Abstract
Antibodies specific for the chicken AE1 anion exchanger have been used to determine the cell-type specific pattern of expression of this electroneutral transporter in the chick chorioallantoic membrane (CAM) during embryonic development. Immunolocalisation analyses demonstrated that the AE1 anion exchanger accumulated in the basolateral membrane of a subset of cells in both the chorionic and allantoic epithelial layers. Double immunostaining indicated that the AE1-positive cells in the chorionic and allantoic epithelia were also positive for the carbonic anhydrase isoform, CAII, which serves as a marker for the villus cavity (VC) cells of the chorionic epithelium and the mitochondria-rich cells of the allantoic epithelium. Immunoelectron microscopy revealed that AE1 accumulated in extensive projections that extended from the lateral membrane of VC cells towards the adjacent capillary covering cells. These results represent the first demonstration of anion exchanger expression in the chick CAM, and they suggest a role for basolateral AE1 in bicarbonate reabsorption that is required in the embryo for maintaining acid-base balance during development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chick chorioallantoic membrane (CAM) is an extraembryonic, three-layered structure derived from distinct embryonic tissues. It consists of the ectodermal chorionic epithelium that is in direct contact with the shell membrane, the intermediate, highly vascularised mesoderm and the endodermal allantoic epithelium that lines the allantoic cavity. Differentiation of distinct cell types in both epithelia results in specialised ion-transporting tissues with proposed roles in several morphogenetic events. The chorionic epithelium is recognised as the site of Ca2+ transport from the eggshell into the embryo (Akins and Tuan 1993), and the allantoic epithelium is thought to be involved in ion and H2O reabsorption from the allantoic fluid (Stewart and Terepka 1969). In addition, the CAM is likely to allow for the maintenance of acid-base balance in the embryo during development (Narbaitz et al. 1995). Indeed, as development proceeds, acid production by the embryo increases and exceeds the limiting rate of CO2 diffusion through the shell and its membranes. This requires a corresponding increase in the concentration of plasma bicarbonate to prevent metabolic acidosis. Although the excretory system can also contribute, the eggshell is regarded as the main source of this extra “non-respiratory” bicarbonate (Dawes and Simkiss 1969; Crooks and Simkiss 1974). In spite of the physiological needs for bicarbonate, there are no data suggesting a role for the chorionic epithelium of the chicken CAM in reabsorbing bicarbonate generated from the eggshell.
Members of the anion exchanger (AE) gene family mediate the exchange of Cl− for HCO3 − in a wide variety of cell types. This electroneutral exchange process plays a role in the maintenance of intracellular pH and in cell volume regulation, and in the transepithelial transport of acid/base (Kopito 1990; Jennings 1992; Alper 1994). The best-characterised mammalian anion exchanger is the erythroid band 3 protein, which is encoded by the AE1 gene. Variant transcripts of the AE1 gene are expressed in chicken erythroid cells (Cox et al. 1995) as well as in type A-intercalated cells of human (Wagner et al. 1987; Kollert-Jons et al. 1993), mouse (Breton et al. 1995), rat (Alper et al. 1989) and chicken (Cox and Cox 1995) kidney.
In addition to AE1, the AE gene family contains at least two additional members, AE2 and AE3. AE2 is widely expressed in nonerythroid tissues including the stomach, kidney, liver, pancreas, salivary glands and male reproductive system (Jons et al. 1994; Brosius et al. 1995; Cox et al. 1996; Garcìa et al. 1998; Stuart-Tilley et al. 1998; Holappa et al. 1999; Jensen et al. 1999; Roussa et al. 1999; Castillo et al. 2000; Roussa 2001). AE3 is primarily expressed in the nervous system, cardiac muscle and gut (Kudrycki et al. 1990; Linn et al. 1992, 1995; Yannoukakos et al. 1994).
In our attempt to gain insight into the mechanisms through which bicarbonate transport through the chick CAM occurs, we carried out immunolocalisation studies using antibodies that recognise chicken AE1 anion exchangers. We found that the expression of AE1 in the chicken CAM is developmentally regulated. In addition, this electroneutral transporter was restricted to the basolateral membrane in the villus cavity (VC) cells of the chorionic epithelium and the mitochondria-rich cells of the allantoic epithelium. This pattern of expression for AE1 is consistent with a role for this transporter in the bicarbonate reabsorption that is required in the embryo for maintaining acid-base balance during development.
Materials and methods
Fertilised eggs from Cobb chickens, kindly provided by a local commercial source (Garbini, Castelplanio, AN), were incubated in a humidified incubator at 99.5°F. Embryos (2–4) were killed daily from day 8 of incubation (E8) to hatching (E20). The CAM was removed together with the relevant shell membrane. The proventriculus was removed from two adult chickens and used for control studies.
Antibodies
Two rabbit polyclonal antibodies specific for chicken AE1 were used in the study. The first antibody was directed against a peptide corresponding to amino acids 185–203 of the chicken erythroid AE1-1b anion exchanger (Cox and Cox 1995). The second antibody was directed against a bacterial fusion protein containing amino acids 1–359 of the chicken AE1-4 anion exchanger (Adair-Kirk et al. 1999).
Monoclonal antibody 2A2-1 was used to detect chicken carbonic anhydrase (CAII; Linser et al. 1984). The 2A2-1 monoclonal antibody was kindly provided by Dr. P.J. Linser (Whitney Laboratory, Florida University, St. Augustine, FL).
Immunohistochemistry
Immediately after removal, pieces of CAM were fixed in Bouin’s solution (30 ml picric acid, 10 ml formaldehyde, 2 ml acetic acid) for 3 h at room temperature. After dehydration in graded ethanols, the samples were cleared in xylene and embedded in paraffin at 56–58°C. Sections (5 μm thick) were mounted on gelatin-coated slides, rehydrated and processed for immunohistochemical staining using the Vectastain ABC kit reagents (Vector Laboratories, Burlingame, CA). After inactivation of the endogenous peroxidase (0.3% H202 in methanol for 30 min) and blocking of endogenous avidin-binding activity (avidin-biotin blocking kit; Vector Laboratories), sections were incubated for 20 min in normal goat serum diluted 1:5 with 1% bovine serum albumin (BSA; Sigma, St. Louis, MO) in 0.05 M phosphate-buffered saline (PBS), pH 7.6. Incubation of sections with the AE1-specific antibodies was performed at dilutions from 1:1,000 to 1:4,000 overnight at room temperature, in a humid chamber. After washing, sections were incubated with biotinylated goat anti-rabbit IgG for 45 min, followed by washing and treatment with the avidin-biotin-peroxidase complex for 45 min. The immunoreactive polypeptides in all sections were visualised by incubation, for 8 min, with VIP substrate (Vector Laboratories). Some of the immunostained sections were counterstained with methylene blue. Finally, sections were dehydrated and mounted in Eukitt.
Immunohistochemical controls were carried out on CAM sections by incubation with preimmune rabbit serum or with PBS plus 1% BSA, in place of the primary antibody. The specificity of the antisera was also checked by using, as a negative control, rehydrated sections from fowl proventriculus fixed in Bouin’s solution and embedded in paraffin. Sections from fowl proventriculus were treated with anti-AE1 antisera, as described above.
A double-labelling procedure allowed simultaneous visualisation of AE1 and the cytosolic isoform of carbonic anhydrase (CAII). In this procedure, sections were first incubated with anti-AE1 antibodies (1:1,000), followed by the routine treatments up to development of the peroxidase marker with VIP, which results in a purple reaction product. Sections were then washed in PBS containing 1% BSA and 0.01% Triton X-100 for 4 h at 4°C and incubated with the anti-chicken CAII monoclonal antibody (1:100) overnight at room temperature. Biotinylated anti-mouse IgG was then applied for 45 min, followed by washing in PBS and incubation with avidin-biotin-peroxidase complex (Sigma) for 45 min. The CAII-immunoreactive sites were visualised with 3,3′-diaminobenzidine (DAB substrate; Vector Laboratories) as a yellow-brown precipitate.
In other sections, double immunolabelling was performed by sequential incubation with the antiserum raised against the bacterial fusion protein containing amino acids 1–359 of chicken AE1-4 and with the 2A2-1 monoclonal antibody, followed by FITC-conjugated goat anti-rabbit IgG and TRITC-conjugated sheep anti-mouse IgG (Sigma), respectively, diluted 1:50 in PBS (1 h). Sections were rinsed in PBS, mounted in glycerol/PBS (1:1) mixture and subjected to analysis by the BioRad MRC-600 confocal laser scanning microscope (BioRad, Hertfordshire, UK). The image acquisition was carried out by BioRad COMOS software.
Controls were performed by replacing the primary antibody at each step with PBS plus 1% BSA or preimmune rabbit serum.
Immunogold labelling for electron microscopy
The CAM was cut in small pieces and immersion fixed in 3% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.3, for 4 h at 4°C. Tissues were then washed and stored overnight in 0.1 M phosphate buffer containing 8% saccharose. After dehydration in graded ethanols, tissues were embedded in Unicryl resin (British Bio Cell International, Cardiff, UK) according to the manufacturer’s instructions. Ultrathin sections (50–60 nm) were cut using an ultramicrotome (Zeiss Ultrotome V) and mounted on 400-mesh uncoated gold grids at room temperature. After rinsing in 0.05 M TRIS-buffered saline (TBS), pH 7.6, the sections were treated with normal goat serum (1:10) in 1% BSA in TBS for 30 min and then incubated with anti-AE1 antibodies, diluted 1:200 with TBS containing 1% BSA, overnight at room temperature in a humid chamber. The following steps were then performed: washing with TBS for 20 min, treatment with 1% BSA in TBS for 30 min and incubation with Auroprobe EM goat anti-rabbit G-10 (10-nm gold-labelled IgG; Amersham Life Science, Aylesbury, UK) at a dilution of 1:40 in TBS containing 0.05% Tween 20, for 90 min. The sections were then washed several times with TBS and distilled water and, finally, they were stained with uranyl acetate and lead citrate. The specimens were examined with a Philips 201C transmission electron microscope.
Control sections were incubated either with 1% BSA in TBS or preimmune serum instead of the primary antibody.
Western blotting analysis
Lysates were prepared by resuspending chicken erythroid cells and CAM membranes from 17-day-old embryos in SDS lysis buffer. The samples were sonicated and microcentrifuged for 5 min. The pellet was discarded and the soluble fraction was electrophoresed on a 6% SDS polyacrylamide gel and transferred to nitrocellulose. The nitrocellulose filter was incubated with the rabbit antibody specific for amino acids 185–203 of the chicken erythroid AE1-1b anion exchanger, or with the rabbit antibody directed against a bacterial fusion protein containing amino acids 1–359 of the chicken AE1-4 anion exchanger. In each instance, the filter was washed and incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase. Immunoreactive polypeptides were detected by enhanced chemiluminescence. Control blotting experiments using preimmune serum did not detect any immunoreactive species in lysates prepared from erythroid cells or CAM membranes (data not shown).
Results
The mature CAM of the chick embryo is characterised by distinct cell types within its epithelia (Fig. 1). The chorionic epithelium is composed of VC cells and capillary covering (CC) cells, while the allantoic epithelium contains basal cells, granule cells and mitochondria-rich cells. Different morphological features and specific biochemical markers of the CAM cell types can first be detected in embryos around E12 (Coleman and Terepka 1972; Gabrielli et al. 2001, 2003).
A schematic representation of the chicken chorioallantoic membrane (CAM) which forms by fusion of chorion and allantois beginning from day 4 of development (A). At maturity (B), the CAM consists of the chorionic epithelium (ce) in contact with the shell membrane (sm), the middle layer (ml) derived from the fusion of mesodermal layers of chorion and allantois, and the allantoic epithelium (ae) which faces the allantoic cavity (ac). Numerous blood vessels (bv) are present in the mesodermal layer or form the intrachorionic blood sinus (bs). 1 Capillary covering (CC) cells, 2 villus cavity (VC) cells, 3 granule cells, 4 mitochondria-rich cells, 5 basal cells
Developmentally regulated and cell type-specific expression of AE1 in the chick CAM
At the earliest developmental stages examined, no AE1 anion exchanger-specific immunoreactivity was detected in the differentiating CAM, except for the AE1 staining observed in the erythrocytes found in the vessels of the mesodermal layer, and in the blood sinus developing towards the shell membrane (Fig. 2A). In the mature CAM, AE1 staining was observed in cells in both the chorionic and allantoic epithelial layers (Figs. 2B, C, 3). However, the intensity of the AE1 anion exchanger-specific staining in the cells of the CAM varied as a function of development. AE1 staining was first detectable in the chorionic epithelium around embryonic stage E12 (Fig. 2B). The staining intensity peaked around E15–16 (Fig. 2C) and it was maintained at this level during subsequent stages of development except for a slight reduction at hatching, which probably was the result of tissue regression (data not shown). Two different AE1 antisera were effective in producing a marked immunostaining. The highest sensitivity was obtained with the antiserum directed against amino acids 1–359 of chicken AE1-4, which was used in subsequent analyses. In the chorionic epithelium, AE1 staining with this antiserum was restricted to the basolateral membrane of positive cells (Fig. 3A). The staining did not extend to the apical membrane which protrudes into the depression between the blood sinus vessels. These apical protrusions are characteristic of the VC cells (Fig. 1). In addition, some of the AE1-negative cells in the chorion possess the distinctive morphological features of CC cells (Gabrielli et al. 2001, 2003). The apical membrane in these cells characteristically elongates towards the shell membrane (Figs. 1, 3A asterisk). In the allantoic epithelium (Fig. 3A inset), AE1 immunostaining was localised to the basolateral membrane of cells whose size and location in the epithelium are typical of mitochondria-rich cells (Gabrielli et al. 2001).
Developmental expression of AE1 anion exchanger in the chicken CAM by immunohistochemical analysis using antibodies raised against a bacterial fusion protein containing amino acids 1–359 of chicken AE1-4. A At E8, there is no AE1 staining in the CAM epithelia. Only erythrocytes of the blood vessels (bv), present either in the middle layer or close to the chorionic epithelium, are stained. B In 12-day-old embryos, AE1 immunoreactivity is detected in some cells of the chorionic epithelium, facing the shell membrane (sm), as well as in the erythrocytes of mesodermal blood vessels (bv) and intrachorionic blood sinus (bs). The allantoic epithelium (ae) is unstained. C Sections of the CAM at E15 display intense labelling of AE1 in cells of the chorionic epithelium, in contact with the shell membrane (sm). An AE1-positive cell can be observed in the allantoic epithelium (asterisk). Erythrocytes of blood vessels (bv) and sinus (bs) are strongly stained. Magnification ×400 in A; ×800 in B, C
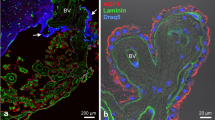
Chicken CAM at E15. A High magnification of the AE1-staining pattern observed in the chorionic epithelium. Strong AE1 labelling is present in the basolateral membrane of cells that possess the structural features of VC cells. An unreactive CC cell is marked with an asterisk. Inset in A illustrates AE1 labelling in the basolateral membrane of a cell of the allantoic epithelium. B Double labelling of AE1 and the carbonic anhydrase isoform, CAII, in the chorionic epithelium and allantoic epithelium. AE1 immunoreactivity (purple) is restricted to the basolateral membrane of cells that also express CAII (yellow-brown). VC Villus cavity cells, sm shell membrane, bs blood sinus, bv mesodermal blood vessels. Magnification ×1,400 in A, B; ×1,150 in insets
Control. No immunoreactivity is observed in the epithelial layers or erythrocytes of a stage E15 CAM following incubation with the AE1 preimmune serum. sm Shell membrane, bv blood vessel. Magnification ×450
Control. Immunolocalisation of AE1 in the fowl proventriculus using antibodies raised against a bacterial fusion protein containing amino acids 1–359 of chicken AE1-4. AE1 is detected in the erythrocytes of the blood capillaries, but it is not observed in the oxyntic-peptic cells of the gastric glands. The peroxidase marker was developed with 3,3′-diaminobenzidine and nickel enhancement (DAB kit; Vector Laboratories). Section was counterstained with methylene blue. Magnification ×450
Several controls were performed to verify the specificity of the staining pattern observed for AE1 in the CAM. First, tissue sections incubated with the AE1 preimmune serum were devoid of staining (Fig. 4). Secondly, tissue sections from the stomach, which expresses the AE2 anion exchanger at high levels (Cox et al. 1996), were also negative for staining with the AE1 antiserum (Fig. 5). These results suggest that the staining observed in the CAM is likely due to the presence of AE1 in this tissue rather than the ubiquitously expressed AE2 anion exchanger.
Double immunolabelling allowed the simultaneous visualisation of the CAII isoform of carbonic anhydrase and the AE1 anion exchanger within cells. This analysis indicated that cells in the chorionic and in the allantoic epithelium that express AE1 also express CAII (Fig. 3B). An identical result was obtained when fluorescently labelled secondary antibodies and confocal laser scanning microscopy were used to localise AE1 and CAII in the cells of the chorionic epithelium. As shown in Fig. 6, AE1 accumulated in the basolateral membrane of VC cells, while CAII exhibited a diffuse cytoplasmic staining pattern. Colocalisation of AE1 and CAII was evident also in erythrocytes of the chorionic blood sinus and mesodermal vessels.
A chicken CAM at E17 was double stained with antibodies against AE1 (A) and CAII (B). Immunoreactive polypeptides were visualised by confocal microscopy. Merged confocal image of the chorionic epithelium (C) shows that the cells which express AE1 in their basolateral membranes also express cytosolic CAII. VC Villus cavity cells, n nucleus, sm shell membrane, e erythrocytes, bs blood sinus. Magnification ×900
Immunoelectron microscopy revealed that AE1 localises to extensive projections in the lateral membrane of chorionic VC cells
Immunoelectron microscopy was used to determine the precise intracellular distribution of AE1 within the VC cells of the chorion. This ultrastructural analysis verified that unique morphological features of the different cell types in the chorionic epithelium could be distinguished first in 12-day-old embryos (Figs. 7A, 8A). The CC cells showed the typical elongation of their supranuclear cytoplasm which extends apically towards the shell membrane and, laterally to form cytoplasmic processes underlying the blood sinus vessels (Fig. 7B). Intermediate-type filaments were present in the CC cell cytoplasm. These cytoskeletal filaments were organised in large bundles mostly located in the supranuclear cell portion, around the nucleus and in association with the numerous junctional complexes between adjacent CC cells. In addition, these filaments were present in the well-developed protrusions that extended from the lateral membranes of CC cells towards the adjacent VC cells (Figs. 7A, B, 10, 11). In Fig. 8B, the distinctive features of the apical portion of a VC cell can be seen. The apical cell membrane is folded in thin microvilli, which protrude into a large depression beneath the inner shell membrane.
The chick chorionic epithelium at E12. Immunogold labelling of AE1 with antibodies raised against a bacterial fusion protein containing amino acids 1–359 of chicken AE1-4. A Overview of a CC cell which shows no AE1 immunoreactivity. B Higher magnification of the CC cell apical portion shows no AE1-specific labelling within the cell and along the cell membranes. Gold particles are associated with erythrocytes of the chorionic plexus (arrows). Large bundles of cytoskeletal elements are present in the CC cell supranuclear cytoplasm (arrowheads). Magnification ×7,500 in A; ×10,000 in B
Chick CAM at E12. A Low magnification of a VC cell of the chorionic epithelium embedded in Unicryl resin and immunolabelled for AE1. B A portion of the apical membrane of the VC cell shown in A. No labelling is present at the peculiar evaginations of the apical cell membrane. Gold particles are associated exclusively with erythrocytes present in the chorionic blood sinus (arrow). C Higher magnification view of the basal area of the VC cell shown in A. A few gold particles are associated with the pronounced infoldings of the VC cell lateral membranes facing an unlabelled adjacent CC cell. Arrowhead indicates a junctional complex between CC cells. Magnification ×7,000 in A; ×12,000 in B; ×28,500 in C
Immunogold labelling of AE1 in the chick CAM at E18. Negative control stained with preimmune serum shows no gold particles in the VC cell (on the left) or CC cell (on the right). Magnification ×19,000
Weak AE1 immunoreactivity was observed in the chorionic epithelium on E12, as indicated by the occurrence of few gold particles that were restricted to the basolateral membranes of some VC cells (Fig. 8C). The basolateral membranes of the adjacent CC cell (Fig. 8C) as well as the apical membranes of both CC cells and VC cells (Figs. 7B, 8B) were mostly free from gold labelling. Control preparations that omitted the primary antibody or used preimmune serum were devoid of gold particles (Fig. 9).
At later developmental stages AE1 expression increased. In 18-day-old embryos (Figs. 10, 11) the VC cells, whose apical membranes were still unreactive (Figs. 10, 11 insets), were strongly labelled in their basolateral membranes after incubation with either the antibody directed against a bacterial fusion protein containing amino acids 1–359 of the chicken AE1-4 (Fig. 10) or the antibody directed against amino acids 185–203 of AE1-1b (Fig. 11). The observed gold particles primarily decorated long evaginations that extended from the lateral membrane of VC cells towards the adjacent CC cells, which were unlabeled.
Immunogold labelling of AE1 in the chick chorionic epithelium of an 18-day-old embryo. The rabbit antibody directed against a bacterial fusion protein containing amino acids 1–359 of the chicken AE1-4 anion exchanger was used. The detailed view of a basal region, belonging to the VC cell in the inset, shows strong labelling associated with the basolateral cell membranes which protrude into the intercellular tissue space. The lateral membranes of the adjacent CC cell are devoid of significant gold labelling. The cytoplasm at these sites often contains bundles of filaments (arrow). Magnification ×42,000; ×12,000 in inset
Immunogold labelling with the AE1-specific peptide antibody in the chick CAM at E18. High magnification of a basal area of a VC cell (in the inset) confirms the localisation of AE1 in the basolateral cell membranes facing an unlabeled CC cell. Magnification ×42,000; ×7,300 in inset
Immunoblotting
Immunoblotting analysis was used to compare the AE1 species in the CAM from 17-day-old embryos with the AE1 variants that have been previously characterised in chicken erythroid cells (Cox et al. 1995). Lysates prepared from erythroid cells and purified CAM membranes were probed with rabbit antibodies raised against a bacterial fusion protein containing amino acids 1–359 of chicken AE1-4. This analysis revealed that multiple AE1 species with a molecular mass ranging from 93 to 117 kDa accumulated in the CAM (Fig. 12A). A few of the larger of these immunoreactive species were distinct in size from the variants detected in erythroid cells indicating that the profile of bands observed in the CAM was not simply the result of erythroid contamination of the CAM membranes. Similar blotting analyses with the antibody directed against amino acids 185–203 of AE1-1b yielded identical results (Fig. 12B). The fact that the sequence between amino acids 185–203 of AE1-1b is not conserved in the AE2 (Cox et al. 1996) and AE3 (Kudrycki et al. 1990) anion exchangers strongly suggests that the multiple species observed in the CAM are all derived from the AE1 gene. Whether the multiple species observed in the CAM are the result of differential glycosylation of a single AE1 variant or are derived from multiple variant AE1 transcripts awaits further analysis.
Western blot analysis of erythrocyte (RBC) and CAM extracts from 17-day-old chicken embryos. Blotting was carried out using rabbit antibodies raised against a bacterial fusion protein containing amino acids 1–359 of chicken AE1-4 (A) or with rabbit antibodies against a peptide corresponding to amino acids 185–203 of the chicken erythroid AE1-1b (B)
Discussion
Other investigators have demonstrated that AE1 anion exchangers are expressed in erythroid cells and in type A-intercalated cells of the kidney in both mammalian and avian species (Drenckhahn et al. 1985; Alper et al. 1989; Kudrycki and Shull 1993; Cox and Cox 1995). The data presented here illustrate that AE1 anion exchangers are also expressed in specific cell types in both the chorionic and the allantoic epithelium of the chicken CAM.
The onset of AE1 expression in the cells of the chorionic epithelium is coincident with the differentiation of distinct cell types characteristic of the mature CAM on day E12 (Coleman and Terepka 1972). The observed patterns of expression of AE1 on E15 in both the chorionic and allantoic epithelia persisted until hatching. In both epithelia, the AE1 immunostaining was restricted to the basolateral membrane of a subset of cells that also expressed the cytosolic isoform of carbonic anhydrase, CAII, a marker for VC cells and mitochondria-rich cells in the chorionic and allantoic epithelium, respectively (Gabrielli et al. 2001). Immunogold labelling confirmed and further elucidated the pattern of intracellular localisation of AE1 in the chorionic epithelium. These analyses revealed that this electroneutral transporter was restricted to convoluted membranes at the basolateral surface of VC cells.
The coexpression of AE1 and carbonic anhydrase in chorionic VC cells suggests a role for AE1 in the maintenance of pH equilibrium in this cell type that has been proposed to secrete H+ apically via a vacuolar-type H+-ATPase (Narbaitz et al. 1995). The regional acidification resulting from H+ secretion would promote solubilisation of the shell mineral calcite generating calcium that would be available for uptake. This pattern of localisation of membrane transporters in VC cells would be identical to that observed for type A-intercalated cells of the kidney collecting ducts where basolateral AE1 anion exchangers and apical proton pumps function co-ordinately in the presence of intracellular carbonic anhydrase (Schwartz et al. 1985; Alper et al. 1989; Brown and Breton 1996).
Based on the functional requirements of the developing embryo as well as the restricted localisation of AE1 in the chorionic epithelium, a highly specialised role may be proposed for VC cells. In addition to contributing to local acidification through apical proton pumps, these cells, via their basolateral AE1 anion exchangers, could contribute to the maintenance of acid-base balance during development by reabsorbing HCO3 − ions into the embryo. Besides the intracellular HCO3 − derived from the activity of the cytosolic carbonic anhydrase, the main source of this HCO3 − supply for the embryo might be the HCO3 − ions released together with calcium ions from the eggshell. Reabsorption of these ions through the chorionic VC cells might explain the gradual increase in plasma bicarbonate concentration during development (Dawes and Simkiss 1971). This proposed reabsorption of bicarbonate by VC cells may be mediated by apical Na+/HCO3 − cotransporters, as already established in several tissues (Romero et al. 1998; Choi et al. 1999; Thévenod et al. 1999; Giffard et al. 2000; Roussa 2001; Park et al. 2002). Alternatively, it is possible that CO2 diffuses across the apical membrane into the VC cells, is converted to HCO3 − by the cytosolic CAII and then leaves the basolateral pole via AE1 anion exchangers. A partial reabsorption of bicarbonate can also be achieved by the allantoic mitochondria-rich cells which are enriched in both apical H+-ATPase and cytosolic CAII (Narbaitz et al. 1995; Gabrielli et al. 2001). The presence of AE1 anion exchangers in the basolateral membranes of these cells provides the complete machinery necessary for proton secretion coupled to bicarbonate reabsorption. This machinery likely contributes to the progressive acidification of the allantoic fluid that has been reported to occur during development (Freeman and Vince 1974). Our results, which show no AE1 immunoreactivity at the apical membrane of mitochondria-rich cells, cannot confirm the suggestion that, similar to the type B-intercalated cells of the kidney collecting ducts and reptilian bladders, the allantois would contain a second type of mitochondria-rich cells which secrete H+ through the base and HCO3 − through the apex (Narbaitz et al. 1995).
In conclusion, our demonstration that AE1 anion exchangers are expressed in specific cell types in the CAM provides further insight into the functional role of these cells during embryonic development. In particular, the finding that basolateral AE1 anion exchangers colocalise with cytosolic carbonic anhydrase in VC cells and mitochondria-rich cells strongly suggests mechanisms of bicarbonate reabsorption at these sites, in order to prevent metabolic acidosis in the developing embryo. Other investigators have shown that CAII associates with the carboxyl terminus of AE1 anion exchangers (Vince and Reithmeier 1998, 2000). Studies on the functional consequences of this interaction supported the idea that the complex of AE1 and CAII forms a “bicarbonate transport metabolon” which would potentiate anion transport activity by minimising the distances of substrate diffusion between CAII and AE1 (Reithmeier 2001; Sterling et al. 2001). Our double immunofluorescence results, showing erythrocyte CAII closely restricted to the cell membranes where AE1 localises, are in line with the metabolon hypothesis. The coexpression of AE1 and CAII in the VC cells and mitochondria-rich cells of the CAM suggests that a metabolon may exist in these cells to maximise bicarbonate transport.
References
Adair-Kirk TL, Cox KH, Cox JV (1999) Intracellular trafficking of variant chicken kidney AE1 anion exchangers: role of alternative NH2 termini in polarized sorting and Golgi recycling. J Cell Biol 147:1237–1248
Akins RE, Tuan RS (1993) Transepithelial calcium transport in the chick chorioallantoic membrane. I. Isolation and characterization of chorionic ectoderm cells. J Cell Sci 105:369–379
Alper SL (1994) The band 3-related AE anion exchanger gene family. Cell Physiol Biochem 4:265–281
Alper SL, Natale J, Gluck S, Lodish HF, Brown D (1989) Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A 86:5429–5433
Breton S, Alper SL, Gluck SL, Sly WS, Barker JE, Brown D (1995) Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR 2 null) mice. Am J Physiol 269:F761–F774
Brosius FC III, Nguyen K, Stuart-Tilley AK, Haller C, Briggs JP, Alper SL (1995) Regional and segmental localization of AE2 anion exchanger mRNA and protein in rat kidney. Am Phys Soc 269:F461–F468
Brown D, Breton S (1996) Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199:2345–2358
Castillo JE, Martinez-Anso E, Malumbres R, De Alava E, Garcìa C, Medina JF, Prieto J (2000) In situ localization of anion exchanger-2 in the human kidney. Cell Tissue Res 299:281–287
Choi I, Romero MF, Khandoudi N, Bril A, Boron WF (1999) Cloning and characterization of a human electrogenic Na+-HCO3 − cotransporter isoform (hhNBC). Am J Physiol 276:C576–C584
Coleman JR, Terepka AR (1972) Fine structural changes associated with the onset of calcium, sodium and water transport by the chicken chorioallantoic membrane. J Membr Biol 7:111–127
Cox KH, Cox JV (1995) Variant chicken AE1 anion exchangers possess divergent NH2-terminal cytoplasm domains. Am J Physiol 268:F503–F513
Cox KH, Adair-Kirk TL, Cox JV (1995) Four variant chicken erythroid AE1 anion exchangers. J Biol Chem 270:19752–19760
Cox KH, Adair-Kirk TL, Cox JV (1996) Variant AE2 anion exchanger transcripts accumulate in multiple cell types in the chicken gastric epithelium. J Biol Chem 271:8895–8902
Crooks RJ, Simkiss K (1974) Respiratory acidosis and eggshell reabsorption by the chick embryo. J Exp Biol 61:197–202
Dawes CM, Simkiss K (1969) The acid-base status of the blood of the developing chick embryo. J Exp Biol 50:79–86
Dawes CM, Simkiss K (1971) The effects of respiratory acidosis in the chick embryo. J Exp Biol 55:77–84
Drenckhahn D, Schluter K, Allen DP, Bennett V (1985) Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science 230:1287–1289
Freeman BM, Vince MA (1974) Development of the avian embryo. A behavioural and physiological study. Chapman and Hall, London
Gabrielli MG, Materazzi G, Cox JV, Menghi G (2001) Specialised cell types in the chorioallantoic membrane express carbonic anhydrase during chick embryogenesis. J Anat 198:229–238
Gabrielli MG, Materazzi G, Bondi AM, Menghi G (2003) Developmental expression of glycocomponents in the chick chorioallantoic membrane. Anat Embryol 207:63–72
Garcìa C, Montuenga LM, Medina JF, Prieto J (1998) In situ detection of AE2 anion exchanger mRNA in the human liver. Cell Tissue Res 291:481–488
Giffard RG, Papadopoulos MC, van Hooft JA, Xu LJ, Giuffrida R, Monyer H (2000) The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J Neurosci 20:1001–1008
Holappa K, Mustonen M, Parvinen M, Vihko P, Rajaniemi H, Kellokumpu S (1999) Primary structure of a sperm cell anion exchanger and its messenger ribonucleic acid expression during spermatogenesis. Biol Reprod 61:981–986
Jennings ML (1992) Anion transport protein. In: Seldin DW, Giebischs G (eds) The kidney physiology and pathophysiology. Raven, New York, pp 503–535
Jensen LJ, Stuart-Tilley AK, Peters LL, Lux SE, Alper SL, Breton S (1999) Immunolocalization of AE2 anion exchanger in rat and mouse epididymis. Biol Reprod 61:973–980
Jons T, Warrings B, Jons A, Drenckhahn D (1994) Basolateral localization of anion exchanger 2 (AE2) and actin in acid-secreting (parietal) cells of the human stomach. Histochemistry 102:255–263
Kollert-Jons A, Wagner S, Hubner S, Appelhans H, Drenckhahn D (1993) Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol 265:F813–F821
Kopito RR (1990) Molecular biology of the anion exchanger gene family. Int Rev Cytol 123:177–199
Kudrycki KE, Shull GE (1993) Rat kidney band 3 chloride/bicarbonate exchanger mRNA is transcribed from an alternative promoter. Am J Physiol 264:F540–F547
Kudrycki KE, Newman PR, Shull GE (1990) cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl−/HCO3 − exchanger. J Biol Chem 265:462–471
Linn SC, Kudrycki KE, Shull GE (1992) The predicted translation product of the cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl−/HCO3 − exchanger. J Biol Chem 267:7927–7935
Linn SC, Askew G, Menon AG, Shull GE (1995) Conservation of an AE3 Cl−/HCO3 − exchanger cardiac-specific exon and promoter region and AE3 mRNA expression patterns in murine and human hearts. Circ Res 76:584–591
Linser P, Perkins MS, Fitch FW, Moscona AA (1984) Comparative characterization of monoclonal antibodies to carbonic anhydrase. Biochem Biophys Res Commun 125:690–697
Narbaitz R, Bastani B, Galvin NJ, Kapal VK, Levine DZ (1995) Ultrastructural and immunocytochemical evidence for the presence of polarized plasma membrane H+-ATPase in two specialised cell types in the chick chorioallantoic membrane. J Anat 186:245–252
Park K, Hurley PT, Roussa E, Cooper GJ, Smith CP, Thévenod F, Steward MC, Case RM (2002) Expression of a sodium bicarbonate cotransporter in human parotid salivary glands. Arch Oral Biol 47:1–9
Reithmeier RAF (2001) A membrane metabolon linking carbonic anhydrase with chloride/bicarbonate anion exchangers. Blood Cells Mol Dis 27:85–89
Romero MF, Fong PY, Berger UV, Hediger MA, Boron WF (1998) Cloning and functional expression of rNBC, an electrogenic Na+-HCO3 − cotransporter from rat kidney. Am J Physiol 274:F425–F432
Roussa E (2001) H+ and HCO3 − transporters in human salivary ducts. An immunohistochemical study. Histochem J 33:77–84
Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thévenod F (1999) Immunolocalization of anion exchanger AE2 and Na+-HCO3 − cotransporter in rat parotid and submandibular glands. Am J Physiol 277:G1288–G1296
Schwartz GJ, Barasch J, Al-Awqati Q (1985) Plasticity of functional epithelial polarity. Nature 318:368–371
Sterling D, Reithmeier RA, Casey JR (2001) A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276:47886–47894
Stewart ME, Terepka AR (1969) Transport functions of the chick chorio-allantoic membrane. 1. Normal histology and evidence for active electrolyte transport from the allantoic fluid, in vivo. Exp Cell Res 58:93–106
Stuart-Tilley AK, Shmukler BE, Brown D, Alper SL (1998) Immunolocalization and tissue-specific splicing of AE2 anion exchanger in mouse kidney. J Am Soc Nephrol 9:946–959
Thévenod F, Roussa E, Schmitt BM, Romero MF (1999) Cloning and immunolocalization of a rat pancreatic Na+ bicarbonate cotransporter. Biochem Biophys Res Commun 264:291–298
Vince JW, Reithmeier RAF (1998) Carbonic anhydrase II binds to carboxyl terminus of human band 3, the erythrocyte Cl−/HCO3 − exchanger. J Biol Chem 273:28430–28437
Vince JW, Reithmeier RAF (2000) Identification of the carbonic anhydrase II binding site in the Cl−/HCO3 − anion exchanger AE1. Biochemistry 39:5527–5533
Wagner SR, Vogel R, Lietzke R, Koob R, Drenckhahn D (1987) Immunochemical characterization of the human band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol 253:F213–F222
Yannoukakos D, Stuart-Tilley AK, Fernandez HA, Fey P, Duyk G, Alper SL (1994) Molecular cloning, expression and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res 75:603–614
Acknowledgements
We are grateful to Dr. P. J. Linser for the monoclonal antibody against chicken carbonic anhydrase and to Garbini S.p.A. for the fertilised eggs. The skilful technical assistance of Stefano Riccioni and Simonetta Cammertoni is gratefully acknowledged. This study was supported by grants from the University of Camerino.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabrielli, M.G., Cox, J.V., Materazzi, G. et al. Cell type-specific and developmentally regulated expression of the AE1 anion exchanger in the chicken chorioallantoic membrane. Histochem Cell Biol 121, 189–199 (2004). https://doi.org/10.1007/s00418-004-0627-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-004-0627-7