Abstract
Pulmonary neuroepithelial body (NEB) receptors in rats receive at least four different nerve fibre populations. In addition to a spinal sensory innervation that contacts NEBs at their basal side, extensive vagal nodose sensory terminals and separate nitrergic and cholinergic nerve endings protrude between NEB cells. In the present study, antibodies against the vesicular glutamate transporter 2 (VGLUT2), a transmembrane protein responsible for loading glutamate into synaptic vesicles, were used to investigate whether some of the nerve terminals contacting NEBs in rat lungs might use glutamate as a neurotransmitter. VGLUT2 immunoreactivity (IR) was detected in extensive intraepithelial arborising nerve terminals that appeared to contact most of the NEBs. Multiple immunostaining showed VGLUT2 IR in the vagal nodose and spinal sensory nerve terminals contacting NEBs, and in another, most likely sensory, intraepithelial nerve fibre population, the origin and further characteristics of which remain to be elucidated. At least part of the VGLUT2-immunoreactive nerve fibres that contact NEBs were shown to be myelinated. The expression of VGLUT2 indicates that glutamate is stored and released as a neurotransmitter in terminals of several pulmonary (sensory) nerve fibre populations that selectively relate to the complex NEB receptors. The present study strongly suggests an involvement of glutamatergic mechanisms in the peripheral transduction of sensory stimuli from the lungs, via the release of glutamate from nerve terminals, thereby modulating the activity of NEB receptor cells or the excitability of afferent nerves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The amino acid glutamate is a major excitatory neurotransmitter in the mammalian central nervous system (CNS). As a non-peptide transmitter, glutamate is loaded from the axoplasma into synaptic vesicles, and released by calcium-dependent exocytosis into the synaptic cleft, where it binds to glutamate receptors (GluRs). Glutamate is removed from the extracellular space by excitatory amino acid transporters (for review see O’Shea 2002). Since glutamate is involved in important metabolic pathways in most cells, it has, until recently, been difficult to unambiguously identify cells that are able to store and release glutamate as a neurotransmitter, especially outside the central nervous system.
The mechanism for accumulating glutamate into synaptic vesicles involves energy-dependent transport via specific transporter proteins (reviewed by Özkan and Ueda 1998), called vesicular glutamate transporters (VGLUTs). The vesicular glutamate uptake system is specific for l-glutamate, is stimulated by physiologically relevant concentrations of Cl− ions, depends on H+ ions (Varoqui et al. 2002) and has been shown to be present in the membrane of synaptic vesicles in ultrastructural studies (Fremeau et al. 2001).
Recently, three VGLUTs have been identified in the CNS. VGLUT1 (Bellocchio et al. 2000; Otis 2001; Takamori et al. 2000) and VGLUT2 (Bai et al. 2001; Fremeau et al. 2001; Herzog et al. 2001; Takamori et al. 2001) appear to be restricted to known glutamatergic neurones and exhibit a striking complementary pattern of expression (Kaneko and Fujiyama 2002), while VGLUT3 appears to be expressed by a number of cell populations that are not traditionally considered to release glutamate (Fremeau et al. 2002; Gras et al. 2002; Schäfer et al. 2002; Takamori et al. 2002). The presence of VGLUTs is both necessary and sufficient for uptake and storage of glutamate and is therefore a good determinant for a glutamatergic phenotype of neurones (Guyenet and Stornetta 2003; Takamori et al. 2000).
Besides in the CNS, immunoreactivity (IR) for VGLUT2 has recently also been described in the peripheral nervous system. Numerous neuronal cell bodies in rat nodose and dorsal root ganglia, as well as their central projections have been reported to be VGLUT2-immunoreactive (-IR; Li et al. 2003; Todd et al. 2003; Tong et al. 2001). Peripheral afferent nerve terminals in the gut (Tong et al. 2001) and in intraganglionic laminar nerve endings (IGLEs) in the oesophagus (Raab and Neuhuber 2003) were also shown to express VGLUT2 IR.
Outside the CNS, the lungs are one of the organs for which evidence exists for a physiological and pathological role of glutamate (Skerry and Genever 2001). The presence of the ionotropic GluRs, N-methyl-d-aspartate (NMDA) receptors R1 (Gill et al. 2000), R2B (Robertson et al. 1998), R2C and R2D (Said et al. 2001), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor GluR2/3 (Gill et al. 2000), and of the metabotropic mGluR2/3 (Gill et al. 2000) has been described in lungs. In isolated perfused rat lungs, activation of NMDA receptors caused high-permeability oedema, due to excitotoxic injury (Said et al. 1996). The apparent link between NMDA receptor activation and airway hyperresponsiveness is suggestive of glutamate signalling to be involved in the pathogenesis of acute bronchial asthma and acute respiratory distress syndrome (Said et al. 2001).
Pulmonary neuroepithelial bodies (NEBs) are receptor-effector units which are most likely able to accommodate various sensory modalities (Adriaensen et al. 2003; Brouns et al. 2003). As highly specialised, extensively innervated clusters of neuroendocrine cells, NEBs appear to be normal components of the airway epithelium of all air-breathing vertebrates investigated so far.
In rat lungs, NEBs receive at least four different nerve fibre populations, two of which are sensory. A population of vagal sensory nerve terminals selectively contacting pulmonary NEBs, with origin in the nodose ganglion, is myelinated, ramifies between NEB cells in a candelabrum-like pattern and can be labelled by calbindin D28 k (CB) and P2X3 purinoreceptor IR (Adriaensen et al. 1998; Brouns et al. 2000; 2003). They form a nerve fibre population that is clearly different from the unmyelinated calcitonin gene-related peptide (CGRP)-IR and substance P-IR nerve fibres that contact NEBs mainly at their basal pole and that find their origin in dorsal root ganglia (DRG). These spinal sensory nerve terminals express capsaicin receptors (vanilloid receptor 1) and are depleted after capsaicin treatment (Brouns et al. 2003).
In addition to the dual sensory innervation, pulmonary NEBs in rats are selectively contacted by separate nitrergic and cholinergic nerve fibre populations. The extensive neuronal nitric oxide synthase (nNOS)-IR nerve terminals that are located intraepithelially, selectively between NEB cells, appear to originate from non-cholinergic neuronal cell bodies located close to the epithelium in intrapulmonary airways (Brouns et al. 2002a). The cholinergic component of the innervation of NEBs appears as thin basal or intraepithelial vesicular acetylcholine transporter (VAChT)-IR nerve terminals, and evidence suggests that they may arise from preganglionic parasympathetic nerves (Adriaensen et al. 2003; Brouns et al. 2002b).
Glutamate and AMPA receptor signalling pathways have been reported to be involved in the transmission of afferent inputs from the airways to the solitary tract, in this way mediating reflex airway constriction (Haxhiu et al. 1997). Because the central projections of many nodose and dorsal root sensory neurones appear to use glutamate as a neurotransmitter, the aim of the present study was to find out whether the peripheral terminals of the vagal nodose and spinal sensory neurones that selectively contact pulmonary NEBs are also able to store and release glutamate as a neurotransmitter. Since glutamate is present in all cells, VGLUT2 immunocytochemistry was selected as a tool to specifically detect glutamatergic neuronal cell bodies, nerve fibres and terminals in lungs. VGLUT2-IR nerve terminals were compared with all nerve fibre populations contacting NEBs that have been characterised so far.
Materials and methods
Animals and tissue processing
Studies were performed on 10-day-old (n=6), 4-week-old (n=2) and adult (n=2) Wistar rats (Iffa Credo, Brussels, Belgium). All animals were kept with their mothers (10-day-old) or in separate acrylic cages (4-week-old and adults) with wood shavings in an acclimatised room (12 h/12 h light/dark cycle; 22±3°C) and were provided with water and food ad libitum. National and international principles of laboratory animal care were followed and the experiments were approved by the local ethics committee of the University of Antwerp.
All rats were killed using an overdose of sodium pentobarbital (Nembutal; 200 mg/kg intraperitoneally). Lungs were intratracheally instilled with 4% paraformaldehyde (in 0.1 M phosphate buffer; pH 7.4), dissected en bloc with the oesophagus, degassed and further fixed for 30 min. All tissues were rinsed in phosphate-buffered saline (PBS; 0.01 M, pH 7.4), stored overnight in 20% sucrose (in PBS; 4°C) and mounted in Tissue Tek (Sakura Finetek Europe, Zoeterwoude, The Netherlands) on a cryostat chuck by freezing in a CO2 chamber. Cryosections (30 μm thickness) were thaw-mounted on poly-l-lysine-coated microscope slides, dried at 37°C (2 h) and processed for immunolabelling.
Immunocytochemical procedures
All incubations were carried out at room temperature in a closed humid incubation chamber. Characteristics and sources of the applied primary antisera are listed in Table 1, and those of secondary antisera in Table 2. The combinations of primary and secondary antisera used for multiple immunocytochemical labelling are listed in Tables 3, 4, 5, 6. Unless indicated otherwise, all primary and secondary antisera were diluted in PBS containing 10% normal goat serum, 0.1% bovine serum albumin, 0.05% thimerosal and 0.01% NaN3 (PBS*). Prior to incubation with the primary antisera, cryostat sections were incubated for 30 min with PBS* containing 1% Triton X-100. After a final wash, all sections were mounted in Citifluor (Ted Pella 19470, Redding, CA, USA).
Conventional immunocytochemical double labelling
Cryostat sections were incubated overnight with a mixture of two primary antibodies raised in different species. After rinsing in PBS the sections were incubated for 1 h with the appropriate secondary antibodies.
Multiple stainings using tyramide signal amplification (TSA)
To obtain enhanced sensitivity and to allow combination of antisera raised in the same species, a biotin-conjugated TSA kit (Perkin Elmer Life Sciences NEL700) was applied. Prior to the immunocytochemical staining procedures, endogenous peroxidase activity in cryostat sections of rat lungs was blocked by hydrogen peroxide (3% in 50% methanol/PBS; 10 min). For the first primary incubation a polyclonal antibody raised in rabbit (see Tables 1, 3, 4, 5, 6) was applied. Sections were then consecutively incubated with biotin-conjugated Fab fragments of goat anti-rabbit immunoglobulins G (diluted 1:500; 1 h) and ExtrAvidin-horseradish peroxidase (diluted 1:1,000 in PBS; 1 h). Between subsequent steps, sections were rinsed in PBS containing 0.05% Tween 20. They were further incubated for 10 min with biotin-conjugated tyramide (diluted 1:100 in ‘amplification solution’), and staining was visualised with either Cy3-conjugated streptavidin (diluted 1:6,000; 10 min) or FITC-conjugated streptavidin (diluted 1:1,000; 10 min). In double immunocytochemical procedures, the sections were subjected to an additional conventional immunostaining with the second primary antibody. In triple immunocytochemical stainings, sections were further processed for a double immunocytochemical staining with two antibodies raised in different species. For the simultaneous detection of VGLUT2/myelin basic protein (MBP)/CB or VGLUT2/CGRP/CB, a triple immunofluorescence staining with antibodies raised in the same species (Brouns et al. 2002c) was applied. TSA-enhanced immunostaining for the ATP receptor P2X3 was performed as previously described (Brouns et al. 2000).
Control experiments for the immunocytochemical procedures
Negative staining controls for all immunocytochemical procedures were performed by substitution of non-immune sera for the primary or secondary antisera. The general specificity of the primary antibodies for their respective antigens was tested by the providing companies, and the selectivity in rat lung sections in particular was extensively proven using positive and preabsorption controls and was described in previous publications (Brouns et al. 2000, 2002a, 2003; Raab and Neuhuber 2003). To check for possible crossreactivity after consecutive multiple staining when using two or three rabbit primary antisera, the results of single immunostaining for the substances were evaluated and compared with those from multiple labelling. Controls for the amplification-based multiple staining were performed by omission of the primary antiserum of the second and third incubation. In addition, non-amplified stainings with primary antibodies, using the same concentrations as for the TSA-enhanced reactions were routinely included. To compare the TSA-enhanced and conventional detection of VGLUT2, a conventional immunolabelling was performed using a lower dilution of the primary antiserum. For the triple immunocytochemical staining using three rabbit antibodies, all control stainings were performed as previously described (Brouns et al. 2002c).
Microscopic analysis
Results were quickly evaluated using an epifluorescence microscope (Zeiss Axiophot) equipped with filters for the visualisation of FITC (Zeiss 17; BP 485-20/FT 510/BP 515-565) and Cy3 (Zeiss 14; LP 510-KP 560/FT 580/LP 590).
To obtain detailed images of the multiple-stained individual nerve endings contacting NEBs, either a Zeiss LSM 510 confocal microscope equipped with an argon laser (488 nm) and two helium–neon lasers (543 and 633 nm), or a Perkin Elmer UltraVIEW confocal microscope equipped with an argon–krypton laser source with three excitation lines (488, 568 and 647 nm) were used for excitation of FITC, Cy3 and Cy5, respectively. Images were processed using 3D-reconstruction facilities [Imaris 2.7 (Bitplane, Zurich, Switzerland) or Volocity software (Improvision, Coventry, UK)].
Results
VGLUT2 immunostaining labels nerve terminals that specifically contact pulmonary NEBs
In 10-day-old, 4-week-old and adult rats, VGLUT2 was detected in nerve terminals that formed conspicuous terminal arborisations at distinct locations in the epithelium of bronchi (Figs. 4b, 5a, 7a, 8a, 12c), bronchioles (Figs. 6a, 9a, 11b, 13b) and alveolar areas (Fig. 1a). In 10-day-old rats (Figs. 1, 2, 5, 6, 9, 11, 12, 13) VGLUT2-IR nerve fibres could be seen to ramify at the base of the epithelium and to protrude between the epithelial cells, in this way giving rise to extensive intraepithelial complexes that were always strongly VGLUT2-IR. In 4-week-old (Figs. 3, 4, 7, 10) and adult (Fig. 8) rats, the staining intensity of nerve fibres approaching NEBs varied from fibre to fibre and could often hardly be detected, while VGLUT2 IR was usually strong in the intraepithelial nerve terminals. No differences in localisation of VGLUT2 were observed between TSA-enhanced (Figs. 1, 3, 4, 5, 7, 8, 9, 10, 13) and conventional (Figs. 2, 6, 11, 12) immunodetection.
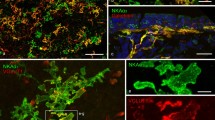
Peripheral lung area of a 10-day-old rat (postnatal day 10; PD10). Maximum value projection of 30 confocal optical sections (1-μm intervals). a Tyramide signal amplification (TSA)-enhanced immunocytochemical staining for vesicular glutamate transporter 2 (VGLUT2; red Cy3 fluorescence) shows nerve fibres (arrows) that approach the epithelium, protrude between the epithelial cells and form an extensive intraepithelial arborisation (arrowheads) of highly fluorescent terminals. b Combination of the red and green channels clearly shows that the intraepithelial nerve terminals coincide with the presence of a neuroepithelial body (NEB), as marked by its calcitonin gene-related peptide immunoreactivity (CGRP IR; green FITC fluorescence). Also note CGRP-immunoreactive (-IR) varicose nerve terminals that contact the basal side of the NEB (open arrows) and appear to colocalise with the more weakly VGLUT2-IR terminals. L Lumen of the airway
Conventional double immunocytochemical staining for VGLUT2 (red Cy3 fluorescence) and CGRP (green FITC fluorescence) showing a CGRP-IR NEB in a rat bronchus (PD10) contacted by VGLUT2+/CGRP− and VGLUT2+/CGRP+ nerve terminals. a Overview. Maximum value projection of 29 confocal optical sections (0.8-μm intervals). b, c Single confocal optical sections of a magnification of the left lower part of the NEB. b Both red fluorescent intraepithelial VGLUT2-IR nerve terminals (arrowheads) and green fluorescent CGRP-IR nerve endings (open arrows) appear to contact CGRP-IR NEB cells. c Red channel showing that also the CGRP-IR nerve terminals (open arrows) express VGLUT2 IR although the staining intensity varies along the fibre
Combined three-channel image of an immunocytochemical triple labelling for VGLUT2 (red Cy3 fluorescence), CGRP (artificial blue colour of Cy5 emission in far-red) and calbindin D28 k (CB; green FITC fluorescence) in a bronchus of a 4-week-old (4W) rat. The imaged CGRP/CB-IR NEB is contacted by spinal sensory CGRP-IR nerve endings (open arrows) and by vagal nodose CB-IR nerve endings (open arrowheads). Strong VGLUT2 IR (arrowheads) is present in nerve terminals protruding between the NEB cells. The CGRP- and CB-IR nerve fibres approaching the NEB reveal just a weak VGLUT2 staining as compared to the intraepithelial terminals. Maximum value projection of 47 confocal optical sections (0.5-μm intervals)
Digital reconstruction of just five optical sections (original data: 49 optical sections; 0.5-μm intervals) of an NEB present in a bronchus of a rat (4W), triple stained for VGLUT2 (red Cy3 fluorescence), CGRP (artificial blue colour of Cy5 emission in far-red) and CB (green FITC fluorescence). a Combined three-channel image showing CGRP/CB-IR NEB cells contacted by a CGRP+/CB+/VGLUT2+ spinal sensory nerve fibre (open arrow) and by a red nerve fibre, the intraepithelial terminals of which (arrowheads) surround an NEB cell (asterisk). Other optical sections (not shown in these images) clearly revealed that the orange VGLUT2+/CB+ nerve terminals that surround NEB cells (open arrowheads) originate from CB+/VGLUT2+ vagal nodose nerve fibres. b Red channel: strong VGLUT2 IR is present in a nerve fibre (open arrow) approaching the NEB and in terminals protruding between the epithelial cells (arrowheads and open arrowheads). c Blue channel: CGRP-IR NEB contacted by a spinal sensory CGRP-IR nerve fibre (open arrow). The marked VGLUT2-IR nerve endings (arrowheads) are CGRP-negative. d Green channel: the VGLUT2-IR nerve endings (arrowheads) lack CB IR, while the NEB cells and the spinal sensory nerve fibre (open arrow) reveal CB IR
Rat PD10, bronchus. Maximum value projection of 31 confocal optical sections (0.8-μm intervals). a A network of VGLUT2-IR nerve fibres (arrows) gives rise to very complex intraepithelial VGLUT2-IR nerve terminals (arrowheads). b Combination of the red and green channels shows that the intraepithelial VGLUT2-IR nerve terminals selectively protrude between CB-IR NEB cells. All CB-IR nerve fibres express a clear VGLUT2 IR (arrows). L lumen of the airway
Rat PD10, bronchiole. Maximum value projection of 52 confocal optical sections (0.5-μm intervals). a Red channel showing an extensive intraepithelial arborisation of VGLUT2-IR nerve endings (arrowheads). b Green channel demonstrating abundant P2X3 receptor-IR intraepithelial vagal sensory nerve endings (open arrowheads). c Combination of both channels shows that all the P2X3-IR nerve terminals express VGLUT2 IR. L lumen of the airway
Immunocytochemical double staining for VGLUT2 and CB in bronchi of a 4-week-old (4W) rat. Some of the vagal nodose CB-IR nerve fibres (arrows) approaching the CB-IR NEB show an almost undetectable VGLUT2 IR. On the other hand, some of the VGLUT2-IR nerve fibres (open arrows) seem to be CB-negative. Maximum value projection of 26 confocal optical sections (0.5-μm intervals). a Arborisation of VGLUT2-IR nerve endings. b CB-IR pulmonary NEB selectively contacted by thick CB-IR vagal nerve fibres. c Combination of both channels. L lumen of the airway
Immunocytochemical double staining for VGLUT2 and CB in bronchi of an adult rat. Some of the vagal nodose CB-IR nerve fibres (arrows) approaching the CB-IR NEB show an almost undetectable VGLUT2 IR. On the other hand, some of the VGLUT2-IR nerve fibres (open arrows) seem to be CB-negative. Maximum value projection of 69 confocal optical sections (0.5-μm intervals). a Red channel showing VGLUT2-IR nerve fibres that give rise to a VGLUT2-IR intraepithelial arborisation. b Green channel showing a CB-IR NEB contacted by profuse CB-IR nerve terminals. c Combination of both channels. L lumen of the airway
Confocal images of an NEB located in the epithelium of a bronchiole (rat PD10). Maximum value projection of 89 confocal optical sections (0.5-μm intervals) after double immunostaining for VGLUT2 and CB. Note an intraepithelial VGLUT2-IR nerve terminal (arrowhead) that does not express CB. a Red channel showing an intraepithelial VGLUT2-IR arborisation. b Green channel showing a CB-IR NEB contacted by CB-IR nerve terminals. L lumen of the airway
Subsequent labelling of lung sections that were processed for VGLUT2 localisation with antibodies against markers for pulmonary NEBs revealed that the intraepithelial VGLUT2-IR arborisations always corresponded with the presence of an NEB (bronchial: Figs. 2, 5, 7, 8, 10, 12; bronchiolar: Figs. 6, 9, 11, 13; alveolar: Fig. 1). Optical sections obtained by confocal microscopy demonstrated that the VGLUT2-IR nerve terminals protrude between and often appear to surround the NEB cells (Fig. 2b, c). Nearly all NEBs, as detected by their CB or CGRP IR, seemed to be contacted by intraepithelial VGLUT2-IR nerve terminals.
Correlation of the VGLUT2-IR terminals with other nerve fibre populations that selectively contact pulmonary NEBs
Immunocytochemical double staining revealed that most of the thin, varicose spinal CGRP-IR nerve fibres selectively contacting CGRP-IR NEBs at their basal side, show VGLUT2 IR (Figs. 1, 2, 3, 4). In these spinal sensory nerve terminals, VGLUT2 IR varied both along the same fibre and between different fibres, and is in most of the fibres weaker than the VGLUT2 staining in intraepithelial vagal sensory nerve terminals. Part of the CGRP-IR NEBs appeared to be contacted by both VGLUT2+/CGRP+ and VGLUT2+/CGRP− nerve terminals.
Using CB or P2X3 receptor staining as markers for the vagal nodose sensory nerve fibre population that forms extensive intraepithelial arborisations between NEB cells, all intraepithelial CB (Figs. 5, 7, 8, 9) and P2X3 receptor-IR (Fig. 6) nerve endings in contact with NEB cells also appeared to show strong VGLUT2 IR. The vagal nodose nerve fibres giving rise to the latter terminals, however, sometimes revealed just a weak VGLUT2 IR, especially in 4-week-old (Figs. 3, 7, 10) and adult rats (Fig. 8). MBP immunostaining showed that CB-IR vagal nodose sensory nerve fibres lose their myelin sheaths in the vicinity of the target NEB, branch and give rise to terminals between the NEB cells. VGLUT2 IR was mostly undetectable in the myelinated part of the fibres, became visible upon termination of the myelin sheaths, showing short strands of nerve fibres approaching NEBs, and was strong in their intraepithelial terminals in NEBs (Fig. 10).
Immunocytochemical triple staining for VGLUT2 (red Cy3 fluorescence), CB (green FITC fluorescence) and myelin basic protein (MBP; artificial blue colour of Cy5 emission in far-red) of a bronchus of a rat (4W). a Overview. The CB-IR NEB is contacted by a CB-IR vagal nodose sensory nerve fibre which is surrounded by an MBP-IR myelin sheath (open arrowheads). VGLUT2 IR is seen in nerve terminals between the NEB cells. Maximum value projection of 45 confocal optical sections (0.5-μm intervals). b, c High magnification details of the framed area in a. The green CB-IR nerve fibre (star) approaching the NEB (asterisk) reveals a blue MBP-IR myelin sheath (open arrowhead) that ends (open arrow) in the immediate neighbourhood of the NEB. The image clearly reveals that VGLUT2 IR is almost undetectable in the approaching nerve fibre, but strong in the terminals that appear after termination of the myelin sheaths (arrowheads). b Combination of the three channels. c Combination of the red and blue channels
Maximum value projection of 24 confocal optical sections (0.8-μm intervals). a Combination of the red, green and blue channels showing a bronchiolar CGRP-IR NEB contacted by CGRP-IR spinal sensory nerve fibres, by intraepithelial VGLUT2-IR and by nNOS-IR nerve terminals. Note that VGLUT2 and nNOS are expressed in different nerve fibres. b Red channel. c Green channel
Maximum value projection of 38 confocal optical sections (0.6-μm intervals). a Overview of an NEB in a bronchus, suggestive of receiving nitrergic nerve terminals from nearby nNOS-IR neuronal cell bodies (asterisk). b. Magnification of a. c, d VGLUT2 IR (c) is not observed in the approaching nNOS-IR (d) nerve fibre (open arrow) or in intraepithelial nerve terminals (open arrowheads)
Images of an NEB present in a terminal bronchiole of a 10-day-old rat, triple-stained for VGLUT2 (red Cy3 fluorescence), vesicular acetylcholine transporter (VAChT; green FITC fluorescence) and CGRP (artificial blue colour of Cy5 emission in far-red). Maximum value projection of 45 confocal optical sections (0.7-μm intervals). a Combination of the red, green and blue channels shows a CGRP-IR NEB contacted by three different nerve fibre populations. b Complex of intraepithelial VGLUT2-IR nerve terminals. c A VAChT-positive nerve fibre (open arrow) appears to give rise to an intraepithelial VAChT-IR nerve ending (open arrowheads). Note the absence of VGLUT2 IR in the approaching cholinergic nerve fibre
Double immunocytochemical staining for VGLUT2 and CB revealed an additional population of intraepithelial VGLUT2-IR nerve terminals in contact with pulmonary NEBs, which apparently did not express CB IR (Figs. 7, 8, 9). Subpopulations of NEBs showed intraepithelial VGLUT2+/CB+ terminals alone (Fig. 5), VGLUT2+/CB+ and VGLUT2+/CB− terminals (Figs. 7, 8, 9) or neither VGLUT2+/CB+ nor VGLUT2+/CB− terminals.
Triple immunocytochemical staining for VGLUT2, CGRP and CB revealed VGLUT2+/CB+/CGRP+, VGLUT2+/CB+/CGRP− and VGLUT2+/CB−/CGRP− nerve terminals selectively in contact with pulmonary NEBs (Figs. 3, 4).
No VGLUT2 IR was observed in intraepithelial nNOS-IR (Figs. 11, 12) or VAChT-IR (Fig. 13) nerve terminals in selective contact with pulmonary NEBs.
Control experiments
Omission of the primary or secondary antibodies resulted in negative staining controls in all immunocytochemical procedures performed.
The staining pattern in single-labelled sections for any of the antigens studied, showed no obvious differences with that observed after multiple labelling.
The oesophagus, present in all sections of rat lungs, showed VGLUT2-IR intraganglionic laminar endings, thereby serving as a positive control.
After non-amplified indirect immunostaining using the same concentrations of primary antisera as used for the TSA-enhanced reaction, no VGLUT2 IR could be detected in rat lungs. In multiple immunolabellings using TSA-enhanced and subsequent conventional staining, omission of the primary antiserum of the second or third incubation abolished all staining in the second or third step. Interference control stainings for the triple immunocytochemical staining with three antibodies raised in rabbits showed that there was neither linking of the third-step secondary antiserum with the second-step primary antiserum, nor of the third-step primary antiserum with the second-step secondary antiserum.
Discussion
Multiple immunostainings in 10-day-old, 4-week-old and adult rats revealed the presence of VGLUT2 IR in different populations of nerve terminals that selectively contact pulmonary NEBs: (1) basal spinal sensory fibres, (2) intraepithelial vagal nodose sensory fibres and (3) at least one other population of intraepithelial nerve terminals. The latter VGLUT2-positive nerve fibre population apparently does not correspond to other already characterised nerve fibre populations. Since the expression of VGLUTs is, at present, regarded as an unequivocal indication of the glutamatergic nature of neurones (Guyenet and Stornetta 2003; Takamori et al. 2000), the detection of VGLUT2 in profuse nerve fibre populations that selectively contact pulmonary NEBs in rats should be considered to reflect the capacity of these terminals to store and release glutamate as a neurotransmitter at the level of NEB cells.
For the localisation of glutamatergic nerve terminals in rat lungs, we applied the same antibody as has been used to demonstrate the presence of VGLUT2 in putative vagal mechanosensor terminals in the rat oesophagus (Raab and Neuhuber 2003), which allowed us to use the oesophageal cross-section present in all of our lung sections as a positive control. The present study did not reveal obvious differences in the VGLUT2 staining pattern between conventional indirect and TSA-enhanced immunocytochemical methods. Both staining procedures can therefore be used to localise VGLUT2 in rat lungs. To allow for the consecutive application of antisera raised in the same species, however, TSA-enhanced visualisation (Brouns et al. 2002c; Hunyady et al. 1996; Shindler and Roth 1996) of VGLUT2, P2X3 or nNOS was combined with conventional immunostaining techniques. The necessity for a biotinylated tyramide amplified staining method to detect P2X3 (Brouns et al. 2000) and nNOS (Brouns et al. 2002a) in nerve terminals in rat lungs has been discussed before.
At all levels of the intrapulmonary airways, subepithelial VGLUT2-IR nerve fibres that branched and formed extensive intraepithelial arborisations invariably coincided with the presence of an NEB. Since rat pulmonary NEBs are contacted by at least three different intraepithelial nerve fibre populations (i.e. vagal nodose sensory, nitrergic and cholinergic), the possibility that the VGLUT2-IR nerve terminals correspond to one or more of these populations had to be taken into account. CB− or P2X3 receptor-expressing vagal nodose sensory intraepithelial nerve terminals selectively contacting NEBs indeed always appeared to contain VGLUT2. VGLUT2/CB labelling, however, revealed that pulmonary NEBs may be contacted by either VGLUT2+/CB+ terminals alone or by additional VGLUT2+/CB− terminals. A minority of the pulmonary NEBs was contacted by neither VGLUT2+/CB+ nor VGLUT2+/CB− terminals. TSA-enhanced nNOS immunostaining combined with conventional VGLUT2 staining revealed that VGLUT2 IR was absent from the intraepithelial nitrergic nerve fibres that arise from intrapulmonary neuronal cell bodies. Also the VAChT-IR cholinergic nerve fibre population that forms basal and intraepithelial nerve endings in a limited number of NEBs was VGLUT2-negative. Triple immunostaining for VGLUT2, CB and CGRP revealed the presence of intraepithelial VGLUT2-positive terminals that contained neither CB nor CGRP. Since the intraepithelial VGLUT2+/CB− nerve terminals selectively contacting NEBs do not correspond with one of the already characterised nerve fibre populations, they represent an additional, so far unreported component of the innervation of NEBs in rat lungs, the origin of which needs further investigation.
In the peripheral nervous system, VGLUT2 IR has been detected in vagal sensory nerve terminals in the gut (Raab and Neuhuber 2003; Tong et al. 2001) and in submucosal neuronal perikarya and their terminals in the guinea-pig ileum (Tong et al. 2001). The majority of the latter were, in contrast to the present findings, found to colocalise VAChT and may therefore be considered cholinergic.
Most DRG neurones that express glutamatergic markers also appear to contain CGRP (Miller et al. 1993; Tong et al. 2001). It has been presumed that some DRG neurones release glutamate and CGRP from the same axon terminals in the spinal cord (Miller et al. 1993). Double immunocytochemical staining for VGLUT2 and CGRP in rat lungs in the present study, revealed VGLUT2 IR in the CGRP-IR varicose spinal sensory nerve terminals that contact NEBs at their basal pole.
It is well-known that the brainstem projection of most sensory neurones in the nodose ganglion use glutamate as a neurotransmitter and that glutamate is an important mediator in the central regulation of breathing, and the development of hypersensitivity, as a consequence of airway ‘sensations’ (Haxhiu et al. 1997). It has further been shown that glutamate is released at central terminals during the acute hypoxic ventilatory response (Burton and Kazemi 2000), an action which pulmonary NEBs may be considered to be involved in. The observation that peripheral terminals of all the so far characterised sensory nerve fibre populations that selectively contact pulmonary NEBs contain VGLUT2 allows us to presume that both central and peripheral nerve terminals of at least the DRG and vagal nodose neurones that project to pulmonary NEBs use glutamate as a transmitter.
Intraepithelial varicose nerve endings showed a strong VGLUT2 IR at all ages. Nerve fibres approaching the NEBs, however, did not always reveal a clear VGLUT2 expression. While the vagal nodose CB-IR sensory nerve fibres approaching NEBs were clearly VGLUT2-IR in 10-day-old rats, they were often hardly detectable in 4-week-old and adult rats. In spinal sensory nerve terminals, the staining intensity varied, both along the same fibre and between different fibres, independent of the age of the rats. VGLUT proteins are assembled in the neuronal cell body and incorporated in the membrane of glutamatergic secretory vesicles, which are then transported and accumulated in the nerve terminals. It might be that VGLUT2 is detected in 10-day-old rats in vagal nodose nerve fibres approaching NEBs because of a higher transport of vesicles along the axon as compared to older animals. The continuously strong VGLUT2 IR in the intraepithelial terminals between the NEB cells seems logical since VGLUT2 is a normal component of the membrane of the glutamate-containing synaptic vesicles (Fremeau et al. 2001) that accumulate in nerve terminals. Synaptic vesicle membranes are largely recycled locally after exocytosis of the transmitter.
Based on the morphological evidence that the vagal nodose component of the innervation of rat pulmonary NEBs consists of myelinated nerve fibres, we recently proposed that at least part of them may belong to the extensive population of myelinated mechanosensors reported in the airways (Adriaensen et al. 2003; Brouns et al. 2003). In the present study, triple immunocytochemical staining for VGLUT2, CB and MBP showed VGLUT2 IR in myelinated nerve endings that contact NEBs, suggestive of the release of glutamate from the myelinated vagal nodose nerve endings that may at least partly be involved in mechanosensation. The latter is supported by reports on the presence of VGLUT2 in mechanosensory IGLEs in the gastrointestinal tract (Raab and Neuhuber 2003) and of glutamate in sensory terminals in muscle spindles (Banks et al. 2002).
Detection of VGLUT2 IR in different sensory nerve fibre populations in contact with NEBs implies the presence in these terminals of glutamatergic synaptic vesicles. Although the presence of synaptic vesicles in sensory nerve endings seems contradictory, it has been regarded as an indication of sensory nerve fibres being able to modulate the associated receptor cells before, after or during transduction of adequate stimuli to afferent nerve discharges. Detailed ultrastructural studies of rabbit (Lauweryns and Van Lommel 1986, 1987) and rat lungs (Van Lommel and Lauweryns 1993) have demonstrated that vagal sensory intraepithelial nerve endings in NEBs carry both afferent-like (mitochondria-rich) and efferent-like (packed with clear synaptic vesicles) nerve endings. It has been speculated that these afferent and efferent nerve endings in NEBs are involved in a mechanism of local modulation of the neuroreceptor, the so-called ‘axon reflex’ (Adriaensen and Scheuermann 1993; Lauweryns and Van Lommel 1986, 1987). The results of the present study suggest that glutamate may be involved in modulating the sensory transduction in NEBs. Since most NEBs appear to be contacted by glutamatergic nerve terminals, an important functional significance may be postulated.
Said and colleagues (1996) showed that in perfused, ventilated rat lungs activation of NMDA receptors triggered acute lung injury. They presumed NMDA receptors in the lung to be located on neuronal tissues. The presence of extensive populations of glutamatergic nerve terminals, however, was not considered. Since many of these terminals were shown to be part of complex sensors, the localisation pattern of glutamate receptors may be complex.
In conclusion, the present study is the first to demonstrate profuse glutamatergic nerve terminals in the lungs, many of which appear to be sensory and selectively contact the complex specialised receptor structures known as NEBs. Vagal nodose sensory, spinal sensory and at least one other population of (sensory?) nerve terminals in contact with NEBs appear to be able to store and release glutamate. Since both glutamate signalling and NEBs have been implicated in the pathogenesis and/or pathology of many lung disorders, the present data suggest that at least some of the physiological actions of glutamate may involve pulmonary NEBs. Detailed localisation and functional studies of glutamate receptors will be necessary to determine the possible interactions between glutamate, sensory nerve terminals, pulmonary NEBs and their environment.
References
Adriaensen D, Scheuermann DW (1993) Neuroendocrine cells and nerves of the lung. Anat Rec 236:70–85
Adriaensen D, Timmermans J-P, Brouns I, Berthoud H-R, Neuhuber WL, Scheuermann DW (1998) Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies. An anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res 293:395–405
Adriaensen D, Brouns I, Van Genechten J, Timmermans J-P (2003) Functional morphology of pulmonary neuroepithelial bodies: extremely complex airway receptors. Anat Rec 270A:25–40
Bai L, Xu H, Collins JF, Ghishan FK (2001) Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem 276:36764–36769
Banks RW, Bewick GS, Reid B, Richardson C (2002) Evidence for activity-dependent modulation of sensory-terminal excitability in spindles by glutamate release from synaptic-like vesicles. Adv Exp Med Biol 508:13–18
Bellocchio EE, Reimer RJ, Fremeau RT, Edwards RH (2000) Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289:957–960
Brouns I, Adriaensen D, Burnstock G, Timmermans J-P (2000) Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X3 receptors. Am J Respir Cell Mol Biol 23:52–61
Brouns I, Van Genechten J, Scheuermann DW, Timmermans J-P, Adriaensen D (2002a) Neuroepithelial bodies: a morphological substrate for the link between neuronal nitric oxide and sensitivity to airway hypoxia? J Comp Neurol 449:343–354
Brouns I, Van Genechten J, Timmermans J-P, Schiebler W, Adriaensen D (2002b) Pulmonary neuroepithelial bodies are selectively contacted by a population of cholinergic motor fibres (abstract). FASEB J 16:A453
Brouns I, Van Nassauw L, Van Genechten J, Majewski M, Scheuermann DW, Timmermans J-P, Adriaensen D (2002c) Triple immunofluorescence staining method with antibodies raised in the same species to study the complex innervation pattern of intrapulmonary chemoreceptors. J Histochem Cytochem 50:575–582
Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans J-P, Adriaensen D (2003) Dual sensory innervation of pulmonary neuroepithelial bodies. Am J Respir Cell Mol Biol 28:275–285
Burton MD, Kazemi H (2000) Neurotransmitters in central respiratory control. Respir Physiol 122:111–121
Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31:247–260
Fremeau RT, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Matisen J, Reimer RJ, Chaudhry FA, Edwards RH (2002) The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A 99:14488–14493
Gill SS, Mueller RW, McGuire PF, Pulido OM (2000) Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol Pathol 28:277–284
Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S (2002) A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci 22:5442–5451
Guyenet PG, Stornetta RL (2003) Glutamatergic expression in autonomic circuitry identified by the presence of vesicular glutamate transporters (abstract). Auton Neurosci 106:1
Haxhiu MA, Erokwu B, Dreshaj IA (1997) The role of excitatory amino acids in airway reflex responses in anesthetized dogs. J Auton Nerv Syst 67:192–199
Herzog E, Bellenchi GC, Cras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S (2001) The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci 21:RC181(1–6)
Hunyady B, Krempels K, Harta G, Mezey E (1996) Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem 44:1353–1362
Kaneko T, Fujiyama F (2002) Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res 42:243–250
Lauweryns JM, Van Lommel A (1986) Effect of various vagotomy procedures on the reaction to hypoxia of rabbit neuroepithelial bodies: modulation by intrapulmonary axon reflexes. Exp Lung Res 11:319–339
Lauweryns JM, Van Lommel A (1987) Ultrastructure of nerve endings and synaptic junctions in rabbit intrapulmonary neuroepithelial bodies: a single and serial section analysis. J Anat 151:65–83
Li JL, Fujiyama F, Kaneko T, Mizuno N (2003) Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibres in the superficial layers of the medullary and spinal dorsal horns of the rat. J Comp Neurol 457:236–249
Miller KE, Douglas VD, Kaneko T (1993) Glutaminase immunoreactive neurons in the rat dorsal root ganglion contain calcitonin gene-related peptide (CGRP). Neurosci Lett 160:113–116
O’Shea R (2002) Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol 29:1018–1023
Otis TS (2001) Vesicular glutamate transporters in cognito. Neuron 29:11–14
Özkan ED, Ueda T (1998) Glutamate transport and storage in synaptic vesicles. Jpn J Pharmacol 77:1–10
Raab M, Neuhuber WL (2003) Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function. Cell Tissue Res 312:141–148
Robertson BS, Satterfield BE, Said SI, Dey RD (1998) N-methyl-d-aspartate receptors are expressed by intrinsic neurons of rat larynx and esophagus. Neurosci Lett 244:77–80
Said SI, Berisha HI, Pakbaz H (1996) Excitotoxicity in the lung: N-methyl-d-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A 93:4688–4692
Said SI, Dey RD, Dickman K (2001) Glutamate signalling in the lung. Trends Pharmacol Sci 22:344–345
Schäfer MK, Varoqui H, Defamie N, Weihe E, Erickson JD (2002) Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem 277:50734–50748
Shindler KS, Roth KA (1996) Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem 44:1331–1335
Skerry TM, Genever PG (2001) Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci 22:174–181
Takamori S, Rhee JS, Rosenmund C, Jahn R (2000) Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407:189–194
Takamori S, Rhee JS, Rosenmund C, Jahn R (2001) Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J Neurosci 21:RC182(1–6)
Takamori S, Malherbe P, Broger C, Jahn R (2002) Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep 3:798–803
Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ (2003) The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17:13–27
Tong Q, Ma J, Kirchgessner AL (2001) Vesicular glutamate transporter 2 in the brain–gut axis. Neuroreport 12:3929–3934
Van Lommel A, Lauweryns JM (1993) Neuroepithelial bodies in the Fawn Hooded rat lung: morphological and neuroanatomical evidence for a sensory innervation. J Anat 183:553–566
Varoqui H, Schäfer MK, Zhu H, Weihe E, Erickson JD (2002) Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 22:142–155
Acknowledgements
We are grateful to Prof. G. Burnstock (director of the Autonomic Neuroscience Institute, Royal Free and University College Medical School) for his invaluable input in the ATP receptor studies. We thank H. De Pauw, R. Spillemaeckers, G. Svensson, F. Terloo and G. Vermeiren for technical assistance, J. Van Daele and D. De Rijck for help with microscopy, imaging and illustrations, D. Vindevogel for aid with the manuscript, and S. Kockelberg for secretarial help. This work was supported by the following research grants: Fund for Scientific Research-Flanders (G.0155.01); NOI-BOF (to D.A.) and BOF-RUCA Small Project (KP02 to I.B.) from the University of Antwerp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brouns, I., Pintelon, I., Van Genechten, J. et al. Vesicular glutamate transporter 2 is expressed in different nerve fibre populations that selectively contact pulmonary neuroepithelial bodies. Histochem Cell Biol 121, 1–12 (2004). https://doi.org/10.1007/s00418-003-0609-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0609-1