Abstract
Expression of phosphodiesterase 5 (PDE5) in the rat testis at several pre and postnatal developmental stages was investigated by immunohistochemistry. The enzyme was localised in vascular smooth muscle cells, as well as in Leydig and peritubular cells. The latter were identified as myoid, based on their immunoreactivity to desmin and α-smooth muscle actin. The presence of PDE5 in myoid cells was confirmed by Western blot analysis and immunohistochemistry performed on highly purified cell fractions, obtained from 16-day-old rats. The expression of PDE5 in these somatic cells of rat testis is discussed in view of the roles played by cGMP signal transduction pathways in the mammalian male reproductive function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the regulation of male reproductive function, cyclic nucleotides are involved in the reduction of spermatogonial division (Hollinger and Hwang 1974) and inhibition of spermiation (Gravis 1978), as well as in the epididimal sperm maturation (Sanborn et al. 1980). They also play key roles in somatic cells of the testis, which directly regulate the microenvironment where germ cells differentiate, besides hormonally controlling testis function. For example, cyclic nucleotides modulate the response to hypophysary gonadotropins in Sertoli cells and hormone production by Leydig cells (Means et al. 1980; Purvis and Hansson 1980).
Less information is so far available on the importance of cyclic nucleotide pathways in other interstitial cells, such as peritubular myoid cells, which in all mammals represent the main component of the seminiferous tubule lamina propria. Nevertheless, in these cells, regarded as smooth muscle cells, based on their ultrastructural features and cytoskeletal arrangement, a functional NO/cGMP pathway, essential in contractility regulation of vascular smooth muscle, can be hypothesised (Sanders et al. 2000).
Intracellular cGMP concentration is known to result from the balance between synthetic guanylate cyclase and phosphodiesterase (PDE) degradative activities. Although several different PDEs can hydrolyze cGMP, the highest rate of cGMP hydrolysis is achieved by phosphodiesterase 5 (PDE5), the activity of which is allosterically enhanced by low substrate concentrations (Ribalkin et al. 2003).
In humans, high amounts of PDE5 mRNA have been detected in several sections of the digestive system, in skeletal and cardiac muscle, and in several endocrine glands, including testis (Yanaka et al. 1998). In rats, PDE5 activity has been detected in lung, spleen, tracheal and vascular smooth muscle cells and platelets (Lincoln et al. 1976; Hamet and Coquil 1978; Coquil et al. 1985) and high levels of transcripts have been found in cerebellum and intestine (Kotera et al. 1999). In specific mammalian body districts, several PDE5-specific inhibitors increased cGMP concentration in vascular myocytes, leading to vessel relaxation. Specifically, Sildenafil inhibits cGMP degradation in mammalian corpus cavernosum smooth muscle (Boolell et al. 1996; Jeremy et al. 1997) and is currently employed in the treatment of human male erectile dysfunction. Despite the obvious interest raised in this item, however, only few reports deal with the cell types that specifically express the enzyme in different organs and tissues. In rodents, PDE5 has been detected in cerebellar Purkinje cells (Kotera et al. 1997), in the epithelium of kidney proximal tubules and collecting ducts, in pancreatic ducts (Kotera et al. 2000; Giordano et al. 2001). More recently, PDE5 expression has been described in the perinatally developing chick dorsal root ganglion (Giordano et al. 2004).
In the present research PDE5 expression was investigated in rat testis, at several pre and postnatal developmental stages, using a highly specific polyclonal antibody, raised against the N-terminal region of bovine PDE5 (Giordano et al. 2001). Immunohistochemistry performed by the avidin-biotin technique in paraffin sections and Western blot analyses demonstrated the presence of this enzyme in Leydig and myoid peritubular cells. Cell populations specifically expressing PDE5 were identified on the basis of their position and through the immunolocalisation of cytoskeletal proteins, namely desmin and α-smooth muscle actin (Virtanen et al. 1986; Tung and Fritz 1990; Palombi et al. 1992).
Materials and methods
Animals
Albino Wistar rats (Charles River, Italy) were kept at 20–22°C, with a dark/light cycle of 12/12 h. Females were placed with males overnight and examined the following morning for the presence of sperm in the vaginal smear; the day of sperm observation was considered as embryonic day 0.5 (E0.5).
All animals were sacrificed by heart resection. Animal housing and handling were conducted according to the European Communities Council Directive of 24 November 1986 (86/609/EEC) and to the Italian Health Ministry guidelines.
Sample collection for immunohistochemistry
Male rats ageing 3, 7, 14 and 60 days (three animals for each developmental stage) were i.p. injected with Farmotal (Amersham Pharmacia Biotech Italia, Milan, Italy) 100 mg/kg b.w., testes were excised and fixed by immersion in Bouin, for 2–6 h, at room temperature (RT). Two pregnant rats at gestational age of E18.5 days were submitted to Farmotal injection, the uterus was quickly removed and immersed in Ringer’s solution, individual foetuses were extracted from the decidua and measured and weighed for confirming the foetal age. Three foetuses for each female were then immersed in Bouin; 10 min later they were cut along a transverse plane and the posterior half was further fixed, for 4 h at RT.
After fixation, specimens were dehydrated in alcohol, embedded in paraffin and sectioned 8 μm thick. Foetuses in particular were serially sectioned along the transversal plane and sections containing a consistent portion of testis were identified and collected.
Purified preparations of peritubular myoid cells from 16-day-old rats were supplied from Prof. F. Palombi (Department of Histology and Medical Embryology, University of Rome ‘La Sapienza’, Rome, Italy). Briefly, the cells were obtained according to Filippini et al. (1993) and spun onto glass slides by cytocentrifugation at 900 rpm for 5 min in a Cytospin 3 Shandon centrifuge. Purification degree, checked in acetone/ethanol-fixed specimens by means of alkaline phosphatase histochemistry (Palombi and Di Carlo 1988) was found to be ≥96%. Paraformaldehyde-fixed samples were utilised for PDE5 and α-SM actin immunohistochemistry through the ABC method.
Sample collection for Western blot analysis
Decapsulated testes and decorticated seminiferous tubules were obtained, according to Geremia et al. (1978), both from prepuberal and from adult rats. Purified preparations of Sertoli cells from 16-day-old rats were obtained and cultured according to Dorrington et al. (1975). Purified preparations of peritubular myoid cells were supplied as mentioned above.
All samples were homogenised in 20 mM Tris–HCl buffer, pH 7.2, containing 0.2 mM EGTA, 5 mM mercaptoethanol, 1 mM PMSF, 5 mM MgCl2 and 2% (v/v) antiprotease cocktail, using a glass homogeniser (15 strokes, 4°C). The homogenates were centrifuged at 14,000×g for 30 min at 4°C, pellets were resuspended in the homogenisation buffer and centrifuged at 14,000×g for 30 min (4°C). The first and the second supernatants were then pooled and denatured for SDS-Page run. Protein determination was performed using the procedure of Lowry et al. (1951).
Western blot analysis
SDS-polyacrylamide gel electrophoresis was performed on 10% slab gels according to Laemmli (1970) and proteins were transferred to nitrocellulose membranes according to Towbin et al. (1979). The blots were incubated for 3 h at RT with 1:1000 diluted anti-PDE5. Alkaline phosphatase-conjugated goat anti-rabbit IgG were used to reveal immunocomplexes and the bands were stained with nitro blue tetrazolium in the presence of 5-bromo-4-chloro-3-indolyl-phosphate. Standard of PDE5 was a crude extract of N18TG2 cells expressing high level of this enzyme isoform (Giordano et al. 2001).
Immunohistochemistry
Freshly deparaffinised sections were treated with 0.3% H2O2 in methanol, for 30 min at RT, to inactivate endogenous peroxidases; after rehydration, they were incubated with citrate buffer pH 6 (10 min at RT, 8 min in microwave oven at 750 W and 30 min at RT) and transferred to PBS containing 0.2% Triton X-100 and 5% non-fat dry milk, for 1 h at RT.
In preliminary experiments PDE5 was immunolocalised by means of both the standard and an amplified ABC procedure (Adams 1992).
According to the former schedule, sections were sequentially incubated in
-
1.
1:200 diluted anti-PDE5 rabbit polyclonal antibody, in PBS containing 0.1% Triton X-100 and 2.5% non-fat dry milk, for 24 h at 4°C;
-
2.
1:200 biotinylated goat anti-rabbit IgG, for 1 h at RT, in PBS containing 1% preimmune goat serum, for 1 h at RT;
-
3.
avidin-biotin-horseradish peroxidase complex, for 1 h at RT;
-
4.
0.05% 3,3′-diaminobenzidine (DAB) in PBS containing 0.01 H2O2 for 2–5 min at RT.
According to the latter schedule, step 3 was followed by incubation in:
-
1.
1:100 diluted biotinylated tyramine, in PBS containing 0.01% H2O2, for 10 min at RT;
-
2.
avidin-biotin-horseradish complex, in PBS containing 1% BSA, for 30 min at RT.
As the two procedures gave quite similar results, the standard one was predominantly utilised.
Cytoskeletal markers were immunolocalised by means of the standard ABC procedure; sections were sequentially incubated in
-
1.
1:800 diluted anti-α-SM actin or 1:100 diluted anti-desmin mouse monoclonal antibodies, in PBS containing 0.1% Triton X-100 and 2.5% non-fat dry milk, for 24 h at 4°C;
-
2.
1:200 biotinylated rabbit anti-mouse IgG, for 1 h at RT, in PBS containing 1% preimmune rabbit serum, for 1 h at RT.
Steps 3 and 4 were performed as described above.
Samples of freshly purified peritubular myoid cells were submitted to the above-described endogenous peroxidase inactivation and microwave treatment, washed in PBS containing 0.05% Triton X-100 and transferred to PBS containing 0.05% Triton X-100 and 3% BSA, for 1 h at RT. PDE5 and α-SM actin were then immunolocalised by means of the above-described standard ABC procedure.
For negative controls, the primary antibody was omitted or substituted with preimmune serum. Moreover, some sections were incubated with the PDE5 antibody preadsorbed (overnight at 4°C) with 150 μg/ml of the PDE5-peptide antigen used to raise PDE5 antibody (Giordano et al. 2001).
Finally, both the standard and the amplified ABC procedure were utilised to verify PDE5 immunolocalisation in sections not submitted to microwave treatment. Unstained and haematoxylin counterstained sections and cell preparations were examined in a Zeiss Axioskop 2, equipped with a video camera. Representative images were electronically captured; contrast and brightness were adjusted by Adobe Photoshop 6.0.
Chemicals
Biotinylated goat anti-rabbit and rabbit anti-mouse IgG, as well as avidin-biotin-HRP (standard ABC kit) were purchased from Vector (Burlingame, CA, USA); anti-α-SM actin and anti-desmin mouse monoclonal antibodies, normal goat and rabbit sera, and DAB were from Sigma Chemical Co. (St. Louis, MO, USA). Anti-PDE5 rabbit polyclonal antibody was raised and purified using a PDE5 N-terminal peptide as already described (Giordano et al. 2001).
Results
Western blot analysis
Immunoblotting using PDE5 specific antibody on different testicular preparation showed the 90 kDa specific bands in control cell extract (neuroblastoma N18TG2) and pure myoid cell preparation (Fig. 1). Lighter bands were found in adult and prepuberal decapsulated testis extracts, while tubules, depleted of interstitial and peritubular cells, and Sertoli cells did not show any crossreacting band.
PDE5 expression in purified cell and tissue preparations from rat testis. Western blot analysis of: adult rat whole testis (AT); decorticated tubules from adult rat testis (Atu); prepuberal rat whole testis (PT); decorticated seminiferous tubules from prepuberal rat testis (Ptu); purified Sertoli cells from prepuberal rat testis (Se); purified myoid cells (M); N18 neuroblastoma cell culture utilised as standard of PDE5 (N18). Each lane was loaded with 100 μg of protein with the exception of lane M, where only 30 μg of myoid extract were utilised
Immunohistochemistry
In sections from rat testis, at all the examined developmental stages, PDE5, α-SM actin and desmin immunoreactivities were always restricted to the cytoplasmic compartment of specific cell populations; all control sections were free of labelling.
At all the examined postnatal stages (Fig. 2), anti-PDE5 selectively labelled Leydig and peritubular cells, besides vascular smooth myocytes. At postnatal day 3, and to a lesser extent at day 7, a specific, though mild, positivity was also detected in spermatogonia, while later the seminiferous epithelium appeared unlabelled. Quite similar results were obtained by means of the standard and the amplified ABC procedure, preceded or not by microwave antigen unmasking. Labelling was absent from sections incubated with immunogen-preadsorbed anti-PDE5 (Fig. 2c).
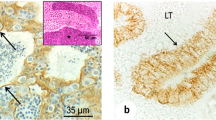
Rat testis PDE5 immunohistochemistry. Microwave treatment, standard ABC method. Haematoxylin counterstaining. Bar 20 μm. a Postnatal day 3. A strong labelling is found on the thick peritubular layer (arrows) and on few interstitial, presumably Leydig, cells (arrowheads). A milder staining is also present in the seminiferous epithelium, where several immunoreactive spermatogonia (asterisks) are observed. b Postnatal day 7. Peritubular (arrows) and Leydig cells are strongly labelled. An extremely mild staining can be detected in few spermatogonia (asterisks). c Postnatal day 7. Control section incubated with immunogen-preadsorbed anti-PDE5 antibody. Labelling is absent from any structure. d Postnatal day 14. Peritubular (arrows) and Leydig (arrowheads) cells are specifically labelled. No staining is found in the seminiferous epithelium. e Adult. Leydig cells are strongly stained. A specific staining can be detected on the extremely thin peritubular layer (arrow). Seminiferous epithelium is unstained
Concerning the peritubular tissue, since this layer is especially defined and thick at postnatal day 3, and undergoes a progressive thinning later, becoming hardly visible in the adult, the immunostaining appeared especially strong in the earlier developmental stages and became extremely faint in the adult testis (Fig. 2).
In sections incubated with the antibody against α-SM actin the positivity was present both in vascular smooth muscle and in the peritubular layer, while following desmin immunolocalisation only the latter tissue was specifically labelled (Fig. 3).
Rat testis. Postnatal day 14. Microwave treatment, standard ABC method. Haematoxylin counterstaining. Bar 20 μm. a PDE5 immunohistochemistry. Peritubular cells are specifically labelled. b Desmin immunohistochemistry. Peritubular cells are strongly labelled. A milder positivity is found in few interstitial, presumably Leydig, cells (arrowhead). c α-SM actin immunohistochemistry. Peritubular cells are specifically labelled
Purified preparations of peritubular myoid cells consisted of roundish cells with peripherally located nuclei. After α-SM actin immunolocalisation, cells were heavily labelled, the positivity characteristically forming a thick cytoplasmic ring (Fig. 4a–c). Virtually all the cells were also labelled by anti-PDE5 in their cytoplasm (Fig. 4d–f). Negative controls were unstained.
Peritubular myoid cells, purified from 16-day-old rats. Microwave treatment, standard ABC method, haematoxylin counterstaining. All the cells present a roundish shape and a peripherally located nucleus. Bar 10 μm. a–c α-SM actin immunocytochemistry. All the cells are heavily labelled. DAB staining is concentrated in the cytoplasm, in the form of a horseshoe-shaped structure; n nucleus. d–f PDE5 immunocytochemistry. Virtually all the cells are heavily immunoreactive. DAB staining is uniformly distributed in the cytoplasm; n nucleus
In 18.5-day-old embryos, PDE5 was immunodetected, besides that in contractile elements of the tunica albuginea, in many interstitial cells, which position inside the intertubular tissue corresponds to the desmin-positive myoid cells described by Palombi et al. (1992) (Fig. 5).
In summary, our results show that in rat testis throughout pre and postnatal development PDE5 is selectively expressed, besides that by the vascular myocytes, by the Leydig cells, both of the foetal and of the adult generation, and the myoid cells.
Discussion
In mammalian testis, cGMP signal transduction pathways, besides being present in vessel endothelium and myocytes (Middendorff et al. 1997b) and being involved in vascular relaxation and blood flow modulation, are also operating in several other cell types and cooperate to plenty other functions, based on autocrine or paracrine mechanisms.
In Leydig cells the ANP/cGMP pathway was proved to cooperatively interact with LH/cAMP in androgen production (Mukhopadhyay et al. 1986; Hipkin and Moger 1991); moreover, Leydig cells were found to contain cGMP and its binding protein cGMP-dependent protein kinase type I and to express the main components of the nitric oxide pathway (nitric oxide synthase and soluble guanylate cyclase), as well as guanylate cyclase B, the C type natriuretic peptide receptor (Davidoff et al. 1997; Middendorff et al. 1997a).
On the other hand, in Sertoli and peritubular cells, where iNOS is stage-specifically expressed (Bauche et al. 1998; O’Bryan et al. 2000) and cGMP, soluble guanylate cyclase, as well as endothelial and neuronal subtypes of NO synthase, were immunohistochemically detected (Middendorff et al. 1997b), the NO production and activity are presumably involved in relaxation of seminiferous tubules and in sperm transport modulation (Middendorff et al. 1997b; Bauche et al. 1998).
In Sertoli cells, where PDE5 expression was undetectable in our experiments, different PDEs are likely to contribute to the cGMP metabolism. Indeed, in these cells cGMP hydrolisis has been shown to occur in a calcium-calmodulin stimulated manner (Conti et al. 1982; Rossi et al. 1985; Conti et al. 1995), which identifies this activity as due to a PDE1 family member. Enzymes of this family, though active towards both cAMP and cGMP, display a substantially higher affinity to the latter nucleotide (Zhao et al. 1997).
Concerning Leydig and peritubular cells, the here reported expression of PDE5 by these cell types both in prepuberal and in adult rat testis is perfectly in agreement with the above-exposed data and gives additional strength to the idea that in mammalian testis cGMP-mediated processes influence not only the vessel dilatation, but also the testosterone synthesis by Leydig cells and the motility of spermatozoa, through the relaxation of peritubular lamina propria cells. In peritubular myoid cells, in particular, besides regulating contractility, which represents a key point in male fertility, cGMP modulation might affect the secretion of a number of substances, including extracellular matrix components (fibronectin, type I and IV collagens, proteoglycans) and growth factors (PModS, TGF beta, IGF-I, activin-A), some of which are known to affect the Sertoli cell function and to be involved in retinol processing (Maekawa et al. 1996).
Finally, the here demonstrated PDE5 expression by both mature and differentiating rat peritubular cells allows to suggest that in mammals the enzyme might play key roles in the acquisition and maintenance of the myoid cell contractile phenotype and more generally in the testis maturation. To this respect, the higher number of peritubular cells present in adult human testis compared to rodent one (Davidoff et al. 1990), together with the species-specific cytoskeletal organisations (Maekawa et al. 1996), should be considered in evaluating the possible effects of PDE5 on the seminiferous tubule contractility in humans.
On this basis, it seems necessary to investigate whether substances interfering with PDE5 activity, as the selective inhibitor sildenafil, currently employed in the treatment of erectile dysfunction, might also affect testicular physiology.
References
Adams JC (1992) Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 40:1457–1463
Bauche F, Stephan JP, Touzalin AM, Jegou B (1998) In vitro regulation of an inducible-type NO synthase in the rat seminiferous tubule cells. Biol Reprod 58:431–438
Boolell M, Gepi-Attee S, Gingell JC, Allen MJ (1996) Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br J Urol 78:257–261
Conti M, Toscano MV, Petrelli L, Geremia R, Stefanini M (1982) Regulation of follicle-stimulating hormone and dibutyryl adenosine 3′,5′-monophosphate of a phosphodiesterase isoenzyme of the Sertoli cell. Endocrinology 110:1189–1196
Conti M, Iona S, Cuomo M, Swinnen JV, Odeh J, Svoboda ME (1995) Characterization of a hormone-inducible, high affinity adenosine 3′-5′-cyclic monophosphate phosphodiesterase from the rat Sertoli cell. Biochemistry 34:7979–7987
Coquil JF, Brunelle G, Guedon J (1985) Occurrence of the methylisobutylxanthine-stimulated cyclic GMP binding protein in various rat tissues. Biochem Biophys Res Commun 127:226–231
Davidoff MS, Breucker H, Holstein AF, Seidl K (1990) Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res 262:253–261
Davidoff MS, Middendorff R, Mayer B, deVente J, Koesling D, Holstein AF (1997) Nitric oxide/cGMP pathway components in the Leydig cells of the human testis. Cell Tissue Res 287:161–170
Dorrington JH, Roller NF, Fritz IB (1975) Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol Cell Endocrinol 3:57–70
Filippini A, Tripiciano A, Palombi F, Teti A, Paniccia R, Stefanini M, Ziparo E (1993) Rat testicular myoid cells respond to endothelin: characterization of binding and signal transduction pathway. Endocrinology 133:1789–1796
Geremia R, d’Agostino A, Monesi V (1978) Biochemical evidence of haploid gene activity in spermatogenesis of the mouse. Exp Cell Res 111:23–30
Giordano D, De Stefano ME, Citro G, Modica A, Giorgi M (2001) Expression of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in mouse tissues and cell lines using an antibody against the enzyme amino-terminal domain. Biochim Biophys Acta 1539:16–27
Giordano D, Giorgi M, Tata AM, Modica A, Augusti-Tocco G (2004) Expression of PDE5 splice variants during ontogenesis of chick dorsal root ganglia. J Neurosci Res 78:815–823
Gravis CJ (1978) Inhibition of spermiation in the syrian hamster using dibutyryl cyclic AMP. Cell Tiss Res 192:241–248
Hamet P, Coquil JF (1978) Cyclic GMP binding and cyclic GMP phosphodiesterase in rat platelets. J Cyclic Nucleotide Res 4:281–290
Hipkin RW, Moger WH (1991) Interaction between cyclic nucleotide second messenger systems in murine Leydig cells. Mol Cell Endocrinol 82:251–257
Hollinger WA, Hwang F (1974) Effect of dibutyryl cyclic AMP on in vitro rat testis DNA, RNA and protein labelling. Endocrinology 94:444–449
Jeremy JY, Ballard SA, Naylor AM, Miller MA, Angelini GD (1997) Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol 79:958–963
Kotera J, Yanaka N, Fujishige K, Imai Y, Akatsuka H, Ishizuka T, Kawashima K, Omori K (1997) Expression of rat cGMP-binding cGMP-specific phosphodiesterase mRNA in Purkinje cell layers during postnatal neuronal development. Eur J Biochem 249:434–442
Kotera J, Fujishige K, Imai Y, Kawai E, Michibata H, Akatsuka H, Yanaka N, Omori K (1999) Genomic origin and transcriptional regulation of two variants of cGMP-binding cGMP-specific phosphodiesterases. Eur J Biochem 262:866–873
Kotera J, Fujishige K, Omori K (2000) Immunohistochemical localization of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in rat tissues. J Histochem Cytochem 48:685–693
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lincoln TM, Hall CL, Park CR, Corbin JD (1976) Guanosine 3′:5′-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci USA 73:2559–2563
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maekawa M, Kamimura K, Nagano T (1996) Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol 59:1–13
Means AR, Dedman JR, Tash JS, Tindall JS, Van Sickle M, Welsh MJ (1980) Regulation of testis sertoli cells by follicle-stimulating hormone. Ann Rev Physiol 42:59–70
Middendorff R, Muller D, Paust HJ, Holstein AF, Davidoff MS (1997a) New aspects of Leydig cell function. Adv Exp Med Biol 424:125–138
Middendorff R, Muller D, Wichers S, Holstein AF, Davidoff MS (1997b) Evidence for production and functional activity of nitric oxide in seminiferous tubules and blood vessels of the human testis. J Clin Endocrinol Metab 82:4154–4161
Mukhopadhyay AK, Schumacher M, Leidenberger FA (1986) Steroidogenic effect of atrial natriuretic factor in isolated mouse Leydig cells is mediated by cyclic GMP. Biochem J 239:463–467
O’Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP (2000) Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod 63:1285–1293
Palombi F, Di Carlo C (1988) Alkaline phosphatase is a marker for myoid cells in cultures of rat peritubular and tubular tissue. Biol Reprod 39:1101–1109
Palombi F, Farini D, Salanova M, de Grossi S, Stefanini M (1992) Development and cytodifferentiation of peritubular myoid cells in the rat testis. Anat Rec 233:32–40
Purvis K, Hansson V (1980) Hormonal regulation of spermatogenesis. Regulation of target cell response. Int J Androl Suppl 3:82–143
Ribalkin SD, Rybalkina IG, Shimizu-Albergina M, Tang X, Beavo JA (2003) PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J 23:469–478
Rossi P, Pezzotti R, Conti M, Geremia R (1985) Cyclic nucleotide phosphodiesterases in somatic and germ cells of mouse seminiferous tubules. J Reprod Fertil 74:317–327
Sanborn BM, Heindel JJ, Robison GA (1980) The role of cyclic nucleotides in reproductive processes. Ann Rev Physiol 42:37–57
Sanders DB, Kelley T, Larson D (2000) The role of nitric oxide synthase/nitric oxide in vascular smooth muscle control. Perfusion 15:97–104
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Tung PS, Fritz IB (1990) Characterization of rat testicular peritubular myoid cells in culture: alpha-smooth muscle isoactin is a specific differentiation marker. Biol Reprod 42:351–365
Virtanen I, Kallajoki M, Narvanen O, Paranko J, Thornell LE, Miettinen M, Lehto VP (1986) Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec 215:10–20
Yanaka N, Kotera J, Ohtsuka A, Akatsuka H, Imai Y, Michibata H, Fujishige K, Kawai E, Takebayashi S, Okumura K, Omori K (1998) Expression, structure and chromosomal localization of the human cGMP-binding cGMP-specific phosphodiesterase PDE5A gene. Eur J Biochem 255:391–399
Zhao AZ, Yan C, Sonnenburg WK, Beavo JA (1997) Recent advances in the study of Ca2+/CaM-activated phosphodiesterases: expression and physiological functions. Adv Second Messenger Phosphoprotein Res 31:237–251
Acknowledgements
We thank Prof. Fioretta Palombi and Dr. Francesca Romano (Department of Histology and General Embryology, University of Rome “La Sapienza”) for supplying purified myoid cells. Thanks are also due to Dr. Sandra Moreno (Department of Biology-LIME, University Roma Tre) for helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scipioni, A., Stefanini, S., Santone, R. et al. Immunohistochemical localisation of PDE5 in Leydig and myoid cells of prepuberal and adult rat testis. Histochem Cell Biol 124, 401–407 (2005). https://doi.org/10.1007/s00418-005-0057-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0057-1