures preferentially localized at the posterior region of the cell (Fig. 8a), which in trichomonads corresponds to lysosomes. An intense labeling was noticed in many intracellular vacuoles after griseofulvin treatment (Fig. 8b–c), suggesting the presence of an autophagic process. A similar labeling pattern was observed in cells incubated with the fluorescent drug DDC, an autophagolysosome marker (Fig. 9a–b). An inhibitory effect of the incorporation of DDC was observed when cells were pre-treated with 3-MA, a specific inhibitor of the early stages of autophagic processes, as seen by fluorescence microscopy (Fig. 9c).
DIC and fluorescence microscopy of T. foetus stained with 20 μg/ml acridine orange. a Control cell presenting intense labeling in structures posteriorly located, probably the lysosomes (arrows). b–c Cells treated with 50 μg/ml griseofulvin for 24 h. Giant cells presenting several stained vacuoles randomly distributed (arrowheads) are seen after drug treatment. Bars 5 μm
Inhibition of DDC incorporation by 3-MA. a Control cells exhibited labeling in compartments posteriorly located (arrows). b Cells treated with 50 μg/ml griseofulvin for 24 h and incubated with DDC show labeled vacuoles randomly distributed throughout the cytoplasm (arrows). c Cells pre-treated with 10 mM 3-MA for 3 h had DDC accumulation prevented. Bar 10 μm
Caspase-like immunoreactivity
In order to check the probable involvement of caspase-3 in trichomonad cell death immunocytochemistry was performed using the CM1 antibody. A group of cells was treated for 3, 6 and 24 h in the presence of griseofulvin, whereas other cells were treated with 0.5 mM H2O2, which were used as positive control (Mariante et al. 2003). Unexpectedly, an activated caspase-3 was not detected after griseofulvin treatment, even after 24 h (Fig. 10).
DIC microscopy of T. foetus after immunocytochemistry for activated caspase-3. a Untreated cells. b Cells treated with 0.5 mM H2O2 for 4 h (positive control). c Cells treated with 50 μg/ml of griseofulvin for 24 h. Note that griseofulvin-treated cells do not present any labeling, in a similar way of the untreated cells, whereas cells treated with H2O2 exhibit an intense positive labeling. Bar 10 μm
Phosphatidylserine (PS) exposition
In order to investigate PS exposition in the outer plasma membrane leaflet, one of the hallmarks of apoptosis (Deshmukh and Johnson 1998), cells were incubated with annexin V, and analyses were performed by flow cytofluorometry. Griseofulvin treatment induced PS exposure within 24 h (Fig. 11). The plasma membrane remained intact since no nucleus PI labeling was detected. Hypotheses of alternative pathways of trichomonads cell death are suggested in Fig. 12.
Flow cytometry analysis of trichomonads cell death. a T. foetus was incubated in the absence (CTRL) or presence (GRISEO) of griseofulvin (50 μg/ml) for 24 h, and PS exposure was analyzed. Percentages of positive cells (squares) are indicated. b Fluorescence intensity of griseofulvin-treated parasites (open histogram). Filled histogram represents basal cell fluorescence in the absence of the drug
Discussion
Griseofulvin is a metabolic product of Penicillium spp. (Develoux 2001). This drug has been mainly used as an antifungal, although its precise mechanism of action is not well known. Although griseofulvin effects on cell death have been reported mainly in fungus, its role on growth inhibition has been demonstrated in Dictyostelium discoideum (Rubino et al. 1982), and in Tetrahymena pyriformis (Sparagano 1995). In addition, this fungicide provoked significant ATP level changes in T. pyriformis (Sparagano 1995), a feature commonly associated with cell death (for review see Huettenbrenner et al. 2003).
Besides inhibition of cell culture growth, and cells’ motility, several morphological alterations were observed in T. foetus after griseofulvin treatment. Many of these alterations have already been previously described in trichomonas after hydrogen peroxide (Mariante et al. 2003), taxol, or nocodazole (Madeiro da Costa and Benchimol 2004) treatments. Among the main alterations, signs of apoptosis, such as nucleus fragmentation, plasma membrane blebbing, and apoptotic bodies formation, and also autophagic characteristics, for example, cytoplasmic vacuolization, were observed.
The nuclear changes here reported suggested the existence/activation of molecules similar to those found in higher eukaryotes, such as endonuclease G, AIF and/or CAD, in T. foetus cell death. Studies at molecular level would help to clarify this hypothesis. Membrane blebbing has also been described during degeneration of T. thermophila induced by staurosporine (Christensen et al. 1998), and in T. foetus treated with drugs that target microtubules (Madeiro da Costa and Benchimol 2004). Apoptotic bodies are related to apoptotic degeneration, and once formed, they are phagocytosed by neighboring cells or macrophages (Wyllie 1981; Savill and Fadok 2000). However, in vitro cultivation of unicellular parasites leads to a necrotic phenotype, since they are not in contact with phagocytic cells.
Vacuolization has been reported during autophagic degeneration of T. thermophila after staurosporine treatment (Christensen et al. 1998), and also in D. discoideum, in a non-apoptotic form of cell death, named paraptosis (Sperandio et al. 2000; Wyllie and Goldstein 2001). Large vacuoles were also reported in G. intestinalis submitted to oxidative stress (Lloyd et al. 2000) in L. donovani after induction with antimicrobial peptides (Bera et al. 2003), and in T. foetus treated with other drugs, such as hydroxyurea, fibronectin, hydrogen peroxide, nocodazole, or colchicine (Benchimol 1999, 2001; Ribeiro et al. 2002; Mariante et al. 2003; Madeiro da Costa and Benchimol 2004).
The vacuole contents displayed abnormal axonemes, probably those originated from flagella and/or axostyle-pelta complex fragmentation. Contrary to previous observations in trichomonads (Noël et al. 2003), the axostyle-pelta complex was not seen depolymerized, but rather fragmented in T. foetus after drug treatment, a finding that agrees with its stable microtubule composition (Ribeiro et al. 2000).
Acridine orange and DDC were used in order to detect autophagolysosomes, structures usually observed during autophagic degeneration. Several vacuoles displayed intense labeling after griseofulvin treatment. It has been reported that 3-MA prevents the autophagic cell death pathway (Seglen and Gordon 1982). Accordingly, in these experiments this drug seemed to prevent the autophagic death pathway since no labeling was observed after DDC incubation in cells previously treated with 3-MA. Together with the observation of endoplasmic reticulum profiles surrounding vacuoles containing cell’s structures, these results suggest an autophagic cell death process in trichomonads.
T. foetus submitted to stressing conditions internalize their flagella to form pseudocysts (Granger et al. 2000; Pereira-Neves et al. 2003), which could be a defensive strategy to environmental changes. The hydrogenosomes have an essential role in the parasite survival, being the site of formation of ATP. They present similarities with mitochondria (reviewed in Muller 1990, 1993) and thus, as trichomonads are amitochondriated, hydrogenosome could be a putative candidate participating in the death of trichomonads cells.
Interestingly, when trichomonads were under griseofulvin treatment, the activated form of caspase-3 was not detected. Although this protease plays a pivotal role during apoptotic cell death in several organisms, other cell death pathways, independent of caspases, may exist. Besides, the same stimuli seem to lead the activation of different cell signaling pathways, dependent or independent on one another (for review see Jia et al. 1997; Guimarães et al. 2003; Huettenbrenner et al. 2003).
It has been shown that a pro-apoptotic drug, such as staurosporine, provokes the exposition of phosphatidylserine (OS) in the outer leaflet of several unicellular organisms, including T. vaginalis (Chose et al. 2002). However, in such organisms the mechanism seems to be caspase-independent since previous treatment with caspase inhibitors failed to prevent this effect (Chose et al. 2002). Here it was analyzed whether or not griseofulvin treatment induces PS translocation to the outer cell plasma membrane. In agreement with Chose et al. (2002), it was found that the drug treatment induced cell membrane PS outside exposition.
The different results obtained with T. foetus after treatment with hydrogen peroxide (Mariante et al. 2003) or griseofulvin (present study) suggest the existence of more than one mechanism of cell demise in these parasites (Fig. 12). The first one, observed after hydrogen peroxide treatment, would count with the participation of caspase-3. In other cell types this protease is known to be activated by either caspase-8, which is itself activated by oligomerization through recruitment by cell death receptors, or caspase-9, which together with ATP and mitochondrial proteins, such as cytochrome c, form the apoptosomes (van Loo et al. 2002). Since trichomonads do not possess mitochondria or cytochrome c, the apoptosome pathway seems unlikely to occur in such organisms. Thus, it is reasonable to suppose that other routes of cell death may be present in the parasite. In addition these routes could occur either through known mechanisms, such as the death receptors pathway, or through still unknown signaling pathways, which could involve the release of hydrogenosome molecules, with functions probably analogous to those of mitochondrial proteins. On the other hand, a second cell death pathway seems to involve the autophagic machinery and the formation of autophagosomes. In the same cell population, or even in a unique cell, features of both apoptotic and autophagic processes are observed. As stated by Jia et al. (1997) and Guimarães et al. (2003) in other cell types, an autophagic route may be required for caspase activation and apoptosis in trichomonads. However, since cell death induced by griseofulvin seems not to involve the caspase pathway, it is reasonable to suppose that another route of cell demise, dependent on the autophagic route, occurs in trichomonads, reinforcing the idea that a cell can switch between apoptosis and autophagy as the dominant form of cell death. In addition, inhibition of autophagosomes formation with 3-MA apparently does not affect the death of cells, a finding that suggests the existence of a mechanism of cell degeneration independent of autophagy.
Finally, nuclear changes observed in T. foetus after drug treatment could be related to the presence of molecules, which could be activated by the caspase pathway or be released from the hydrogenosomes to the nucleus following a death stimulus. However, these proposals need further studies in order to be confirmed.
One could argue that many of the cell changes described in this work—such as some morphological alterations and inhibition of cell growth and motility—could be solely due to the antimicrotubular effect of griseofulvin. Although this possibility cannot be ruled out, other evidences point out to the presence of a cell death program machinery. Besides typical apoptotic and autophagic morphological features—such as nuclear alterations, membrane blebbing, apoptotic bodies, and intense vacuolization, PS translocation to the outer leaflet would not be caused solely by cytoskeleton changes. The mechanisms underlying PS exposure are just beginning to be elucidated, but it seems to be mediated by the activation of scramblase transporters. These proteins enhance lipid transport across plasma membrane, and are externally maintained as a consequence of the inhibition of ATP-dependent flipases, which normally brings PS back to the inner membrane leaflet—and by the polyamine metabolism. Polyamines are essential for cell survival and proliferation, and decrease in their intracellular levels correlates with apoptosis (reviewed in Moreira and Barcinski 2004). Thus, griseofulvin seems to act by not only affecting the cytoskeleton but also seems to have an important implication in the molecular pathways of cell death in trichomonads.
In conclusion, a possible alternative execution route during T. foetus cell death has been shown. Altogether, the results suggest that both caspase-dependent and independent pathways of cell demise may exist in the amitochondriate trichomonads.
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- DAPI:
-
4′,6-diamidino-2-phenylindole dihydrochloride
- AIF:
-
Apoptosis-inducing factor
- CAD:
-
Caspase-activated DNase
References
Arnoult D, Tatischeff I, Estaquier J, Girard M, Sureau F, Tissier JP, Grodet A, Dellinger M, Traincard F, Kahn A, Ameisen JC, Petit PX, (2001) On the evolutionary conservation of the cell death pathway: mitochondrial release of an apoptosis-inducing factor during Dictyostelium discoideum cell death. Mol Biol Cell 12:3016–3030
Arnoult D, Akarid K, Grodet A, Petit PX, Estaquier J, Ameisen JC (2002) On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ 9:65–81
Benchimol M (1999) Hydrogenosome autophagy: an ultrastructural and cytochemical study. Biol Cell 91:165–174
Benchimol M (2001) Hydrogenosome morphological variation induced by fibronectin and other drugs in Trichomonas vaginalis and Tritrichomonas foetus. Parasitol Res 87:215–222
Bera A, Singh S, Nagaraj R, Vaidya T (2003) Induction of autophagic cell death in Leishmania donovani by antimicrobial peptides. Mol Biochem Parasitol 127:23–35
Chang HY, Yang X (2000) Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev 64:821–846
Chose O, Noel C, Gerbod D, Brenner C, Viscogliosi E, Roseto A (2002) A form of cell death with some features resembling apoptosis in the amitochondrial unicellular organism Trichomonas vaginalis. Exp Cell Res 276:32–39
Christensen ST, Chemnitz J, Straarup EM, Kristiansen K, Wheatley DN, Rasmussen L (1998) Staurosporine-induced cell death in Tetrahymena thermophila has mixed characteristics of both apoptotic and autophagic degeneration. Cell Biol Int 22:591–598
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Deshmukh M, Johnson EM Jr (1998) Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron 21:695–705
Develoux M (2001) Griseofulvin. Ann Dermatol Venereol 128:1317–1325
Diamond LS (1957) The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 43:488–490
Dunn WA Jr (1990) Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol 110:1923–1933
Gordon MB, Howard L, Compton DA (2001) Chromosome movement in mitosis requires microtubule anchorage at spindle poles. J Cell Biol 152:425–434
Granger BL, Warwood SJ, Benchimol M, de Souza W (2000) Transient invagination of flagella by Tritrichomonas foetus. Parasitol Res 86:699–709
Guimarães CA, Benchimol M, Amarante-Mendes GP, Linden R (2003) Alternative programs of cell death in developing retinal tissue. J Biol Chem 278:41938–41946
Huettenbrenner S, Maier S, Leisser C, Polgar D, Strasser S, Grusch M, Krupitza G (2003) The evolution of cell death programs as prerequisites of multicellularity. Mutat Res 543:235–249
Jia L, Dourmashkin RR, Allen PD, Gray AB, Newland AC, Kelsey SM (1997) Inhibition of autophagy abrogates tumor necrosis factor alpha induced apoptosis in human T-lymphoblastic leukemia cells. Br J Haematol 98:673–685
Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL (2002) Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ 9:53–64
Lloyd D, Harris JC, Maroulis S, Biagini GA, Wadley RB, Turner MP, Edwards MR (2000) The microaerophilic flagellate Giardia intestinalis: oxygen and its reaction products collapse membrane potential and cause cytotoxicity. Microbiology 146:3109–3118
Madeiro da Costa RF, Benchimol M (2004) The effect of drugs on cell structure of Tritrichomonas foetus. Parasitol Res 92:159–170
Mariante RM, Guimarães CA, Linden R, Benchimol M (2003) Hydrogen peroxide induces caspase activation and programmed cell death in the amitochondrial Tritrichomonas foetus. Histochem Cell Biol 120:129–141
Moreira ME, Barcinski MA (2004) Apoptotic cell and phagocyte interplay: recognition and consequences in different cell systems. Acad Bras Ciênc 76:93–115
Muller M (1993) The hydrogenosome. J Gen Microbiol 139:2879–2889
Muller M (1990) Structure. In: Honigberg BM (eds) Trichomonads parasitic in humans. Springer, Berlin Heidelberg New York, pp 5–35
Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 18:3659–3668
Noël C, Gerbod D, Delgado-Viscogliosi P, Fast NM, Ben Younes A, Chose O, Roseto A, Capron M, Viscogliosi E (2003) Morphogenesis during division and griseofulvin-induced changes of the microtubular cytoskeleton in the parasitic protist, Trichomonas vaginalis. Parasitol Res 89:487–494
Pereira-Neves A, Ribeiro KC, Benchimol M (2003) Pseudocysts in trichomonads—new insights. Protist 154:313–329
Ravagnan L, Roumier T, Kroemer G (2002) Mitochondria, the killer organelles and their weapons. J Cell Physiol 192:131–137
Ribeiro KC, Monteiro-Leal LH, Benchimol M (2000) Contributions of the axostyle and flagella to closed mitosis in the protists Tritrichomonas foetus and Trichomonas vaginalis. J Eukaryot Microbiol 47:481–492
Ribeiro KC, Arnholdt AC, Benchimol M (2002) Tritrichomonas foetus: induced division synchrony by hydroxyurea. Parasitol Res 88:627–631
Rubino S, Unger E, Fogu G, Cappuccinelli P (1982) Effect of microtubule inhibitors in the tubulin system of Dictyostelium discoideum. Z Allg Mikrobiol 22:127–131
Savill J, Fadok V (2000) Corpse clearance defines the meaning of cell death. Nature 407:784–788
Seglen PO, Gordon PB (1982) 3-Methyladedine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA 79:1889–1892
Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, Rakshit S, Sen T, Majumder HK (2004) Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ 11:924–936
Sparagano OA (1995) Griseofulvin: generation time and ATP changes in the ciliate Tetrahymena pyriformis. Life Sci 57:897–901
Sperandio S, de Belle I, Bredesen DE (2000) An alternative, no apoptotic form of programmed cell death. Proc Natl Acad Sci USA 97:14376–14381
Stennicke HR, Salvesen GS (2000) Caspases—controlling intracellular signals by protease zymogene activation. Biochim Biophys Acta 1477:299–306
van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ 9:1031–1042
Wyllie AH (1981) Cell death: a new classification separating apoptosis from necrosis. In: Bowen ID, Lockshin RA (eds) Cell death in biology and pathology. Chapman and Hall, New York, pp 9–34
Wyllie AH, Goldstein P (2001) More than one way to go. Proc Natl Acad Sci USA 98:11–13
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Associação Universitária Santa Úrsula (AUSU). The authors thank Dr. Keith Gull for the TAT-1 antibody and Dr. Anu Srinivasan for the CM1 antibody.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mariante, R.M., Vancini, R.G. & Benchimol, M. Cell death in trichomonads: new insights. Histochem Cell Biol 125, 545–556 (2006). https://doi.org/10.1007/s00418-005-0098-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0098-5