Abstract
The glutamate transporter GLT1 is essential in limiting transmitter signaling and restricting harmful receptor overstimulation. It has been shown recently that GLT1 exists in two forms, the generic GLT1 and a 3′-end-spliced variant of GLT1 (GLT1v), both with similar transport characteristics. To differentiate clearly the cellular distribution of both GLT1 forms in the cortex, specific cRNA probes for non-radioactive in situ hybridization were generated and applied to adult rat brain sections. The results were complemented by western and northern blot analyses and by immunocytochemical investigations using specific peptide antibodies against both GLT1 forms. The study confirmed that generic GLT1 mRNA was expressed predominantly in astrocytes and, to a small extent, in neurons, whereas GLT1 protein was detected only in cell membranes of astrocytes. On the other hand, GLT1v mRNA and protein were demonstrated predominantly in neurons and in non-astrocytic glial cells irrespective of the cortical areas studied. A cytoplasmic granular staining of neurons and astrocytes predominated in the demonstration of GLT1v protein. It is concluded that the cellular expression of the two GLT1 forms is complementary. The cytoplasmic vesicular distribution of GLT1v may represent an endogenous protective mechanism to limit glutamate-induced excitotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the CNS the high-affinity glutamate transporter GLT1 (EAAT2) seems to be very important for the elimination of glutamate from the extracellular space, which is the crucial step in terminating receptor activation and thus in protecting neurons from glutamate excitotoxicity (for review see Danbolt 2001). The importance of GLT1 is underlined by studies on mice lacking GLT1. These mice show elevated brain glutamate levels, lethal spontaneous seizures, and selective neuronal degeneration (Tanaka et al. 1997).
The findings concerning the cellular expression of GLT1 are enigmatic. Both GLT1 mRNA (Torp et al. 1994, 1997; Schmitt et al. 1996; Berger and Hediger 1998) and GLT1 protein (Danbolt et al. 1992; Levy et al. 1993; Rothstein et al. 1994; Lehre et al. 1995; Schmitt et al. 1996; Milton et al. 1997) are shown to be localized in astrocytes. Additionally, GLT1 mRNA has been demonstrated by in situ hybridization (ISH) in subsets of neurons in various brain regions (Torp et al. 1994, 1997; Schmitt et al. 1996; Berger and Hediger 1998), whereas so far GLT1 protein has not been detected in neurons of the adult brain (Danbolt et al. 1992; Levy et al. 1993; Rothstein et al. 1994; Lehre et al. 1995; Milton et al. 1997).
In a previous study we were able to clone the cDNA of a variant of GLT1 (GLT1v) from rat brain, which is generated by alternative splicing at the 3′-end of the GLT1 cDNA. In contrast to generic GLT1, GLT1v mRNA and protein are preferentially expressed in neurons of the CNS and PNS (Schmitt et al. 2002). This finding may offer an explanation for the controversy concerning GLT1 localization in neurons. The spliced 3′-end of GLT1v is very short and for this reason ISH using large cRNA probes, which contain long sequences complementary to homologous parts of generic GLT1 and GLT1v mRNA (cf. literature cited above), may detect both GLT mRNA forms. Therefore, it could be supposed that GLT1 mRNA detected in neurons in previous investigations may alternatively be GLT1v mRNA. In a recent study (Chen et al. 2002) the existence of the 3′-end-spliced GLT1 variant (designated in this study as GLT1b) in rat brain was confirmed by immunocytochemistry and it was shown that the properties of glutamate transport mediated by both GLT1 forms expressed in COS-7 cells and in neuronal cultures are indistinguishable.
In the present study, we addressed the question of GLT1 mRNA localization in neurons using non-radioactive ISH with a generic GLT1-specific cRNA probe. For this purpose it was necessary to clone the whole (non-translated) GLT1-3′-cDNA (not described in the original sequence analysis of Pines et al. 1992) and furthermore to generate a cRNA probe, which does not additionally detect GLT1v mRNA. With these probes, ISH was carried out in various cortical areas of the adult rat brain (hippocampus and entorhinal, parietal, temporal, and perirhinal cortex). The investigations were complemented by immunocytochemistry for GLT1 and GLT1v proteins using generic GLT1 (Schmitt et al. 1996)- and GLT1v (Schmitt et al. 2002)-specific peptide antibodies.
Materials and methods
Animals and tissue sources
The brains of 20 male Wistar rats (purchased from Charles River, Sulzfeld, Germany; aged 8–12 weeks) were used for RNA preparation, northern blotting, immunoblotting, and immunocytochemistry on plastic embedded material, as described below. Additionally, frontal and horizontal blocks of brains (10 rats) of between 5 and 10 mm thickness were used for ISH and immunohistochemistry on cryostat sections.
Cloning of cDNA encoding generic GLT1 3′-end
If not indicated otherwise, all standard procedures were performed as described by Sambrook et al. (1989) and Schmitt et al. (1996) and will be mentioned here only in brief. Total RNA from the rat brain was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction (Chomczynski and Sacchi 1987). First a 3′-RACE was carried out for cloning of the untranslated 3′-region of GLT1. The first-strand synthesis of the cDNA was performed for 50 min at 54°C in a reaction volume of 20 μl containing 5 μg total RNA, 0.5 pM oligo(dT)17-adapter-primer (GACTCGAGTCGACATCGAT17), 1 mM of each dNTP, 40 U RNase OUT, 50 mM TRIS acetate (pH 8.4), 8 mM magnesium acetate, 75 mM potassium acetate, 5 mM dithiothreitol, and 15 U reverse transcriptase Thermoscript RT (Life Technologies, Karlsruhe, Germany). Two primers, GLT1b (Fig. 1a): 5′-CTCCCCAGCGTATCTTATG-3′ (complementary to nt 1753–1771 of the GLT1 sequence; Pines et al. 1992) and the adapter primer: 5′-GACTCGAGTCGACATCGA-3′ (see above) purchased from MWG Biotech, Ebersberg, Germany, were used to amplify a rat brain GLT1 cDNA fragment of the 3′-end.
Nucleotide (a) and deduced amino acid sequences (b) of the 3′-region of generic glutamate transporter GLT1 and a 3′-end-spliced variant of GLT1 (GLT1v) (italic letters). Vertical lines indicate identical nts or amino acids. From nt 1654 up to the 3′-end the generic GLT1 sequence differs from the GLT1v sequence. The primer (Pr) sequences used for cloning and cRNA generation (a) and peptide amino acid sequences used for immunization (Schmitt et al. 1996, 2002) (b) are shaded
Ten percent of the reverse transcription mixture was utilized for a polymerase chain reaction in a final volume of 50 μl containing 0.8 pM of each primer, 0.4 mM of each dNTP, 1 U Taq-Polymerase, 50 mM KCl, 5 mM MgCl2, 0.08% Nonidet P 40, and 10 mM TRIS-HCl (pH 8.8) (MBI Fermentas, St. Leon-Rot, Germany). For amplification the following profile was used: denaturation 40 s/94°C, annealing 2 min/52°C, extension 2 min 15 s/72°C, 40 cycles, and final elongation for 10 min at 72°C. The resulting cDNA was cloned into the pGEM-T vector (Promega, Madison, USA) and then transfected and propagated in JM109 competent cells (Promega). The sequence analysis (performed by MWG Biotech) of the cloned cDNA showed a complete sequence homology between nt 1753 and 1800 to the known generic GLT1 sequence (Fig. 1a; Pines et al. 1992) and, compared to GLT1v, a short 3′-untranslated region (nt 1719–1944).
Generation of a digoxigenin (DIG)-labeled cRNA probe
All procedures for the preparation of DIG-labeled cRNA probes were performed as described by Schmitt et al. (1996, 1997) and Kugler and Schmitt (1999). It will be described here only in brief. A generic GLT1 cDNA fragment (3′-end) contained in the pGEM-T vector (see above) was amplified using the PCR procedure mentioned above (cf. cloning of cDNA). The primers were GLT1a (Fig. 1a): 5′-AACTCTGGCGGCCAATG-3′ (complementary to nt 1653–1669 of the generic GLT1 sequence; Pines et al. 1992) and GLT1c (Fig. 1a): 5′-TGGCAAAGACACGGCACG-3′ (complementary to nt 1890–1907 of the cloned generic GLT1 sequence). The resulting cDNA was cloned into the pGEM-T vector, followed by transfection and propagation in JM109 competent cells (Promega). The identity of the cloned cDNA (GLT1 3′-end) was verified by restriction analysis and partial DNA sequencing (Sanger et al. 1977).
To produce a DIG-labeled antisense (sense) probe, plasmids were linearized by NcoI (NotI) restriction, phenol–chloroform extracted, precipitated, and transcribed by SP6 RNA polymerase (T7 RNA polymerase) according to the manufacturer's manual (Boehringer, Mannheim, Germany). Usually 1.2 μg cDNA template yielded 10–30 μg labeled cRNA, incorporating approximately one DIG-11-UTP at every 20th nucleotide. cRNA probes were analyzed on an agarose gel (1%). For comparison, a DIG-labeled GLT1v cRNA (complementary to nt 1654–2043 of the GLT1v sequence; antisense and sense probes; Schmitt et al. 2002) and a DIG-labeled GLT1 cRNA (complementary to nt -38–1776 of the GLT1 sequence; antisense and sense probes; Schmitt et al. 1996) were used.
Northern blotting
For northern blotting the whole brain, neocortex (including periarchicortex) and hippocampus were dissected and the total RNA of each preparation was isolated as described above. The northern blotting was performed essentially according to the instructions of Boehringer for non-radioactive hybridization (the DIG system user's guide for filter hybridization; Boehringer). Briefly, probes of RNA (50 μg) were separated in a standard formaldehyde agarose gel (1%) and transferred onto nylon membranes (positively charged; Boehringer). Prehybridization was performed using DIG Easy Hyb hybridization solution from Roche Diagnostics (Mannheim, Germany). Subsequently, the blots were placed in the prehybridization solution containing 200 ng/ml DIG-labeled antisense (sense) cRNA (see above) overnight at 62°C. The blots were washed in 2×SSC and 0.1% SDS at room temperature and afterwards in 0.5×SSC and 0.1% SDS at 62°C. After equilibration in washing buffer (0.1 M maleic acid, 0.15 M NaCl, 0.3% Tween 20, pH 7.5) at room temperature, the membranes were transferred into a blocking solution (0.1 M maleic acid, 0.15 M NaCl, 1% blocking reagent, pH 7.5) for at least 30 min. After incubation with sheep anti-DIG–peroxidase conjugated antibody (1:1,000; Boehringer) for 2 h the membranes were rinsed with washing buffer. Bound immunoglobulins were visualized using the enhanced luminol chemiluminescence technique (Amersham, Braunschweig, Germany).
In situ hybridization
Frontal and horizontal blocks of brains of between 5 and 10 mm thickness were frozen in liquid nitrogen-cooled isopentane. Fourteen-micrometer-thick frontal and horizontal cryostat sections were cut at approximate interaural levels 6.7–4.5 mm and 6.4–2.4 mm, respectively, according to the rat brain stereotaxic atlas of Paxinos and Watson (1986), then mounted on precoated glass slides (Superfrost Plus; Menzel, Braunschweig, Germany) and thawed. The sections were fixed for 5 min in freshly prepared 4% formaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Finally the sections were transferred to ethanol and stored at 4°C for 1–2 days.
The sections were removed from ethanol and subsequently rehydrated in a graded series of ethanol, transferred to 2×SSC and treated with 5 μg proteinase K in 10 mM TRIS-HCl buffer (pH 7.5) for 5 min. After washing with 2×SSC, the sections were incubated with freshly prepared 0.25% acidic anhydride in 0.1 M triethanolamine, washed again with 2×SSC, and covered with the hybridization solution containing the DIG-labeled antisense (sense) cRNA probes (see above) (final concentration 1 to 15 ng/μl) and 550 μg/ml salmon testes DNA (Sigma, Deisenhofen, Germany) in 4×SSC, 1×Denhardt's solution (Sambrook et al. 1989), 10% dextran sulfate, and 50% deionized formamide at 55°C or 60°C for 16–18 h. Posthybridization washes were done stepwise with 2×SSC (at room temperature), 50% formamide in 2×SSC (at 55°C or 58°C), and then again with 2×SSC (at room temperature). Next, the sections were treated with 40 μg/ml ribonuclease A (50 Kunitz units/mg; Boehringer) in a solution containing 500 mM NaCl, 10 mM TRIS-HCl (pH 8), and 1 mM EDTA at 37°C for 30 min to remove unhybridized single-strand RNAs. After this treatment, the sections were incubated with the same buffer without RNase A at 58°C or 60°C for 30 min.
Subsequently, the sections were rinsed in TRIS-buffered saline (TBS; 100 mM TRIS and 150 mM NaCl, pH 7.5) for 5 min, incubated with TBS containing 1% blocking reagent (DIG Nucleic Acid Detection kit; Boehringer; 30 min), followed by 0.3% Triton X-100 in TBS (15 min). After incubation with 1.5 U/ml sheep anti-DIG alkaline phosphatase (aP) conjugated (Boehringer) in TBS containing 0.3% Triton X-100 for 60 min, the sections were washed in TBS and transferred to a 0.1 M TRIS buffer containing 100 mM NaCl and 50 mM MgCl2 (pH 9.5) for 5 min prior to the aP visualization (see below).
In some experiments, following the aP visualization, several brain sections were used for the immunocytochemical detection of glial fibrillary acidic protein (GFAP, as specific marker protein of astrocytes; Bignami et al. 1972) and of neuron-specific nuclear protein (NeuN; Mullen et al. 1992). In the immunohistochemical demonstration of NeuN, ISH was performed with HCl instead of proteinase K for denaturation/degradation of proteins. For detection of GFAP the PAP method (peroxidase-antiperoxidase complex 1:100; mouse monoclonal antibody against GFAP 1:2,000; Dako, Hamburg, Germany; Sternberger et al. 1990) was applied. For the immunocytochemical detection of NeuN, a mouse monoclonal antibody against NeuN (1:50; Chemicon International, Hofheim, Germany), a biotinylated goat anti-mouse IgG as a secondary antibody (1:200; Dako), and avidin-biotinylated horseradish peroxidase (HRP) complex (Dako) were used. The histochemical reaction for HRP detection was carried out with 3-amino-9-ethylcarbazole (Sigma) as a chromogen, as well as glucose oxidase (Sigma) and β-d-glucose (Sigma) as described by Asan (1998).
Detection of alkaline phosphatase
The procedure used was described recently (Asan and Kugler 1995). The incubation medium contained 0.4 mM 5-bromo-4-chloro-3-indolylphosphate (Boehringer), 100 mM sodium chloride, 50 mM MgCl2, and 0.4 mM tetranitro blue tetrazolium chloride (Serva, Heidelberg, Germany) in 100 mM TRIS-HCl buffer at pH 9.5.
Controls for ISH
Substitution of the antisense cRNA probe by an equivalent amount of labeled sense cRNA probe led to a lack of staining (Fig. 4d). Omission of labeled cRNA probes from the respective hybridization mixtures resulted in completely unstained sections.
Antibodies and immunoblotting
Antibodies
Two antisera were used which we generated in rabbits against GLT1 peptides at the C-terminus as described in detail elsewhere. One antiserum was directed against the peptide AANGKSADCSVEEEPWKREK (amino acids 554–573 of the generic GLT1 protein; Schmitt et al. 1996), which is specific for generic GLT1 (Fig. 1b Peptide 1). The other antiserum was directed against the peptide KVPFPFLDIETCI (amino acids 550–562 of the GLT1v protein; Gen Bank accession AAK 98779), which is specific for GLT1v (Fig. 1b Peptide 2; Schmitt et al. 2002). The antisera were affinity purified using the synthetic peptides immobilized by transfer to nitrocellulose paper (Schleicher and Schüll, Darmstadt, Germany). The bound immunoglobulins were eluted by low pH (pH 2.8) or with PBS warmed up to 56°C, and the protein content was determined spectrophotometrically (Drenckhahn et al. 1993). Mouse monoclonal antibodies against GFAP and β-tubulin were purchased from Dako and Sigma, respectively. β-Tubulin is highly expressed in oligodendrocytes (as well as in neurons) (Schaeren-Wiemers et al. 1995).
Immunoblotting
For immunoblotting, whole brain, neocortex (including periarchicortex), and hippocampus were dissected and homogenized at 4°C in 10 mM NaH2PO4 (pH 7.2) containing 2 mM MgCl2, aprotinin (5 μg/ml), leupeptin (2 μg/ml), pepstatin (2 μg/ml), and phenylmethylsulfonyl fluoride (100 μg/ml). The homogenate was centrifuged at 1,000 g for 10 min and the 1,000 g supernatant was centrifuged at 100,000 g for 1 h. The protein contents of supernatants and pellets were determined by the BioRad protein assay (BioRad, Munich, Germany) and afterwards used for immunoblotting. Proteins (5–75 μg per lane) were electrophoretically separated on 10% gels by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, the proteins were transferred electrophoretically to nitrocellulose membranes (Burnette 1981). Strips of the nitrocellulose membranes were incubated for 24 h at 4°C with the affinity-purified GLT1 peptide antibodies (approximately 1.8 μg/ml). Bound immunoglobulins were visualized using peroxidase-conjugated goat anti-rabbit IgG (1:3,000; BioRad, Richmond, Canada; blotting grade) and the enhanced luminol chemiluminescence technique (Amersham). Preabsorption of the antibody with an excess of the peptide used for immunization abolished binding to the protein bands.
Immunostaining
For immunostaining fresh frozen (a) and freeze-dried tissues (b) were used. (a) Frontal and horizontal blocks of the brain (as described above) were frozen in liquid nitrogen-cooled isopentane. Ten-micron-thick sections were cut in a cryostat and mounted on glass slides (Superfrost Plus; Menzel). The sections were treated with PBS-buffered freshly prepared 1% formaldehyde (pH 7.4) for 1 min and, after several washes with PBS, with acetone for 1 min at room temperature. (b) Frozen tissues pieces (as described in a) of cerebral cortex and hippocampus were freeze-dried and embedded in the Epoxy resin Quetol 651 (Science Services, Munich, Germany). Semithin sections (1 μm) were mounted on glass slides. The resin was removed by placing the slides for 5 min in sodium methylate solution (Fluka, Neu-Ulm, Germany; Major et al. 1961).
All tissue sections were preincubated for 3 h at room temperature with 0.1% bovine serum albumin (Aurion BSA-C; Biotrend, Cologne, Germany), 10% normal goat serum, and 0.05% Tween 20 (Ferrak, Berlin, Germany) in PBS, pH 7.4. The immunostaining of semithin plastic sections and cryostat sections was performed in different ways. The plastic sections were incubated for 24–48 h at 4°C with the primary antibody diluted in the preincubation solution (anti-GLT1 peptides, 15 μg/ml; anti-GFAP, 1:10,000; anti-β-tubulin, 1:750). After several washes with PBS, the semithin plastic sections were incubated for 60 min at room temperature with indocarbocyanin (Cy3)-labeled secondary antibody (1:800; goat anti-rabbit IgG; Dianova, Hamburg, Germany) for detection of GLT1 proteins, and with carbocyanin (Cy2)-labeled secondary antibody (1:300; goat anti-mouse IgG; Dianova) for the detection of GFAP and β-tubulin. The sections were examined with an Olympus BH-2 fluorescence microscope (Olympus, New Hyde Park, N.Y., USA) equipped with Zeiss optics and an appropriate filter combination for selective visualization of Cy2 and Cy3 fluorescence (BH II DFC 6; Olympus). The cryostat sections were incubated for 24–48 h at 4°C with anti-GLT1 antibodies (15 μg/ml), each diluted in the preincubation solution. After several washes with PBS, the cryostat sections were incubated for 90 min at room temperature with aP-labeled secondary antibody (goat antiserum to rabbit immunoglobulin 1:100; Sigma). The detection was carried out using the aP detection medium (see above).
Controls were performed with primary antibody, previously absorbed with an excess of the corresponding GLT1 peptide used for immunization or without the primary antibody. No staining was observed under these conditions.
Results
Cloning of cDNA encoding the generic GLT1-3′-end
A fragment of the untranslated generic GLT1 cDNA-3′-end was isolated using total RNA from rat brain, performing 3′-RACE and amplification with a 5′-primer specific to generic GLT1 (PrGLT1b; Fig. 1a) and the 3′-adaptor primer. Sequence analysis of the cloned cDNA fragment (191 nt) showed identity between nt 1753 and 1800 to the known untranslated generic GLT1 sequence (Pines et al. 1992). The following untranslated generic GLT1 sequence reached to nt 1944 (Fig. 1a). The untranslated 3′-end of generic GLT1 (from nt 1720 to 1944) was completely different from that of GLT1v (from nt 1688 to 2109) and was therefore used for the generation of generic GLT1-specific cRNA probe.
Northern blotting
Using a cRNA probe complementary for the most part (nt -38 to 1776) of the GLT1 sequence (Schmitt et al. 1996), which is almost identical in generic GLT1 and GLT1v, northern blot of the whole brain showed a major band at ca 11 kb and a weaker band at ca 12.5 kb (Fig. 2 lane 1). Upon probing with a GLT1v-specific cRNA (nt 1654–2043; Schmitt et al. 2002) and a generic GLT1-specific cRNA (nt 1653–1907; this study), only a band at ca 11 kb (Fig. 2 lanes 2–4) and at ca 12.5 kb (Fig. 2 lanes 5–7), respectively, were detected in whole brain, cortex, and hippocampus. The strongest generic GLT1 and GLT1v labeling was observed in the cortex (Fig. 2). Substitution of the antisense by the sense cRNA probes resulted in a complete lack of staining (not shown).
Northern blot analysis of RNA from whole brain (b), cerebral cortex (c), and hippocampus (h) using a large GLT1 cRNA probe (complementary to generic GLT1 and GLT1v sequences; lane 1), a short GLT1-3′-cRNA probe (specific for generic GLT1; lanes 2–4), and the GLT1v cRNA probe (specific for GLT1v; lanes 5–7). The amount of total RNA loaded per lane was 50 μg. Determination of the mRNA size was carried out by comparison with ribosomal RNA bands (ca 2 and 5 kb). Using GLT1 cRNA whole brain (lane 1) shows a main band at ca 11 kb and a weaker band at ca 12.5 kb. Using generic GLT1-3′-cRNA (lanes 2–4) a band at ca 11 kb and using GLT1v cRNA (lanes 5–7) a band at ca 12.5 kb is detected
Western blot analysis (10% SDS-PAGE) of whole brain (b), cerebral cortex (c), and hippocampus (h), using affinity-purified antibodies against generic GLT1 peptide (lanes 1–3 in a and b; peptide 1, cf. Fig. 1b) and GLT1v peptide (lanes 4–6 in a and b; peptide 2, cf. Fig. 1b). a For probing, the 100,000 g pellet was used. The amount of protein loaded per lane was 50 μg. Generic GLT1 and GLT1v antibodies labeled a ca 70-kDa band and sometimes aggregates of those polypeptides (in a and b). b For probing the 1,000 g supernatant (lanes 1, 4), the 100,000 g pellet (lanes 2, 5), and the 100,000 g supernatant (lanes 3, 6) of the whole brain (b) were used. In the 100,000 g supernatant no labeling was observed (lanes 3, 6)
Western blotting
In Western blots of the 100,000 g pellet of tissue homogenates (whole brain, cortex, and hippocampus; Fig. 3), the affinity-purified antibodies against the GLT1v peptide (Peptide 2 in Fig. 1b; Schmitt et al. 2002) or the generic GLT1 peptide (Peptide 1 in Fig. 1b; Schmitt et al. 1996) labeled a band at ca 70 kDa and, sometimes, a band of lower electrophoretic mobility, presumably aggregates of the 70-kDa protein. Detection reaction times were optimized for the 70-kDa band and, therefore, the higher molecular weight band was not detected in all cases. The strongest generic GLT1 and GLT1v labeling was observed in the hippocampus (Fig. 3a).
A similar labeling pattern was observed in 1,000 g supernatant and 100,000 g pellet of whole brain (Fig. 3b lanes 1, 2, 4, 5), whereas no labeling was detected in the 100,000 g supernatant (Fig. 3b lanes 3, 6). Preabsorption of the antibodies with an excess of the corresponding peptides abolished binding to the protein bands.
In situ hybridization
Using the generic GLT1-specific probe (complementary to the untranslated 3′-end of generic GLT1), the labeling pattern was identical to that observed upon application of the cRNA probe complementary to a part of the GLT1 sequence (Schmitt et al. 1996), which is almost identical in generic GLT1 and GLT1v (Fig. 4a, b). Both probes led to intense staining of glial cells (Fig. 4a, b, e, i, k), which were identified by GFAP immunostaining to be astrocytes (Fig. 4n, o). Frequently a faint labeling of most neuronal populations was observed in the cortical regions and their layers studied (hippocampus and entorhinal, perirhinal, parietal, and temporal cortex). The weakly stained perikarya were proven to be neurons, using double labeling with anti-NeuN antibody which labels specifically the nuclei of neurons (Fig. 4k, l). However, in some areas a more intense labeling of neuronal perikarya was observed, for example, in CA 3/4 pyramidal cells of the hippocampus proper (Fig. 4a, b) and in perikarya of the deep layer VI of perirhinal, parietal, and temporal cortex. Other cell types were not detected by GLT1 ISH using both probes.
In situ hybridization (ISH) using various GLT1 cRNA probes: the large GLT1 cRNA probe (complementary to generic GLT1 and GLT1v sequences; a, e, i, k, n), the short GLT1-3′-cRNA probe (specific for generic GLT1; b), and the GLT1v cRNA probe (specific for GLT1v; c, f, g, m, p). a, b The large and the short GLT1 cRNA probes show the same cellular labeling pattern in the dentate gyrus of the hippocampus. There is a strong staining of astrocytes in the neuropil layers and pyramidal cells of CA3 (py). c Using the GLT1v cRNA probe, there is a strong staining of granule cells (gr) and pyramidal cells (py) in the hippocampus. d Control. No labeling is observed using the sense GLT1v cRNA probe in the dentate gyrus. e Generic GLT1 mRNA is preferentially detected in astrocytes of the temporal and parietal cortex from layers I to VI (arrowhead and dotted line delineate the border between cortical areas). Arrow points to labeled subpial astrocytes. f Using the GLT1v cRNA probe, labeling is observed in neurons of the temporal and parietal cortex from layers II to VI. g, h A micrograph pair showing a section from layer VI (parietal cortex) after ISH using the GLT1v cRNA probe in the first (g), and after additional neuron-specific nuclear protein (NeuN) immunostaining in the second figure (h). After ISH (g), the nuclei of neurons (arrows) are not labeled, but are stained after additional immunolabeling using anti-NeuN antibody (h, arrows). i Generic GLT1 mRNA is detected in astrocytes of the perirhinal and medial entorhinal cortical areas throughout the layers I–VI. The lightly to moderately stained neurons cannot be seen at this magnification. k, l A micrograph pair showing a section from layer IV (perirhinal cortex) after ISH using the large GLT1 cRNA probe in the first (k), and after additional NeuN immunostaining in the second figure (l). After ISH (k), the nuclei of lightly to moderately stained neurons (arrowheads) are not labeled, but are stained after additional NeuN immunolabeling (l, arrowheads). Arrows in k and l point to an astrocyte labeled by ISH. m GLT1v mRNA is demonstrated in neurons of the perirhinal and medial entorhinal cortical areas from layers II to VI. n, o A micrograph pair showing a section from the fimbria hippocampi after ISH using the large GLT1 cRNA probe in the first (n), and after additional glial fibrillary acidic protein (GFAP) immunostaining in the second figure (o). GFAP-immunoreactive processes (o) allow identification of ISH-reactive cells (n) as astrocytes (arrows). p ISH reaction product (using the GLT1v cRNA probe) is detected in glial cells of the fimbria hippocampi, which are typically arranged in rows (arrowheads), and therefore appear to be oligodendrocytes. as Antisense, s sense, mo molecular layer of the dentate gyrus (hippocampus), gr granular cell layer of the dentate gyrus (hippocampus), lm lacunosum-molecular layer of the hippocampus proper, ra radiatum layer of the hippocampus proper, py pyramidal cell layer of the hippocampus proper, Te1 area 1 of the temporal cortex, Par2 area 2 of the parietal cortex, MEA medial entorhinal area, Prh perirhinal cortex. Scale bars 30 μm in g, h, n–p; 50 μm in k, l; 325 μm in a–f, i, m
Using the GLT1v-specific probe, GLT1v cRNA was predominantly detected in neuronal cell bodies contrasting the strong astrocytic labeling using the GLT1 probes (Fig. 4c, f–h, m). The stained perikarya were proven to be neurons, using double labeling with anti-NeuN antibody (Fig. 4g, h). The neuronal GLT1v staining intensities ranged from moderate to strong and unlabeled neuronal perikarya could not be detected with certainty in the cortical regions studied.
In the gray matter astrocytes appeared almost non-reactive, which was apparent preferentially in neuropil layers [for example, layer I of various cortical areas (Fig. 4f, m), dentate gyrus molecular layer and lacunosum-molecular layer of the hippocampus proper (Fig. 4c)]. Only a few scattered GLT1v mRNA-reactive cells in white matter were identified as astrocytes by colocalization of GLT1v mRNA and GFAP immunoreactivity (not shown). Labeled glial cells were found in fiber tracts, which were typically arranged like pearls on a string, a morphological characteristic of oligodendrocyte localization (Fig. 4p). GLT1v mRNA was also detected in further glial cell types, i.e., in ependymal cells and epithelial cells of the choroid plexus as described previously (Schmitt et al. 2002).
Immunocytochemistry
To detect specifically generic GLT1 and GLT1v, affinity-purified peptide antibodies were used. In thick cryostat sections (12 μm), labeling patterns for generic GLT1 and GLT1v were very similar showing moderate to strong staining in the neuropil layers of the hippocampus (Fig. 5a, c) and in the various layers of perirhinal (Fig. 5h, k), temporal, and parietal cortex. In the dentate gyrus, the inner molecular layer showed a weaker staining in the demonstration of GLT1 compared to GLT1v (Fig. 5a, c). There was an obvious border between the perirhinal cortex with a strong immunolabeling and the entorhinal cortex with a moderate staining in layers II–VI (Fig. 5h, k).
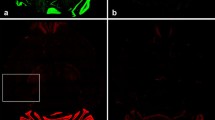
Distribution of generic GLT1 and GLT1v immunoreactivities in 12-μm-thick cryostat sections (a, c, h, k) detected by alkaline phosphatase-labeled secondary antibody and in semithin plastic sections (b, d, e–g, l, m–p) detected by immunofluorescence staining. a–g Hippocampus. A moderate to strong immunolabeling is observed in the neuropil layers of the dentate gyrus (a, c). Note the weaker immunostaining of inner molecular layer (imo) in the detection of generic GLT1 (a). In the granule cell layer (b) and in the pyramidal cell layer of the cornu ammonis sector 3 (e) neurons lack generic GLT1 immunolabeling. GLT1v is detected in the cytoplasm of neurons (n, neuronal nucleus), in the granule (d) and pyramidal cell layers of the cornu ammonis sector 1 (f) and sector 3 (g). h, k A strong immunoreaction for both GLT forms is observed in the perirhinal cortex (Prh) and moderate (layers II–VI) to strong reaction (layer I) in the medial entorhinal cortex (MEA). i No generic GLT1 immunolabeling is observed in neurons (p, pyramidal cells; layer III of the medial entorhinal cortex). l The cytoplasm of neurons is immunostained for GLT1v (n, pyramidal cell nucleus; layer III of the medial entorhinal cortex). Note granular staining in the cytoplasm. m, n Micrograph pair taken from neuron-free hippocampal alveus double-labeled for generic GLT1 (m) and GFAP (n). The glial cell is labeled for generic GLT1 and GFAP, and therefore seems to be an astrocyte (as). o, p Micrograph pair taken from neuron-free hippocampal alveus double-labeled for GLT1v (o) and β-tubulin (p). A glial cell is labeled for GLT1v and β-tubulin, and therefore seems to be an oligodendrocyte (ol). The arrows point to an oligodendrocyte process. The asterisks are localized in the nucleus of an unstained glial cell, which may be an astrocyte. Punctuate immunoreactivity surrounding the glial cells represents staining for GLT1v (o) and β-tubulin (p) in cross-sectioned nerve fibers. For abbreviations, see also legend to Fig. 4. Scale bars 10 μm in m–p; 20 μm in b, d–g, i, l; 325 μm in a, c, h, k
The cellular and subcellular distribution of generic GLT1 and GLT1v was studied in 1-μm-thick plastic sections of the cortical regions under study. These semithin sections allowed a better resolution of immunolabeled structures than thick cryostat sections. A cytoplasmic (sometimes fine granular) staining of neurons and glial cells predominated in the demonstration of GLT1v (Fig. 5d, f, g, l, o). On the other hand, using generic GLT1-specific peptide antibodies the plasma membranes were labeled while the cytoplasm of neurons and astrocytes was completely unstained (Fig. 5b, e, i, m).
In the hippocampus, granule (Fig. 5d) and pyramidal cells (Fig. 5f, g) and scattered cells in the neuropil displayed GLT1v immunoreactivity in their cytoplasm. The same subcellular distribution of immunostaining was observed in the various neurons of the other cortical areas studied, for example, in pyramidal cells (Fig. 5l). Comparing the same neuronal cell types in the demonstration of generic GLT1, no immunoreactivity was detected (Fig. 5b, e, i).
In fiber tracts we observed a moderate to strong immunostaining of glial cells (Fig. 5m, o). In the demonstration of generic GLT1, we found a labeling of glial cell membranes (soma and processes). We identified these glial cells by double immunolabeling with GFAP antibody to be astrocytes (Fig. 5m, n; Schmitt et al. 1996). On the other hand, GLT1v-immunolabeled glial cells of the white matter were only occasionally labeled for GFAP immunoreactions, but mainly double-labeled using a monoclonal antibody against β-tubulin (Fig. 5o, p). It is known that β-tubulin is highly expressed in oligodendrocytes and neurons (Schaeren-Wiemers et al. 1995). As the fiber tracts studied are free of neurons (proven by the lack of immunodetection of neurons using the neuron-specific anti-NeuN antibody), it can be assumed that the β-tubulin-positive cells are oligodendrocytes. Furthermore, the axoplasm of nerve fibers was frequently labeled for GLT1v (Fig. 5o), but not for GLT1 (Fig. 5n).
Discussion
Expression of generic GLT1 in neurons
To show whether generic GLT1 is really expressed in neurons, we have cloned the untranslated 3′-end of the generic GLT1 cDNA, which is completely different from the 3′-end of the variant GLT1 (GLT1v; Schmitt et al. 2002) and generated a generic GLT1-specific cRNA probe for non-radioactive ISH. Northern blot analyses supported our suggestion that a large cRNA probe complementary to long sequences homologous in generic GLT1 and GLT1v mRNA detects both mRNA forms while the shorter probes are specific for the different mRNAs. Nevertheless, comparative ISHs in brain sections using the generic GLT1 specific or the generic GLT1/GLT1v detecting large cRNA probe (Schmitt et al. 1996) revealed the same labeling pattern, i.e., a generally strong labeling of astrocytes and a usually weak labeling of neurons. These data confirm the expression of GLT1 mRNA in neurons as described previously using large cRNA probes (Torp et al. 1994, 1997; Schmitt et al. 1996; Berger and Hediger 1998) and indicate that, under conditions of ISH, labeling using large GLT1 probes preferentially represents generic GLT1 mRNA distribution. An explanation for this could be that ISH detection reaction times are optimized for the prevailing astrocytic signals in the brain and may be cut too short to enable detection of the neuronal GLT1v mRNA. Specific probes for GLT1v mRNA, however, generally do not label astrocytes (Schmitt et al. 2002; this study), and thus the neuronal signal for the variant is detected.
Our comparative immunocytochemical studies using a generic GLT1-specific peptide antibody confirmed that although generic GLT1 mRNA is expressed in neurons, generic GLT1 protein is only detectable in astrocytes, but not in neurons of the normal adult brain (Danbolt et al. 1992; Levy et al. 1993; Rothstein et al. 1994; Lehre et al. 1995; Milton et al. 1997). There are various suggestions to explain this discrepancy (Danbolt 2001). Previous in vitro expression studies (Lin et al. 1998; Guo et al. 2002) have shown that aberrant GLT1 mRNAs may produce, for unknown reasons, a dominant negative effect on normal GLT1 resulting in loss of GLT1 protein. Whether the strong expression of the variant GLT1 (GLT1v) mRNA (although not aberrant) in neurons (Schmitt et al. 2002) could exert a similar dominant negative effect on generic GLT1 production remains to be determined.
Complementary cellular expression of generic GLT1 and GLT1v
Our immunocytochemical results indicate a differential cellular distribution of the two GLT1 proteins in the brain areas studied, as has been suggested for other CNS areas (Schmitt et al. 2002). Generic GLT1 protein is restricted to astrocytes, whereas variant GLT1 protein is localized primarily in non-astrocytic glial cells and in neurons (Schmitt et. al. 2002). Remarkably, neuronal localization of GLT1v protein is found not only in glutamatergic but also in other neuronal populations (for example, GABAergic: Purkinje cells of the cerebellum; Schmitt et al. 2002).
These findings are in some contrast to other immunocytochemical investigations localizing GLT1 protein forms. Thus, Reye et al. (2002) reported immunolabeling for both GLT1 proteins in astrocytes but failed to find labeling in neurons. Chen et al. (2002), on the other hand, localized GLT1v (designated by the authors as GLT1b) protein in neurons and astrocytes. The combination of ISH and immunocytochemistry carried out in the present study gave closely similar cellular distribution patterns for generic GLT1 mRNA and protein, and identical patterns for GLT1v mRNA and protein. This leaves little doubt that neurons possess the capacity to and indeed to produce GLT1v, while astrocytes synthesize generic GLT1.
Our results again support the important role of GLT1 in the CNS: all main neural cell populations express this high affinity glutamate transporter. The differential distribution of the two forms (generic GLT1 in astrocytes and GLT1v in neurons and non-astrocytic glial cells) indicates possible subtle functional differences in the different cell types.
Subcellular distribution of GLT1v
Concerning possible functional differences, an important observation is that at the subcellular level GLT1v immunolabeling revealed a preferentially cytoplasmic staining of neurons (and glial cells) (see also Schmitt et al. 2002), whereas GLT1 is predominantly found in plasma membranes of astrocytes (Danbolt et al. 1992; Levy et al. 1993; Rothstein et al. 1994; Lehre et al. 1995; Schmitt et al. 1996; this study). In well-preserved semithin sections, the immunoreactions labeled granular profiles in the cytoplasm. At the electron microscopic level it has also been shown that immunoreaction product is often located in the cytoplasm, presumably associated with cytoplasmic membranes (Chen et al. 2002). A localization of GLT1v in vesicle membranes is supported by the immunoblot finding that GLT1v, like GLT1, could only be detected in the membrane fraction of brain homogenates. A vesicular localization is also reported for other transporters and is regarded as a possible mechanism in the posttranslational regulation of transporter expression (for review, see Danbolt 2001). For EAAC1 it has been shown that trafficking of the glutamate transporter from storage vesicles to the cell membrane is very rapid (Dowd and Robinson 1996; Davis et al. 1998).
In this respect, another important finding is that GLT1v contains at the spliced 3′-end a class I PDZ binding motif (T-C-I; Schmitt et al. 2002). It has been demonstrated that C-terminal PDZ domain binding motifs are involved in intracellular trafficking of proteins (see, for example, Daw et al. 2000; Scott et al. 2001; Torres et al. 2001) and in keeping membrane proteins at the correct locations by means of anchoring proteins (for review, see Danbolt 2001). A trafficking of the variant GLT1 (GLT1v) from storage vesicles to the cell membrane may represent an endogenous protective mechanism to limit glutamate-induced excitotoxicity in neurons.
References
Asan E (1998) The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol 142:1–121
Asan E, Kugler P (1995) Qualitative and quantitative detection of alkaline phosphatase coupled to an oligonucleotide probe for somatostatin mRNA after in situ hybridization using unfixed rat brain tissue. Histochem Cell Biol 103:463–471
Berger UV, Hediger MA (1998) Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol 198:13–30
Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43:429–435
Burnette WN (1981) Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radionated protein A. Anal Biochem 112:195–203
Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA (2002) Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci 22:2142–2152
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Danbolt NC, Storm-Mathisen J, Kanner BI (1992) An [Na++K+]coupled l-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience 51:295–310
Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB (1998) Multiple signalling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci 18:2475–2485
Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaae JTR (2000) PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28:873–886
Dowd LA, Robinson MB (1996) Rapid stimulation of EAAC1-mediated Na+-dependent l-glutamate transport activity in C6 glioma by phorbol ester. J Neurochem 67:508–516
Drenckhahn D, Jöns T, Schmitz F (1993) Production of polyclonal antibodies against proteins and peptides. Methods Cell Biol 37:7–56
Guo H, Lai L, Butchbach MER (2002) Human glioma cells and undifferentiated primary astrocytes that express aberrant EAAT2 mRNA inhibit normal EAAT2 protein expression and prevent cell death. Mol Cell Neurosci 21:546–560
Kugler P, Schmitt A (1999) The glutamate transporter EAAC1 is expressed in neurons and glial cells in the rat nervous system. Glia 27:129–142
Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC (1995) Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15:1835–1853
Levy LM, Lehre KP, Rolstad B, Danbolt NC (1993) A monoclonal antibody raised against an [Na++K+]-coupled l-glutamate transporter purified from rat brain confirms glial localization. Fed Eur Biochem Soc Lett 317:79–84
Lin CLG, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD (1998) Aberrant RNA processing in a neurodegenerative disease: the cause for aberrant EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20:589–602
Major HD, Hampton JC, Rosario B (1961) A simple method for removing the resin from epoxy-embedded tissue. J Biophys Biochem Cytol 9:909–910
Milton ID, Banner SJ, Ince PG, Piggott NH, Fray AE, Thatcher N, Horne CHW, Shaw PJ (1997) Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study. Mol Brain Res 52:17–31
Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic, San Diego
Pines G, Danbolt NC, Bjørås M, Zhang Y, Bandahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI (1992) Cloning and expression of a rat brain l-glutamate transporter. Nature 360:464–467
Reye P, Sullivan R, Scott H, Pow DV (2002) Distribution of two splice variants of the glutamate transporter GLT-1 in rat brain and pituitary. Glia 38:246–255
Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW (1994) Localization of neuronal and glial glutamate transporters. Neuron 13:713–725
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanger F, Nicklen S, Coulsen AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467
Schaeren-Wiemers N, Schaefer C, Valenzuela DM, Yancopoulos GD, Schwab ME (1995) Identification of oligodendrocytes- and myelin-specific glues by a differential screening approach. J Neurochem 65:10–22
Schmitt A, Asan E, Püschel B, Jöns T, Kugler P (1996) Expression of the glutamate transporter GLT1 in neural cells of the rat central nervous system: non-radioactive in situ hybridization and comparative immunocytochemistry. Neuroscience 71:989–1004
Schmitt A, Asan E, Püschel B, Kugler P (1997) Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J Neurosci 17:1–10
Schmitt A, Asan E, Lesch K-P, Kugler P (2002) A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience 109:45–61
Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD (2001) An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 21:3063–3072
Sternberger LA, Hardy PH, Cuculis J, Meyer HG (1990) The unlabeled antibody method of immunohistochemistry: preparation and properties of soluble antigen–antibody complex (horseradish peroxidase–anti-horseradish peroxidase) and its use in identification of spirochaetes. J Histochem Cytochem 18:315–333
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702
Torp R, Danbolt NC, Babaie E, Bjørås M, Seeberg E, Storm-Mathisen J, Ottersen OP (1994) Differential expression of two glial glutamate transporters in the rat brain: an in situ hybridization study. Eur J Neurosci 6:936–942
Torp R, Hoover F, Danbolt NC, Storm-Mathisen J, Ottersen OP (1997) Differential distribution of the glutamate transporters GLT1 and rEAAC1 in rat cerebral cortex and thalamus: an in situ hybridization analysis. Anat Embryol 195:317–326
Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG (2001) Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30:121–134
Acknowledgements
The authors are indebted to PD Dr. Esther Asan for helpful criticism and thank Erna Kleinschroth for her excellent technical assistance. The study was supported by a grant from the Deutsche Forschungsgemeinschaft to Peter Kugler.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kugler, P., Schmitt, A. Complementary neuronal and glial expression of two high-affinity glutamate transporter GLT1/EAAT2 forms in rat cerebral cortex. Histochem Cell Biol 119, 425–435 (2003). https://doi.org/10.1007/s00418-003-0530-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0530-7