Abstract
Chemokines are important mediators of chemotaxis, cell adherence, and proliferation and exert specific functions in bone remodeling. Despite the potential intriguing role of chemokines in the regulation of osteoclast (OC) functions, little is known about the expression of chemokines and their receptors in human OCs at different stages of differentiation. Therefore, we analyzed the expression of CXC chemokine receptors (CXCR1, CXCR2, CXCR3, CXCR4 and CXCR5) and ligands (CXCL8, CXCL10, CXCL12 and CXCL13) both at molecular and protein levels, in human OCs grown on plastic or calcium phosphate-coated slides at different stages of differentiation. Real-time PCR showed that CXCR1, CXCR2, CXCR3, CXCR4, CXCR5 and CXCL8 were expressed in undifferentiated cells and significantly decreased during OC differentiation. By contrast, CXCL10 and CXCL12 were strongly upregulated from day 0 to day 8 in cells grown on calcium phosphate-coated slides. Immunocytochemistry showed that OCs grown on plastic expressed CXCR3, CXCR4, CXCR5, CXCL8 and CXCL12, while they were negative for CXCR1, CXCR2 and CXCL10. Interestingly, both at molecular and protein levels CXCL10 and CXCL12 significantly increased only when cells were differentiated on calcium phosphate-coated slides. These data suggest that the selection of a substrate that better mimics the tridimensional structure of bone tissue, thus favoring OC maturation and differentiation, may be necessary when studying osteoclastogenesis in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is a highly metabolically active tissue where the remodeling process is an active coupling of bone formation and resorption, mainly due to two different cell types: osteoblasts, also called bone-forming cells (Ducy et al. 2000), and osteoclasts (OCs) (Teitelbaum 2000), or bone-resorbing cells. These cells are responsible for the maintenance of bone tissue homeostasis through cell–cell contact or production of soluble factors. OCs are multinucleated cells formed by the fusion of mononuclear progenitors of the monocyte–macrophage cell lineage. Osteoclastogenesis is promoted by two molecules: the macrophage–colony-stimulating factor (M-CSF) and the receptor for activation of nuclear factor kappa B ligand (RANKL), which is expressed by osteoblastic and bone marrow stromal cells and is essential for macrophage maturation (Takahashi et al. 2002). Bone resorption is a multistep process initiated by the proliferation of immature OC precursors, the commitment of these cells to the OC phenotype, and, finally, the degradation of bone by mature OCs (Väänänen and Zhao 2002).
Some reports (Delima et al. 2002; Graves et al. 2002; Volejnikova et al. 2002) and our recent findings (Lisignoli et al. 1999, 2000, 2002) have demonstrated high levels of cytokines and chemokines in inflamed bone tissue suggesting a specific role in the pathophysiology of bone tissue remodeling. Moreover, it has been reported that some chemokines, such as CCL3α, are capable of inducing an increase in osteoclastogenesis so favoring the progression of bone erosion (Scheven et al. 1999; Han et al. 2001; Lean et al. 2002). Chemokines are a family of small peptides that regulate differentiation, proliferation, morphology, and cell movement as well as other activities. Based on the position of the first two cysteine residues and the chromosomal location of the corresponding gene, two main chemokine families have been identified: CXC and CC (Baggiolini 2001; Zlotnik and Yoshie 2001). Chemokines act on different cell types interacting with seven transmembrane-domain glycoprotein receptors coupled to G protein signaling pathway (Baggiolini 2001; Mackay 2001; Zlotnik and Yoshie 2001). CXC chemokines mainly studied are: CXCL8 (also called IL8), CXCL10 (also called IFNγ-inducible protein 10, IP-10), CXCL12 (also called stromal-derived factor 1, SDF-1), and CXCL13 (also called B cell attracting, BCA-1). CXCL8 binds two different receptors CXCR1 and CXCR2, while CXCL10, CXCL12, and CXCL13 bind only one receptor: CXCR3, CXCR4 and CXCR5, respectively. The CXC chemokines are a unique family of cytokines that regulate angiogenesis (Belperio et al. 2000) since the NH2 terminus of the majority of CXC chemokines contains a three amino acid motif (Glu-Leu-Arg: the ELR motif) that is a potent promoter of angiogenesis. Moreover, CXC chemokines are characteristically heparin-binding proteins.
Despite OCs exerting specific functions in the bone tissue remodeling, little is known about the expression of chemokines and their receptors on human OCs at different stages of differentiation, probably due to the difficulty of obtaining in vitro a sufficiently pure population. Moreover, it is well known that the nature of the substrate on which cells are grown can modulate the expression of different factors (Fuller and Chambers 1989; Hentunen et al. 1994; Fuller et al. 2000). OC differentiation differs at least quantitatively on bone versus plastic cultures and also a recent report showed that mice OCs on calcium phosphate-coated slides express high levels of chemokines and their receptors (Lean et al. 2002). Therefore, we analyzed the expression of some CXC receptors/ligands (CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCL8, CXCL10, CXCL12 and CXCL13) in human OCs at different stages of differentiation. Human OCs were grown both on plastic and calcium phosphate-coated slides and we found a significant upregulation, both at molecular and protein levels, of CXCL10 and CXCL12 only when OCs were differentiated on calcium phosphate-coated slides.
Materials and methods
Osteoclast culture
Human OCs were isolated from buffy coats obtained from healthy donors (total ten: seven male, three female; mean age: 44 years). Peripheral blood mononuclear cells were separated by gradient centrifugation on Histopaque 1077 (Sigma, St. Louis, MO, USA). Cells were recovered, washed twice, and CD11b-positive cells were then purified by immunomagnetic labeling using the Midi MACS cell separation system (Miltenyi Biothec, Bergisch Gladbach, Germany).
Cells were resuspended in minimum essential medium alpha (Invitrogen, San Giuliano Milanese, Italy) supplemented with 10% FBS (Invitrogen), l-glutamine, penicillin/streptomycin (Sigma; complete medium) and were seeded in six-well plates. Cells were left to adhere for 1 h at 37°C, and then the supernatant was removed and complete medium with RANKL (100 ng/ml; Peprothec, Rocky Hill, NJ, USA) and M-CSF(10 ng/ml; R&D, Minneapolis, MN, USA) was added. Every 3 days medium was changed.
Osteoclast phenotypical characterization
Cells, 4×105/cm2, were seeded both on 24-well plastic plates and on calcium phosphate-coated slides (BD Biosciences, Bedford, MA, USA). After 8 days the cells were fixed in ethanol/acetone (1:1) and stained for tartrate-resistant acid phosphatase (TRAP) using a commercial kit (Sigma).
For F-actin ring analysis, cells were fixed with 3.7% paraformaldehyde (PFA) in PBS, pH 7.4, containing 3% sucrose for 10 min at room temperature. OCs were then washed three times with PBS, permeabilized with 1% Triton X-100 for 5 min, and incubated with FITC-phalloidin for 30 min. OCs were analyzed using an inverted brightfield microscope for TRAP or a fluorescence microscope for F-actin ring analysis.
Assessment of bone resorption
Cells, 4×105/cm2, were seeded on calcium phosphate-coated slides and after 4 and 8 days of culture washed twice with PBS and immersed in 6% sodium hypochlorite for 5 min. After air-drying the pits of the calcium phosphate-coated slides were examined by reflected light microscopy.
RNA extraction and real-time PCR
Purified CD11b-positive cells (4×105/cm2) were seeded either on plastic wells (in 24-well plates) or on calcium phosphate-coated slides (in 24-well plates). Total cellular RNA was isolated both from CD11b-positive cell suspensions (day 0) and from cell cultures (day 4 and day 8) using the RNeasy Mini kit (Qiagen, Milan, Italy) according to the manufacturer’s instructions, and treated with DNase I (DNA-free kit; Ambion, Austin, TX, USA) to prevent amplification of genomic DNA. Total RNA (1 μg) was reverse transcribed in a 50-μl reaction using MMLV reverse transcriptase and random hexamers, following the manufacturer’s protocol (Perkin Elmer, Norwalk, CT, USA). One microliter of cDNA (corresponding to 20 ng total RNA) was used for real-time PCR. Primers for PCR amplification were those described in the literature [glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and CXCR4; Blanco et al. 1995; Aiuti et al. 1999] or generated from GenBank sequences using Primer 3 software (Rozen and Skaletsky 2000) or the LightCycler Probe Design software (Roche Molecular Biochemicals, Mannheim, Germany; Table 1). BLASTN searches were conducted on all primer nucleotide sequences to ensure gene specificity. The housekeeping gene GAPDH was used as an endogenous control to normalize for intersample variations in the amount of total RNA.
Real-time PCR was run in a LightCycler instrument (Roche Molecular Biochemicals) using the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) with the following protocol: initial activation of HotStar Taq DNA polymerase at 95°C for 15 min, 40 cycles of 94°C for 15 s, 58°C for 20 s, and 72°C for 12 s. The increase in PCR product was monitored for each amplification cycle by measuring the increase in fluorescence caused by the binding of SYBR Green I dye to dsDNA. The crossing point values (i.e., the cycle number at which the detected fluorescence exceeds the threshold value) were determined for each sample and specificity of the amplicons was confirmed by melting curve analysis and agarose gel electrophoresis. For each target gene, mRNA levels were normalized to GAPDH and expressed as fold change values relative to undifferentiated CD11b-positive cells.
Immunocytochemistry for chemokines and their receptors
Cells were plated in eight-well chamber slides at a density of 4×105/cm2. After 8 days, the chamber slides were washed twice with PBS and fixed in 4% PFA for 30 min. Slides were incubated with 3% hydrogen peroxide solution at room temperature for 30 min followed by 30 min with normal goat serum and human immunoglobulin (3 mg/ml) to block both endogenous peroxidase and non-specific staining.
Cells were then incubated at room temperature for 1 h with 10 μg/ml of the following MoAb: anti-human-CXCR1, -CXCR2, -CXCR4, -CXCL8, -CXCL10 (all from Pharmingen, San Diego, CA, USA), -CXCR3, -CXCR5, -CXCL12, and -CXCL13 (all from R&D Systems, Minneapolis, MN, USA). Slides were washed twice with TRIS-buffered saline (TBS) pH 7.2 and then incubated with undiluted affinity-purified goat anti-mouse immunoglobulins conjugated with peroxide-labeled polymer (Envision kit; Dako, Glostrup, Denmark) at room temperature for 30 min. A peroxide reaction using diaminobenzidine (Sigma) as a substrate was performed. Negative staining control experiments were performed either by omitting the primary antibody or by using a control isotype-matched antibody. Slides were counterstained with hematoxylin, dehydrated, mounted with Permount, and evaluated in a brightfield microscope (Axiophot; Zeiss, Milan, Italy). For intracellular chemokine detection, 0.1% saponin was added to the washing buffer. For immunodetection of CXCL10 and CXCL12 we used as secondary antibody the affinity-purified goat anti-mouse immunoglobulins conjugated with alkaline phosphatase-labeled polymer (Envision kit; Dako) and we developed the immune reaction using new fuchsin as substrate.
Immunocytochemistry of CXCL10 and CXCL12 of OCs grown on calcium phosphate-coated slides
Since real-time PCR showed that CXCL10 and CXCL12 mRNA were differently expressed by OCs when grown on plastic or calcium phosphate-coated slides, we performed immunocytochemistry for these two chemokines also on calcium phosphate-coated slides. In particular, 4×105 cells/cm2 were seeded on calcium phosphate-coated slides and immunocytochemistry was performed after 8 days as described above.
Immunochemical analysis of CXCL10 and CXCL12 on OCs in bone biopsies
To confirm in an “ex vivo” model that OCs in bone resorbing lacunae were positive to CXCL10 and CXCL12, we analyzed trabecular bone tissue biopsies from three rheumatoid arthritis patients. Biopsies were immediately fixed in a freshly prepared 9:1 mixture of B5 solution (mercuric chloride-containing fixative)/40%formaldehyde at room temperature for 2 h and decalcified, as previously reported (Lisignoli et al. 2000). Briefly, slides were incubated with monoclonal anti-human CXCL10 (1:25; BD Biosciences Pharmingen) or anti-CXCL12 (R&D Systems) diluted in TBS containing 0.25% BSA, 0.1% NaN3, and 1.5% normal rabbit serum at room temperature for 1 h. Then, slides were washed twice with TBS 0.04 M pH 7.6 containing 0.1% saponin, incubated with undiluted affinity-purified goat anti-mouse immunoglobulins conjugated with peroxide-labeled polymer (Envision kit; Dako) at room temperature for 30 min, and developed as described above.
Statistical analysis
The Mann-Whitney test was used to compare chemokine receptors and ligands expression at day 0 versus day 4 and day 8 of OCs grown on plastic or calcium phosphate-coated slides. Moreover, we compared at day 4 or day 8, OCs grown on plastic versus calcium phosphate-coated slides.
Results
Osteoclast characterization
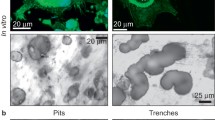
Osteoclasts grown both on plastic and calcium phosphate-coated slides were analyzed for TRAP staining and actin ring. As shown in Fig. 1a, b, mature OCs were highly positive to TRAP when grown both on plastic and calcium phosphate-coated slides. Interestingly, after 8 days, OCs grown on calcium phosphate-coated slides showed an increased number of nuclei compared to OCs grown on plastic. OCs grown on plastic or calcium phosphate-coated slides formed F-actin rings, a specific OC structure involved in sealing the OCs to mineralized matrix, in both culture conditions tested (Fig. 1c, d). Pit resorption assay clearly demonstrated that isolated OCs were functionally active. In particular, as shown in Fig. 1e, f, the number of pits formed by mature OCs significantly increased from day 4 to day 8.
Phenotypical characterization at day 8 of osteoclasts (OCs) grown on plastic (a, c) and calcium phosphate-coated slides (b, d) by TRAP staining (a, b) and actin labeling by FITC-phalloidin (c, d). Magnification ×250. Pit resorption assay of OCs differentiated on calcium phosphate-coated slides at day 4 (e) and day 8 (f). Magnification ×125
CXC chemokine ligand/receptor expression
mRNA expression
The modulation of the gene expression of four CXC chemokines and five CXC receptors was monitored during osteoclastic differentiation of CD11b-positive cells obtained from five different donors and cultured on plastic wells or calcium phosphate-coated slides. Real-time PCR was used to determine the relative mRNA expression in cultured cells compared with undifferentiated CD11b-positive cells and to evaluate differences between the two culture conditions.
For CXC receptors we found a general decrease in transcript abundance from starting CD11b-positive cells (day 0) to day 4 and day 8 cultured cells (Fig. 2). CXCR1, after the initial drop from day 0 values (P<0.01), did not show significant differences between day 4 and day 8 mRNA expression. For CXCR2, CXCR3, and CXCR5 the lowest mRNA expression was found on day 8 both for cells grown on plastic and for cells grown on calcium phosphate-coated slides. An opposite trend was observed for CXCR4 mRNA, with the lowest values on day 4 and higher values on day 8 in both conditions tested. For CXC receptor mRNAs no significant differences were found between plastic and calcium phosphate-cultured cells, except for CXCR5 on day 4 that was higher for plastic than for calcium phosphate (P<0.01).
Real-time PCR analysis of CXCR1, CXCR2, CXCR3, CXCR4 and CXCR5 mRNA expression in differentiating OCs cultured for 4 and 8 days on plastic and calcium phosphate-coated slides (Bone slides) on day 4 and day 8. Data were normalized to GAPDH and expressed as fold difference values relative to undifferentiated CD11b-positive cells (day 0). Asterisks P<0.01 day 0 versus day 4 or day 8 of OCs grown on plastic or calcium phosphate-coated slides; solid diamonds P<0.01 day 4 or day 8 of OCs grown on plastic versus calcium phosphate-coated slides
CXC chemokine mRNAs were also significantly modulated during differentiation (Fig. 3). For CXCL8 mRNA we found a significant decrease, both in plastic and calcium phosphate-cultured cells (P<0.01), from day 0 to day 8. On the contrary, CXCL10 and CXCL12 transcripts were strongly upregulated from day 0 to day 4 (approximately 1.5-fold and fourfold increase, respectively) and day 8 (approximately fourfold and sevenfold increase, respectively). Interestingly, this positive trend was found only in cells grown on calcium phosphate-coated slides (P<0.01 at day 4 and P<0.001 at day 8), while mRNA from plastic cultures was either strongly decreased (CXCL10) or unvaried (CXCL12). CXCL13 mRNA was detected only at trace levels in a few samples (data not shown).
Real-time PCR analysis of CXCL8, CXCL10 and CXCL12 mRNA expression in differentiating OCs cultured for 4 and 8 days on plastic and calcium phosphate-coated slides (Bone slides) on day 4 and day 8. Data were normalized to GAPDH and expressed as fold difference values relative to undifferentiated CD11b-positive cells (day 0). Asterisks P<0.01, solid squares P<0.001 day 0 versus day 4 or day 8 of OCs grown on plastic or calcium phosphate-coated slides; solid diamonds P<0.01 day 4 or day 8 of OCs grown on plastic versus calcium phosphate-coated slides
Immunocytochemical analysis
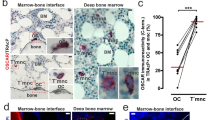
The data obtained by real-time PCR prompted us to investigate the CXC chemokine ligands and receptors at the protein level. As shown in Figs. 4 and 5, cells grown for 8 days on plastic were negative for CXCR1, CXCR2, CXCL10, and CXCL13 and positive for CXCR3, CXCR4, CXCR5, CXCL8, and CXCL12. We observed that less differentiated OCs (with at least three nuclei) or mature OCs (with more than five nuclei) were both positive to these chemokine receptors or ligands. Since CXCL10 and CXCL12 expression was significantly different between plastic and calcium phosphate-coated slides at the molecular level, we also performed the immunocytochemical analysis for these two chemokines on calcium phosphate-differentiated OCs. As shown in Fig. 5, CXCL12 was more highly expressed at the protein level in OCs grown on calcium phosphate-coated slides compared to plastic. Interestingly, CXCL10 immunostaining that was negative when the cells were grown on plastic, was positive on mature OCs grown on calcium phosphate-coated slides (Fig. 5).
Immunocytochemical analysis of CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCL8 and CXCL13 on OCs grown on plastic for 8 days. Magnification ×125. Negative control (−Control). Short arrows were use to indicate less differentiated OCs (with at least three nuclei), long arrows were used for mature OCs (with more than five nuclei)
CXCL10 expression on OCs in bone tissue biopsies
To confirm the relevance of CXCL10 and CXCL12 positivity in OCs grown on calcium phosphate-coated slides, we analyzed OCs in bone tissue biopsies of rheumatoid arthritis patients. As shown in Fig. 6, mature OCs present in resorbing bone lacunae were highly positive to CXCL10 and CXCL12, as found in OCs grown on calcium phosphate-coated slides.
Discussion
Chemokines are important inflammatory mediators that regulate different cell activities (Baggiolini 2001; Mackay 2001; Zlotnik and Yoshie 2001). In inflamed bone tissue chemokines are highly expressed by different cell types and exert specific functions on bone tissue remodeling (Lisignoli et al. 1999, 2000, 2002; Delima et al. 2002; Graves et al. 2002; Volejnikova et al. 2002). OCs are cells derived from the monocytic cell lineage that resorb the bone tissue when it reaches maturation. Chemokines are implicated in different activities, like chemotaxis, cell adhesion, and expression of proteolytic enzymes, that are important for OCs to exert their functions. In fact, some reports already demonstrated a direct role for some chemokines, like CCL3 in inducing osteoclastogenesis (Scheven et al. 1999; Han et al. 2001) or CCL9 in stimulating cytoplasmic motility and polarization (Lean et al. 2002). Despite the potential intriguing role of chemokines in the regulation of OC function, few data are available on chemokine expression by human OCs at different stages of differentiation.
In this study we investigated in vitro the expression of chemokines and receptors of the CXC family in human OCs obtained by CD11b-positive cells, a subpopulation of human peripheral blood mononuclear cells which is specifically upregulated under some pathological conditions involving an increased OC function (i.e., rheumatoid arthritis; Nielsen et al. 1999). Furthermore, OCs were grown onto two different substrates, plastic and calcium phosphate-coated slides, since studies from different authors have revealed different patterns of protein expression under these conditions (Fuller et al. 2000; Lean et al. 2002). Our study demonstrates that human OCs express different CXC chemokine receptors and ligands, during differentiation, when grown on plastic or calcium phosphate-coated slides. In particular, at the mRNA level we found that undifferentiated CD11b-positive cells express CXCR1, CXCR2, CXCR3, CXCR4 and CXCR5 and these receptors are downregulated during OC differentiation from day 0 to day 8. The same trend was also observed for CXCL8, while CXCL10 and CXCL12 significantly increased from day 0 to day 8 only when CD11b-positive cells were differentiated on calcium phosphate-coated slides. Analysis performed at the protein level on OCs grown on plastic demonstrated a positive expression only for CXCR3, CXCR4, CXCR5, CXCL8 and CXCL12. An upregulated expression of CXCL10 and CXCL12 was found by immunocytochemistry when cells where differentiated on calcium phosphate-coated slides.
We are the first to demonstrate a distinct chemokine receptor/ligand expression pattern on human OCs, thus confirming that in vitro analysis of OCs requires the right selection of the substrate for growing cells. Calcium phosphate synthetic material seems to be the better substrate for OC in vitro analysis, since the mechanism responsible for bone turnover is better mimicked. Immunostaining of CXCL10 and CXCL12 performed in our study confirms that this pattern of expression is closer to the in vivo expression by OCs. In fact, immunostaining of specimens obtained from rheumatoid arthritis patients revealed high expression levels for both CXCL10 and CXCL12 in multinucleated OCs present in areas of bone resorption.
These differences in chemokine receptors/ligands by OCs grown on plastic or calcium phosphate synthetic material, may be explained, according to findings obtained by others (Leeuwenburgh et al. 2001; Heymann et al. 2001; Monchau et al. 2002), by the following evidence: (1) a better adherence of OCs to calcium phosphate-coated slides than to plastic, (2) a number of OCs formed on bone substantially greater than on plastic, (3) a better development of typical bone OC features, such as polarized dome shape and a ruffled border, in the presence of calcium phosphate, and (4) the ability of OCs to simultaneously resorb and phagocytose the synthetic calcium phosphate matrix. Mature OCs need these conditions to perform their natural functions in the bone. The demonstration that the expression of CXCL10 and CXCL12 by mature human OCs is dependent on the growing substrate chosen clearly suggests that for studying in vitro OC generation and function it is important to establish culture conditions (such as the use of mineralized substrate) that mimic the bone microenvironment. The different expression of chemokine receptors and ligands between undifferentiated and mature OCs suggests a specific response of the cells to the same chemokine stimulation and contributes to better define the true phenotypic features of human OCs. Analysis of chemokine expression might be included in the phenotypic characterization of mature OCs and, among them, CXCL10 and CXCL12 should be clearly expressed by true OCs, along with the typical markers of differentiation (TRAP, calcitonin receptor, F-actin ring). Moreover, our data on chemokine production by OCs suggests that these cells not only represent a target for chemokine stimulation, but may actively take part in the process of recruitment and activation of immune cells in the bone microenvironment.
During OC differentiation, undifferentiated CD11b-positive cells lose the expression of different CXC receptors, leading to a reduced repertoire of responsiveness by mature OCs to inflammatory chemokines. Loss of expression of chemokine receptors is consistent with the observation (Sauty et al. 2001) that they are often downregulated after mononuclear cells have passed through the endothelial cells and have been committed to a more differentiated phenotype in the inflamed tissue. Mature OCs expressed CXCL8 but not its receptors CXCR1 and CXCR2, while only few samples expressed traces of CXCL13 but were highly positive to its receptor CXCR5. By contrast, the differentiation process leads to a significant increase of expression of CXCL10 and CXCL12, two chemokines that exert a specific role during inflammation, as well as of their specific receptors CXCR3 and CXCR4 suggesting a differential autocrine/paracrine mechanism of action of these factors during the process of differentiation. In fact, recent findings from our group (Grassi et al. 2003) and others (Yu et al. 2003) demonstrate a role for the chemokine CXCL12 in recruitment of OC precursors, stimulation of MMP-9 production by these cells, and increasing OC ability to resorb the bone matrix. Moreover, the expression of CXCL10 in bone tissue biopsies and its important biological function on human osteoblasts (Lisignoli et al. 2003a) and on the recruitment and proliferation of T lymphocytes has been described (Lisignoli et al. 2003b).
In conclusion, our findings demonstrate a differential expression of CXC chemokine receptors and ligands during OC maturation. Our data provide new insight into the understanding of OC development and homing, as well as the methods used to differentiate in vitro OCs from precursor cells, and support the hypothesis of a role for chemokines in the regulation of OC function.
References
Aiuti A, Turchetto L, Cota M, Cipponi A, Brambilla A, Arcelloni C, Paroni R, Vicenzi Bordigon C, Poli G (1999) Human CD34(+) cells express CXCR4 and its ligand stromal cell-derived factor-1. Implications for infection by T-cell tropic human immunodeficiency virus. Blood 94:62–73
Baggiolini M (2001) Chemokines in pathology and medicine. J Intern Med 250:91–104
Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM (2000) CXC chemokines in angiogenesis. J Leukoc Biol 68:1–8
Blanco FJ, Geng Y, Lotz M (1995) Differentiation-dependent effects of IL-1 and TGF-beta on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J Immunol 154:4018–4026
Delima AJ, Karatzas S, Amar S, Graves DT (2002) Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J Infect Dis 186:511–516
Ducy P, Schinke T, Karsenty G (2000) The osteoblast: a sophisticated fibroblast under central surveillance. Science 289:1501–1504
Fuller K, Chambers TJ (1989) Bone matrix stimulates osteoclastic differentiation in cultures of rabbit bone marrow cells. J Bone Miner Res 4:179–183
Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ (2000) A role for TGF-β1, in osteoclast differentiation and survival. J Cell Sci 113:2445–2453
Grassi F, Cristino S, Toneguzzi S, Piancentini A, Facchini A, Lisignoli G (2003) CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J Cell Physiol (in press)
Graves DT, Alsulaimani F, Ding Y, Marks SC Jr (2002) Developmentally regulated monocyte recruitment and bone resorption are modulated by functional deletion of the monocytic chemoattractant protein-1 gene. Bone 31:282–287
Han J-H, Chol SJ, Kurihara N, Koide M, Oba Y, Roodman GD (2001) Macrophage inflammatory protein-1α is an osteoclastogenic factor in myeloma that is independent of receptor activity of nuclear factor kB ligand. Blood 97:3349–3353
Hentunen TA, Cunningham NS, Vuolteenaho O, Reddi AH, Väänänen HK (1994) Osteoclast recruiting activity in bone matrix. Bone Miner 25:183–198
Heymann D, Guicheux J, Rousselle AV (2001) Ultrastructural evidence in vitro of osteoclast-induced degradation of calcium phosphate ceramic by simultaneous resorption and phagocytosis mechanisms. Histol Histopathol 16:37–44
Lean JM, Murphy C, Fuller K, Chambers TJ (2002) CCL9/MIP-1 γ and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem 87:386–393
Leeuwenburgh S, Layrolle P, Barrere F, de Bruijn J, Schoonman J, van Blitterswijk CA, de Groot K (2001) Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro. J Biomed Mater Res 56:208–215
Lisignoli G, Toneguzzi S, Pozzi C, Piacentini A, Riccio M, Ferruzzi A, Gualtieri G, Facchini A (1999) Proinflammatory cytokines and chemokine production and expression by human osteoblasts isolated from patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 26:791–799
Lisignoli G, Piacentini A, Toneguzzi S, Grassi F, Cocchini B, Ferruzzi A, Gualtieri G, Facchini A (2000) Osteoblasts and stromal cells isolated from femora in rheumatoid arthritis (RA) and osteoarthritis (OA) patients express IL-11, leukaemia inhibitory factor and oncostatin M. Clin Exp Immunol 119:346–353
Lisignoli G, Toneguzzi S, Grassi F, Piacentini A, Tschon M, Cristino S, Gualtieri G, Facchini A (2002) Different chemokines are expressed in human arthritic bone biopsies: IFN-gamma and IL-6 differently modulate IL-8, MCP-1 and RANTES production by arthritic osteoblasts. Cytokine 20:213–238
Lisignoli G, Toneguzzi S, Piacentini A, Cattini L, Lenti A, Tschon M, Cristino S, Grassi F, Facchini A (2003a) Human osteoblasts express functional CXC chemokine receptors 3 and 5: activation by their ligands, CXCL10 and CXCL13, significantly induces alkaline phosphatase and beta N-acetylhexosaminidase release. J Cell Physiol 194:71–79
Lisignoli G, Toneguzzi S, Piacentini A, Cristino S, Cattini L, Grassi F, Facchini A (2003b) Recruitment and proliferation of T lymphocytes is supported by IFNγ- and TNFα-activated human osteoblasts: involvement of CD54 (ICAM-1) and CD106 (VCAM-1) adhesion molecules and CXCR3 chemokine receptor. J Cell Physiol (in press)
Mackay CR (2001) Chemokines: immunology’s high impact factors. Nat Immunol 2:95–101
Monchau F, Lefevre A, Descamps M, Belquin-Myrdycz A, Laffargue P, Hildebrand HF (2002) In vitro studies of human and rat osteoclast activity on hydroxyapatite, beta-tricalcium phosphate, calcium carbonate. Biomol Eng 19:143–152
Nielsen H, Petersen AA, Skjodt H, Horslev-Petersen K, Bendtzen K (1999) Blood levels of CD11b+ memory T lymphocytes are selectively upregulated in patients with active rheumatoid arthritis. APMIS 107:1124–1130
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana, Totowa, NJ, pp 365–386
Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD (2001) CXCR3 internalization following T cell–endothelial cell contact: preferential role of IFN-inducible T cell α chemoattractant (CXCL11). J Immunol 167:7084–7093
Scheven BAA, Milne JS, Hunter I, Robins SP (1999) Macrophage-inflammatory protein-1α regulates preosteoclast differentiation in vitro. Biochem Biophys Res Commun 254:773–778
Takahashi N, Udagawa N, Takami M, Suda T (2002) Cells of bone. Osteoclast generation. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology, vol 1. Academic, San Diego, pp 109–126
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Väänänen K, Zhao H (2002) Osteoclast function. Biology and mechanisms. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology, vol 1. Academic, San Diego, pp 127–139
Volejnikova S, Marks SCJr, Graves DT (2002) Tumor necrosis factor modulates apoptosis of monocytes in areas of developmentally regulated bone remodeling. J Bone Miner Res 17:991–997
Yu X, Huang Y, Collin-Osdoby P, Osdoby P (2003) Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res 18:1404–1418
Zlotnik A, Yoshie O (2001) Chemokines: a new classification system and their role in immunity. Immunity 12:121–127
Acknowledgements
This work was partially supported by grants from Istituti Ortopedici Rizzoli, Bologna, Italy, and MIUR, Università degli Studi di Bologna, Italy. The authors wish to thank Mrs. Patrizia Rappini and Graziella Salmi for the assistance in the preparation of the manuscript, Mr. Keith Smith for editing, and Mr. Luciano Pizzi for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grassi, F., Piacentini, A., Cristino, S. et al. Human osteoclasts express different CXC chemokines depending on cell culture substrate: molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem Cell Biol 120, 391–400 (2003). https://doi.org/10.1007/s00418-003-0587-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0587-3