Abstract
Limited information exists about the putative role and expression in human skeletal muscle cells of the 88-kDa integral membrane protein fatty acid translocase (FAT), highly homologous to the human leucocyte differentiation factor CD36. Therefore, we investigated in healthy male individuals the muscle (m. vastus lateralis) fibre type specific expression and subcellular localisation of FAT/CD36. For this purpose four different monoclonal antibodies raised against human and mouse FAT/CD36 were used. Acetone or methanol/acetone fixation were tested. Serial cryosections were cut at −20°C, thaw-mounted on uncoated glass slides and air-dried before processing indirect immunofluorescence assays. Images were examined in a Nikon ER800 microscope, digitally captured, processed and analysed by LUCIA laboratory software. Three antibodies showed that FAT/CD36 was: (1) most abundantly expressed in capillary endothelium, (2) colocalised with caveolin-3, which indicates that FAT/CD36 is in the sarcolemma, or its close vicinity, and (3) abundantly expressed in (or in the close vicinity) of the sarcolemma and intracellular structures of type-1 muscle fibres, and much less abundantly in the sarcolemma of type-2 muscle fibres. One of the antibodies raised against mouse CD36 also detected myosin heavy chain 1, which makes it unsuitable in skeletal muscle research. The fixation (acetone or methanol/acetone) was found to be highly important for the result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From studies in rodents, there is firm evidence that membrane-associated proteins are involved in the cellular uptake of long chain fatty acids (LCFAs). Five different membrane-associated proteins have been identified as potential FA receptors and/or transporters (Abumrad et al. 1993; Fujii et al. 1987; Schaffer and Lodish 1994; Schwieterman et al. 1988; Sorrentino et al. 1988; Stremmel 1988; Trigatti et al. 1991). Of these, a 88-kDa integral membrane protein gave evidence for FA transport across the plasma membrane (Abumrad et al. 1993). This putative membrane fatty acid translocase (FAT) appears to be highly homologous (85%) to the human leukocyte differentiation antigen CD36 (glycoprotein IV; Abumrad et al. 1993; Endemann et al. 1993).
The putative role of FAT/CD36 on LCFA uptake in rodent skeletal muscle has been studied extensively. Overexpression of FAT/CD36 in murine skeletal muscle correlates with increased LCFA uptake (Ibrahimi et al. 1999), whereas the uptake of LCFAs in giant vesicles of rat muscle sarcolemma correlates highly with FAT/CD36 expression (Bonen et al. 1998). Furthermore, kinetic studies showed that maximal palmitate transport was 1.8-fold greater in giant vesicles obtained from oxidative (soleus) than in vesicles obtained from glycolytic (EDL) muscles (Bonen et al. 1998). In addition, work by Bonen and co-workers also suggests that FAT/CD36 resides both in the sarcolemma and intracellular membrane stores (Bonen et al. 2000). Results of an elegant study of Zhang and co-workers confirmed the sarcolemmal localisation of FAT/CD36 but were unable to detect FAT/CD36 in the cytoplasmic structures of the white gastrocnemius muscle of the rat (Zhang et al. 2003). Upon contraction and/or insulin stimulation FAT/CD36 partly translocates from these intracellular stores to the sarcolemma (Bonen et al. 2000; Luiken et al. 2002). Moreover, the increase in LCFA uptake after electrically stimulating muscle was positively correlated with an increase of cell surface concentrations of FAT/CD36 (Bonen et al. 1999).
Recently, the possible role of FAT/CD36 in LCFA transport in humans has been uncovered. It was found that patients with one or more mutations of the FAT/CD36 gene demonstrated a decreased or no myocardial LCFA accumulation (Okamoto et al. 1998; Tanaka et al. 1997, 2001). Information about the putative role of FAT/CD36 in human skeletal muscle, however, is very scanty. Only two authors report on this subject matter. Tunstall and co-workers found that 9 days of endurance training increased LCFA uptake and FAT/CD36 expression in homogenates of vastus lateralis muscle of healthy volunteers (Tunstall et al. 2002), whereas Cameron-Smith and co-workers revealed that in highly endurance trained subjects 5 days of high fat diet further increased FAT/CD36 expression (Cameron-Smith et al. 2003). Since in obesity and type-2 diabetes oxidation of LCFAs is impaired, while triacylglycerol accumulation in type-1 skeletal muscle fibres is increased (Goodpaster et al. 2000; Kelley and Goodpaster 2001), which suggests a mismatch between transsarcolemmal uptake and oxidation, precise information about which cells express the FAT/CD36 molecule is needed. In particular, no morphological studies have addressed the localisation of FAT/CD36 in human skeletal muscle.
The mechanism by which FAT/CD36 facilitates LCFA uptake is virtually unknown. Recently, Stremmel and co-workers suggested a possible role for caveolae in this process. Caveolae are microdomains of the plasma membrane that have been implicated in signal transduction (Stremmel et al. 2001). A family of three caveolae membrane proteins (caveolin-1 to -3) has been identified. Of these, caveolin-3 is exclusively expressed in skeletal muscle (Song et al. 1996). In addition, from experiments with C2C12 myotubes and extracts from mouse skeletal muscle it emerged that caveolin-3 is also involved in glucose metabolism (Scherer and Lisanti 1997).
In view of these considerations and new developments, the purpose of the present study was threefold: (1) to investigate the subcellular localisation of FAT/CD36 in individual muscle fibres of healthy and fit subjects, (2) to investigate the muscle fibre type specific expression of FAT/CD36 and (3) to investigate whether FAT/CD36 colocalises with caveolin-3. In order to examine these issues we used four different antibodies raised against FAT/CD36.
Materials and methods
Ten healthy subjects volunteered for this study. Their mean (±SD) age (in years), body mass index (kg.m−2) and peak oxygen uptake (ml−1.kg−1.min) were 32.7±6.7, 24.2±2.0 and 51.1±11.4, respectively. All subjects gave their written informed consent before entering the study, which was approved by the Medical Ethics Committee of our institution. After an overnight fast, percutaneous muscle biopsies (m. vastus lateralis) were performed under local anaesthesia (Xylocaine 2%) with a modified Bergström needle (Maastricht Instruments, Maastricht, The Netherlands). After careful removal of fat tissue and blood, muscle for histology was mounted on a glass slide with a drop of embedding compound (Tissue-Tek; Sakura Finetek Europe, Zoeterwoude, The Netherlands; Miles Laboratories, USA) and rapidly frozen in isopentane cooled to its melting point with liquid nitrogen. Subsequently, these samples were put in an aluminium cryo-vial and stored at −70°C until use.
Serial cryosections (5 μm thick) were cut at −20°C using a Leica cryomicrotome (Leica, Nussloch, Germany). Cryosections were thaw-mounted on uncoated glass slides and air-dried for at least 30 min before processing for double immunofluorescence.
Primary antibodies
Four different antibodies directed to FAT/CD36 were used in this study: (1) 131.4, a mouse IgG1 monoclonal antibody and (2) a mouse IgG1 monoclonal antibody MO25, both raised against human CD36 in one of our laboratories (Matsuno et al. 1996), (3) SC-7309 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), a mouse IgM monoclonal antibody reactive to CD36 of human origin, and (4) anti-murine CD36 (ABM-5524, clone 63; Cascade BioScience, Winchester, MA, USA), a mouse IgA monoclonal antibody raised against mouse CD36. Other antibodies used in this study were: (5) a mouse IgG1 monoclonal antibody reactive with caveolin-3 (clone 26; BD Biosciences Pharmingen, Alphen a/d Rijn, The Netherlands), (6) a purified anti-cytochrome c monoclonal antibody (clone 6H2.B4; BD Biosciences Pharmingen), (7) a mouse IgM monoclonal antibody raised against adult human slow myosin heavy chain [A4.840, developed by Dr. Blau (Cho et al. 1993)] and (8) a mouse IgG1 monoclonal antibody directed against adult human slow myosin heavy chain [A4.951 developed by Dr. Blau (Hughes et al. 1993)]. The last two antibodies referred to as A4.840 and A4.951 were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA.
Validation of FAT/CD36 antibodies in western blotting
Approximately 25 frozen human muscle sections of 20 μm thickness were cut at −20°C and sampled in an ice-cold tube. Two hundred microlitres of ice-cold PBS, containing 1 mM PMSF and 1 mM EDTA, pH 7.4, was added. After vortexing for 10 s, the sample was then sonicated for 3×5 s. Subsequently, 2 vol muscle homogenate and 1 vol SDS sample buffer (2.3% SDS, 62.5 mM TRIS-HCl pH 6.8, 10% glycerol, 5% β-mercaptoethanol and 0.05% bromophenol blue) were boiled for 4 min followed by centrifugation for 5 min at 12,000 g. Twelve percent polyacrylamide SDS gels were loaded with equal amounts (25 μg) of protein. After electrophoresis using a Mini-Protean 3 electrophoresis cell (BioRad Laboratories, Veenendaal, The Netherlands), blotting was performed using a Mini Trans-Blot electrophoretic transfer cell (BioRad Laboratories). Proteins were transferred to a nitrocellulose membrane (0.45 μm; BioRad Laboratories) for 1 h at 100 V in a cold (4°C) buffer containing 25 mM TRIS, pH 8.8, 192 mM glycine and 20% methanol. After protein transfer, nitrocellulose membranes were blocked for 20 min with 5% non-fat dry milk in 0.05% Tween 20/PBS (131.4; CD36-CascBiosc.), with 3% BSA/TBS (MO25) or with 3% BSA in 0.5% Triton X-100/PBS (sc-7309). Thereafter, antibody incubation was performed with gentle shaking overnight at room temperature at the appropriate dilution (Table 1). After the primary antibody incubation, the membranes were incubated for 60 min with a horseradish peroxidase-conjugated rabbit anti-mouse Ig (Dako, Glostrup, Denmark) at a dilution of 1:10,000. The membranes were then washed for 1.5 h in 0.05% Tween 20/PBS (131.4; CD36-CascBiosc.), 0.05%Tween 20/TBS (MO25) or 0.5% Triton X-100/PBS (sc-7309) and again for 10 min in PBS. Subsequently, they were treated for 1 min with enhanced chemiluminescence substrate (Super Signal West Dura Extended Duration Substrate; Pierce/Perbio Science, Etten-Leur, The Netherlands). Finally, nitrocellulose membranes were exposed to X-ray film (CL-Xposure Film; Pierce/Perbio Science) for 1 min.
Immunofluorescence assay
Routine indirect (double-) immunofluorescence assays were performed. Two fixation methods were tested, i.e. 5 min acetone, and 5 min methanol directly followed by 1 min acetone. Sections were incubated for 2 h at room temperature or overnight at 4°C with the primary antibodies in the required dilution (Table 1). After washing the slides three times for 5 min with PBS, sections were incubated for 30 min at room temperature with the appropriate secondary antibodies: goat anti-mouse (GAM) IgM, IgG1 conjugated with Alexa Fluor 488 or Alexa Fluor 555 (Molecular Probes Europe, Leiden, The Netherlands), or goat anti-mouse IgA conjugated with fluorescein isothiocyanate (GAMIgAFITC; Southern Biotechnology, Birmingham, AL, USA). Sections were washed again three times for 5 min in PBS and mounted in Mowiol-TRIS pH 8.5 (Calbiochem, Omnilabo International, Etten-Leur, The Netherlands) containing 0.5 μg/ml 4′-6′-diamino-2-phenylindole (DAPI; Molecular Probes Europe).
Images were examined in a Nikon ER800 microscope (Uvikon, Bunnik, The Netherlands) using a Nikon Plan-Apo DM-20× objective (N.A. 0.75) and a Nikon Plan-Apo DM-40× objective (N.A. 0.95). Images were digitally captured using a 1.3 Megapixel (1,300×1,030, HXV) Basler A101C progressive scan colour CCD colour camera, driven by LUCIA laboratory image processing and analysis software (Laboratory Imaging, Prague, Czech Republic). To ensure valid computer-assisted quantitative analysis of the sections, special care was taken to capture most images with identical camera settings (i.e. the same exposure time, gain and offset settings). In some cases, however, we diminished exposure time by 50% in order to obtain the sarcolemmal localisation of FAT/CD36 and caveolin-3 more precisely.
Results
Validation of the FAT/CD36 antibodies
FAT/CD36 is a highly glycosylated membrane protein of approximately 88 kDa, involved in the transmembrane transport of long-chain fatty acids. In this study we have used four different monoclonal antibodies, all directed to FAT/CD36. Western blotting analyses of human m. vastus lateralis revealed an approximately 88-kDa protein complex for all FAT/CD36 monoclonal antibodies studied (Fig. 1). For comparison, cultured human adipocytes were used (Fig. 1 lane e). However, the FAT/CD36 antibody obtained from Cascade Bioscience also showed crossreactivity with a 200-kDa protein, corresponding to myosin heavy chain.
Western blotting with anti-FAT/CD36 antibodies of human m. vastus lateralis (lanes a–d) and cultured human adipocytes (lane e). The four FAT/CD36 antibodies studied, i.e. lane a sc-7309, lane b MO25, lane c 131.4 and lanes d, e ABM-5524, each showed a protein band or complex at approximately 88 kDa. Note the 200-kDa myosin heavy chain (MyHC) protein band in lane d using the ABM-5524 antibody from Cascade BioScience
FAT/CD36 expression in human m. vastus lateralis
Immunofluorescence assays were performed to study the distribution of FAT/CD36 in human vastus lateralis muscle. The fixation method of the cryosections appeared to be important. Two FAT/CD36 antibodies, i.e. MO25 and sc-7309, required only an acetone fixation to obtain a positive staining pattern. In contrast, the other FAT/CD36 antibodies, i.e. from Cascade Bioscience and 131.4, showed a more intense staining pattern after methanol/acetone fixation (Table 1).
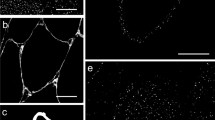
The four FAT/CD36 antibodies tested essentially showed the same, i.e. a strong expression of FAT/CD36 in the small capillaries, representing the presence of FAT/CD36 in vascular endothelium (Fig. 2A–D) and a lesser expression in the sarcolemma or its close vicinity of all muscle fibres. It can be observed that sarcolemmal staining of FAT/CD36 was more intense in type-1 than in type-2 muscle fibres, irrespective of the antibody used (Fig. 3A, C). In Fig. 2D a picture at higher magnification is shown of the sarcolemma of some skeletal muscle cells. The strong staining of the sarcolemma and the thickening at its inner site is readily visible. In the cytoplasm a more or less punctate staining pattern is visible with the FAT/CD36 antibody sc-7309 (Fig. 2D). Double immunostaining with a FAT/CD36 (sc-7309) and a cytochrome c antibody showed no colocalisation of FAT/CD36 with cytochrome c (data not shown).
Immunofluorescence staining of human vastus lateralis muscle incubated with monoclonal FAT/CD36 antibodies 131.4 (A; the strong green positive structures in between the myofibrils are the capillaries), MO25 (B) and sc-7309 (C, D). The nuclei (blue) are stained with DAPI. Note in D the thickening of the sarcolemma and the punctate staining pattern in the cytosol
However, the Cascade Bioscience FAT/CD36 antibody revealed a strong intracellular signal only in fibres identified by myosin heavy chain 1 (MHC1) staining (data not shown). Western blotting of skeletal muscle homogenates showed a second band at a molecular mass (200 kDa) corresponding with MHC1. Therefore, this antibody is unsuitable for use in this tissue.
Colocalisation of FAT/CD36 with caveolin-3
To investigate more precisely the expression of FAT/CD36 in transverse muscle cells, double staining was performed using a caveolin-3 monoclonal antibody. The transmembrane protein caveolin-3, which is exclusively expressed in skeletal and cardiac muscle cells, is localised at the inner surface of the plasma membrane. As shown (Fig. 3E, F), only the membranes of muscle cells are stained with the caveolin-3 antibody. In contrast to the FAT/CD36 distribution no endothelial cells of small capillaries are detected with the caveolin-3 antibody. Double immunofluorescence staining of FAT/CD36 with caveolin-3 shows colocalisation of both proteins in the sarcolemma (Fig. 4A–C).
Immunofluorescence staining of serial sections of MyHC-1 (A, B), FAT/CD36 (sc-7309; C, D) and caveolin-3 (E, F). To show the sarcolemmal location of FAT/CD36 and caveolin-3 more precisely, the exposure time was 50% lower as compared with Fig. 2. This causes an almost complete disappearance of cytoplasmic signal. Nuclei (B, D, F) are stained with DAPI (blue). Note that FAT/CD36 is more abundantly expressed in type-1 muscle fibres, whereas caveolin-3 seems to be more abundantly expressed in type-2 muscle fibres. I Type-1 muscle fibres, II type-2 muscle fibres
Fibre type specific expression of FAT/CD36 and caveolin-3
To show whether the expression of FAT/CD36 differs between slow (type-1) and fast (type-2) muscle cells, immunofluorescence staining was performed with FAT/CD36 antibodies and a monoclonal antibody directed to MHC1 (slow muscle fibres). Compared with type-2 muscle fibres, sarcolemmal staining with FAT/CD36 antibodies of type-1 muscle fibres was more intense (Fig. 3A–D). In contrast, caveolin-3 seems to be more abundantly expressed in type-2 muscle fibres (Fig. 3E, F).
Discussion
Using immunofluorescence microscopy, we showed for the first time in human skeletal muscle that FAT/CD36 is expressed both at the sarcolemma and in the cytoplasm. This confirms and extends the results of other researchers, using homogenates of human skeletal muscles (Cameron-Smith et al. 2003; Tunstall et al. 2002). The novel findings of the present study are: (1) FAT/CD36 is ubiquitously expressed in human endothelial cells and human muscle cells, but is relatively more abundant in endothelial cells, (2) FAT/CD36 colocalises with caveolin-3, (3) the expression of FAT/CD36 is more abundant in type-1 fibres, whereas the expression of caveolin-3 is more abundant in type-2 muscle fibres and (4) FAT/CD36 resides both in the sarcolemma and/or in its close vicinity, where the signal is very strong, and has a punctate pattern in the cytoplasm. We were able to exclude the possible colocalisation with mitochondria.
The four antibodies employed in the present study basically revealed comparable results, namely the strongest signal in endothelium of blood vessels and a weaker signal in the sarcolemma or its close vicinity. The weakest signal was observed within the cytoplasm, albeit only with three out of four antibodies tested (one of the antibodies revealed false-positive results, i.e. it colocalised with MHC1).
The finding of a more abundant expression of FAT/CD36 in endothelial cells of capillaries was recently also reported by Zhang and co-workers (Zhang et al. 2003). The results of the present study and those of Zhang et al. strongly support a role of FAT/CD36 in the transport of long-chain fatty acids across the endothelial membrane into the interstitial space as suggested by Van der Vusse and co-workers (Van der Vusse et al. 1998, 2002).
The present findings on FAT/CD36 expression in human skeletal muscle confirm and extend the results of earlier studies performed in rodents, showing a more abundant expression of FAT/CD36 in oxidative, compared with glycolytic fibres (Bonen et al. 1998; Van Nieuwenhoven et al. 1995). Our results are also partly in line with data provided by Zhang and co-workers who showed in their extensive study using a new rat-specific antibody (UA009) that FAT/CD36 is expressed in oxidative muscle fibres (soleus, diaphragm; Zhang et al. 2003). However, in contrast to the present results, Zhang and co-workers were unable to detect the antigen in white fibres of the gastrocnemius muscle, either at the sarcolemma or in the cytoplasm (Zhang et al. 2003).
From our experiments using immunofluorescence microscopy it is clear that FAT/CD36 in human muscle is associated with the sarcolemma of both type-1 and type-2 muscle fibres, and also is present in punctate clusters in the cytoplasm in the vicinity of the sarcolemma of type-1 fibres. Since no colocalisation with cytochrome c was observed, these clusters are not associated with mitochondria.
The finding of the existence of an intracellular pool of FAT/CD36 contrasts with the results of Zhang and co-workers who were unable to identify an intracellular FAT/CD36 pool in rat skeletal muscle (Zhang et al. 2003). The reason for this discrepancy might be the different fixations of the muscle specimens employed in both studies. Whereas Zhang and co-workers used ethanol, we showed in the present study that signal is lost by the use of alcoholic fixation. However, data from other researchers employing the giant vesicles technique obtained from rodent muscles also indicate the existence of an intracellular pool of FAT/CD36 (Bonen et al. 2000; Koonen et al. 2002; Luiken et al. 2002, 2003). These studies demonstrated that upon contraction and insulin stimulation part of the intracellular pool translocates, akin to the glucose transporter GLUT-4, from intracellular domains to the sarcolemma. Recently, it has been established, both in rat and human muscle cells, that GLUT-4 resides in intracellular vesicles (Borghouts et al. 2000; Ploug et al. 1998). Whether FAT/CD36 colocalises with GLUT-4 remains to be established.
The involvement of caveolae or their marker protein caveolin-3 in cellular fatty acid uptake has been suggested by the results of a number of recent studies (Pohl et al. 2002; Ring et al. 2002; Stremmel et al. 2001). Clearly, the resolution of a light microscope does not allow us to detect single caveolae, but the strikingly similar distribution of FAT/CD36 and caveolin-3 at the sarcolemma is suggestive for a role of caveolae in sarcolemmal fatty acid transport. This notion is supported by the results of recent studies in adipocytes, showing that caveolae indeed contain FAT/CD36 (Souto et al. 2003).
In conclusion, the results of this study reveal that FAT/CD36 and caveolin-3 are differently expressed among human muscle fibres of healthy, fit, male subjects. The density of FAT/CD36 signal decreases in the following order: endothelium of capillaries > sarcolemma of type-1 fibres > sarcolemma of type-2 fibres and cytoplasm of type-1 fibres. Furthermore, FAT/CD36 seems to colocalise with caveolin-3, which suggests a role for caveolae in transsarcolemmal fatty acid transport.
References
Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA (1993) Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 268:17665–17668
Bonen A, Luiken JJ, Liu S, Dyck DJ, Kiens B, Kristiansen S, Turcotte LP, et al (1998) Palmitate transport and fatty acid transporters in red and white muscles. Am J Physiol 275:E471–E478
Bonen A, Dyck DJ, Ibrahimi A, Abumrad NA (1999) Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am J Physiol 276:E642–E649
Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN (2000) Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem 275:14501–14508
Borghouts LB, Schaart G, Hesselink MK, Keizer HA (2000) GLUT-4 expression is not consistently higher in type-1 than in type-2 fibres of rat and human vastus lateralis muscles: an immunohistochemical study. Pflugers Arch 441:351–358
Cameron-Smith D, Burke LM, Angus DJ, Tunstall RJ, Cox GR, Bonen A, Hawley JA, et al (2003) A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr 77:313–318
Cho M, Webster SG, Blau HM (1993) Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J Cell Biol 121:795–810
Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA (1993) CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 268:11811–11816
Fujii S, Kawaguchi H, Yasuda H (1987) Purification of high affinity fatty acid receptors in rat myocardial sarcolemmal membranes. Lipids 22:544–546
Goodpaster BH, Theriault R, Watkins SC, Kelley DE (2000) Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49:467–472
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158:183–199
Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, et al (1999) Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem 274:26761–26766
Kelley DE, Goodpaster BH (2001) Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941
Koonen DP, Coumans WA, Arumugam Y, Bonen A, Glatz JF, Luiken JJ (2002) Giant membrane vesicles as a model to study cellular substrate uptake dissected from metabolism. Mol Cell Biochem 239:121–130
Luiken JJ, Dyck DJ, Han XX, Tandon NN, Arumugam Y, Glatz JF, Bonen A (2002) Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am J Physiol Endocrinol Metab 282:E491–E495
Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF (2003) Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52:1627–1634
Matsuno K, Diaz-Ricart M, Montgomery RR, Aster RH, Jamieson GA, Tandon NN (1996) Inhibition of platelet adhesion to collagen by monoclonal anti-CD36 antibodies. Br J Haematol 92:960–967
Okamoto F, Tanaka T, Sohmiya K, Kawamura K (1998) CD36 abnormality and impaired myocardial long-chain fatty acid uptake in patients with hypertrophic cardiomyopathy. Jpn Circ J 62:499–504
Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E (1998) Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol 142:1429–1446
Pohl J, Ring A, Stremmel W (2002) Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J Lipid Res 43:1390–1399
Ring A, Pohl J, Volkl A, Stremmel W (2002) Evidence for vesicles that mediate long-chain fatty acid uptake by human microvascular endothelial cells. J Lipid Res 43:2095–2104
Schaffer JE, Lodish HF (1994) Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79:427–436
Scherer PE, Lisanti MP (1997) Association of phosphofructokinase-M with caveolin-3 in differentiated skeletal myotubes. Dynamic regulation by extracellular glucose and intracellular metabolites. J Biol Chem 272:20698–20705
Schwieterman W, Sorrentino D, Potter BJ, Rand J, Kiang CL, Stump D, Berk PD (1988) Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A 85:359–363
Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, et al (1996) Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271:15160–15165
Sorrentino D, Stump D, Potter BJ, Robinson RB, White R, Kiang CL, Berk PD (1988) Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J Clin Invest 82:928–935
Souto RP, Vallega G, Wharton J, Vinten J, Tranum-Jensen J, Pilch PF (2003) Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J Biol Chem 278:18321–18329
Stremmel W (1988) Fatty acid uptake by isolated rat heart myocytes represents a carrier-mediated transport process. J Clin Invest 81:844–852
Stremmel W, Pohl L, Ring A, Herrmann T (2001) A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids 36:981–989
Tanaka T, Okamoto F, Sohmiya K, Kawamura K (1997) Lack of myocardial iodine-123 15-(p-iodiphenyl)-3-R,S-methylpentadecanoic acid (BMIPP) uptake and CD36 abnormality: CD36 deficiency and hypertrophic cardiomyopathy. Jpn Circ J 61:724–725
Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, et al (2001) Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res 42:751–759
Trigatti BL, Mangroo D, Gerber GE (1991) Photoaffinity labeling and fatty acid permeation in 3T3-L1 adipocytes. J Biol Chem 266:22621–22625
Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D (2002) Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab 283:E66–E72
Van der Vusse GJ, Glatz JF, Van Nieuwenhoven FA, Reneman RS, Bassingthwaighte JB (1998) Transport of long-chain fatty acids across the muscular endothelium. Adv Exp Med Biol 441:181–191
Van der Vusse GJ, van Bilsen M, Glatz JF, Hasselbaink DM, Luiken JJ (2002) Critical steps in cellular fatty acid uptake and utilization. Mol Cell Biochem 239:9–15
Van Nieuwenhoven FA, Verstijnen CP, Abumrad NA, Willemsen PH, Van Eys GJ, Van der Vusse GJ, Glatz JF (1995) Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun 207:747–752
Zhang X, Fitzsimmons RL, Cleland LG, Ey PL, Zannettino AC, Farmer EA, Sincock P, et al (2003) CD36/fatty acid translocase in rats: distribution, isolation from hepatocytes, and comparison with the scavenger receptor SR-B1. Lab Invest 83:317–332
Acknowledgements
Jan F.C. Glatz is Netherlands Heart Foundation Professor of Cardiac Metabolism. Joost J.F.P. Luiken is the recipient of a VIDI-Innovational Research Grant from the Netherlands Organization for Scientific Research (NWO-ZonMw grant 016.036.305). The authors thank Michael Magagnin for his assistance in some of the western blots.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keizer, H.A., Schaart, G., Tandon, N.N. et al. Subcellular immunolocalisation of fatty acid translocase (FAT)/CD36 in human type-1 and type-2 skeletal muscle fibres. Histochem Cell Biol 121, 101–107 (2004). https://doi.org/10.1007/s00418-003-0615-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0615-3