Abstract
Purpose
To evaluate the efficacy of intraoperative slow-release dexamethasone implant (DEX) combined with removal of idiopathic epiretinal membrane (ERM).
Methods
In this observational retrospective study, data of 40 patients with phakic eyes affected by idiopathic ERM were analysed. All patients underwent cataract phacoemulsification, 25-gauge (G) pars plana vitrectomy (PPV), ERM removal with DEX implant (“DEX YES” group, #20) or without DEX implant (“DEX NO” group, #20). We collected data on best-corrected visual acuity (BCVA) < 20/40 Snellen charts, central macular thickness (CMT) ≤ 400 μm (measured by SD-OCT) and integrity of sub-foveal ellipsoid/myoid zone. BCVA, CMT and intraocular pressure (IOP) were evaluated at baseline as well as 15, 30 and 90 days after surgery.
Results
In the “DEX YES” group, statistically significant BCVA improvement was observed at 15, 30 and 90 days (p < 0.001), while in the “DEX NO” group, improvements were observed only at 30 and 90 days (p < 0.001). In both groups, CMT significantly decreased at each follow-up visit (p < 0.001), and no statistically significant increase of IOP was detected at each follow-up visit.
Conclusions
In this study, DEX accelerated the improvement of BCVA at 15 days after surgery. However, no evidence of further anatomical (CMT) and functional (BCVA) DEX effectiveness combined with removal of idiopathic ERM by 25-G PPV at 30 and 90 days follow-up was observed.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epiretinal membranes (ERMs) are a non-vascularized fibrocellular contractile proliferation, composed of accessory retinal glial cells, fibrous astrocytes and Müller cells, which form over the surface of the internal limiting membrane (ILM) in the macular area [1, 2]. Iwanoff first described them in 1865, and Gass proposed an ophthalmoscopic classification [3, 4]. The spectral-domain optical coherence tomography (SD-OCT) has allowed clinicians to more accurately diagnose and characterize several vitreomacular interface disorders [5, 6], as proposed by the International Vitreomacular Traction Study Group [7]. From the physiopathological point of view, the first pathogenic hypothesis is the migration of glial cells on the retinal surface through microscopic breaks of ILM after an anomalous posterior vitreous detachment (PVD) [8, 9], while an alternative hypothesis is that the vitreoschisis plays a crucial role in ERM development as postulated by Sebag et al. [10]. ERM can be classified as idiopathic, primary (due to an anomalous PVD) or secondary to retinal detachment, uveitis, retinal vascular occlusions or trauma [11]. The prevalence of ERM is between 4 and 12.8% according to the study population [12, 13]. Both sexes are equally affected. Bilateral involvement occurs in up to 10–20% of cases, usually with asymmetry [2, 14]. The decrease in best-corrected visual acuity (BCVA), with or without metamorphopsia, is the main indication for surgical treatment by pars plana vitrectomy (PPV), and different prognostic factors for visual acuity (VA) improvements after ERM surgery have been described. Among them, a short duration of symptoms before surgery, preoperative VA, central macular thickness (CMT) and integrity of the sub-foveal ellipsoid as well as myoid zone of the photoreceptors at baseline can potentially assist clinicians to identify the optimal time to perform surgery and to predict postoperative outcomes [15, 16]. However, ERM traction causes inflammation, exudates and leukocyte response in the macular region; in addition, sometimes, after PPV and ERM removal, a residual intraretinal oedema is still present, limiting possibilities for a complete visual function recovery [17, 18]. Growing evidence suggests a possible inflammatory pathogenesis of residual macular oedema (MO) after PPV.
Intravitreal slow-release dexamethasone implant (DEX) (Ozurdex; Allergan, Inc., Irvine, CA) is currently approved by the Food and Drug Administration (FDA) to treat secondary MO caused by retinal vein occlusion, non-infectious uveitis and diabetic macular oedema, which is refractory to anti-VEGF treatments [19, 20]. To date, literature evidence suggests efficacy of off-label DEX injections after PPV for ERM in case of persistent MO [21, 22]. However, little is known about the benefits of intraoperative DEX injections for a rapid MO improvement and visual acuity, particularly in case of ERM with low CMT and integrity of sub-foveal ellipsoid and myoid zone of the photoreceptors.
The aim of this study was to evaluate the efficacy of DEX combined with removal of idiopathic ERM by 25-gauge PPV in patients with mild ERM and in the absence of MO, in terms of BCVA improvement, macular thickness reduction and intraoperative DEX safety.
Methods
This was an observational retrospective study conducted in accordance with the principles of the Declaration of Helsinki, and all patients signed a specific informed consent for the use of their data. Patients were considered based on information retrieved in electronic medical record. We included 40 patients divided into two groups: 20 eyes underwent 25G PPV and no Ozurdex implant (“DEX NO” group); 20 underwent 25G PPV with Ozurdex implant (“DEX YES” group).
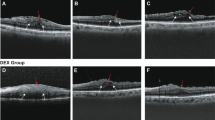
More detailed, only data of patients diagnosed with primitive symptomatic ERM documented by SD-OCT (RTVue-XR, Optovue) were analysed. We considered as eligible patients’ eyes affected by wide adhesion (> 1.500 mc) ERM, CMT ≤ 400 μm, measured by SD-OCT, associated with integrity of sub-foveal ellipsoid and myoid zone of the photoreceptors and presence of severe metamorphopsia and absence of MO, judged based on subjective symptoms and tested with the Amsler grid chart. We included patients with BCVA less than 20/40 Snellen charts and ocular axial length less than 27.00 mm (optical biometry) associated with cataracts.
Specifically, the integrity of the sub-foveal ellipsoid and myoid zone and absence of MO was evaluated by two independent observers and compared by Cohen’s kappa coefficient. The interobserver agreement of image analyses was 0.92 (k = 0.225, p < 0.01).
The exclusion criteria included concomitant or previous macular diseases (diabetic macular oedema, branch retinal vein occlusion or central retinal vein occlusion and age-related macular degeneration) as well as secondary epiretinal membranes (traumatic ERM and/or ERM associated with previous retinal laser retinopexy or associated with lamellar and/or full thickness macular hole). Previous vitreoretinal surgery (i.e., for retinal detachment or vitreous haemorrhages), previous cataract surgery and presence of glaucoma were also considered exclusion criteria as well as low-quality OCT images due to dioptric media opacity.
Follow-up visits were performed after 15, 30 and 90 days.
The following parameters were collected: BCVA with Snellen charts, slit-lamp examination, IOP measurement (Goldmann applanation tonometry), dilated fundus examination and measurement of CMT by SD-OCT. Primary endpoints included changes in BCVA and in CMT after 15, 30 and 90 days follow-up compared with the preoperative value. Secondary endpoints included high-IOP development and occurrence of adverse events (vitreous haemorrhage, retinal detachment, DEX migration into the anterior chamber and endophthalmitis).
Surgical procedure
Experienced surgeons (AS, TC, LV, FG, FB and SR) performed all interventions under local anaesthesia (peribulbar block). Patients underwent a standard phacoemulsification and IOL (single piece) implant and a three port 25-G PPV using the constellation vitrectomy system (Alcon Laboratories).
All procedures at are unit included core vitrectomy, posterior hyaloid removal, ERM and internal limiting membrane (ILM) peeling. A posterior vitreous detachment was induced intraoperatively if vitreous was not detached from the posterior pole. Membraneblue-dual (TrypanBlue 0.15% + Brilliant Blue 0.025% + PEG 4%, DORC, Zuidland, The Netherlands) was used to stain the ERM first and the ILM after, to facilitate the rhexis technique for ERM and ILM peeling. Specifically, the ERM and ILM removal was performed up two-three disk diameters centred on the fovea. At the end of the peeling procedure, a detailed examination of the retinal periphery and endo-laser on rhegmatogenous areas was performed. At the end of the surgery, after a balanced saline solution to air exchange, in “DEX YES” selected eyes, DEX was cautiously injected to avoid any damage to the retina, and the correct positioning of the device was checked (Video). Finally in both groups, if uncomplicated, the surgical procedure was concluded performing a hydration of the sclerotomies [23]. In DEX YES group, patients were recommended to avoid face-down position to prevent Ozurdex dislocation into the anterior chamber. While in DEX NO group, patients were suggested to observe prone position only on the day of the surgery.
In both groups, in the postoperative period, patients were treated using topical antibiotics (chloramphenicol) 4 times per day for 7 days and topical non-steroidal anti-inflammatory drugs (bromfenac) twice daily for 4 weeks. Only in “DEX NO” group, dexamethasone drops were used 4 times a day for 7 days to taper down weekly in 1 month.
Video slow release dexamethasone implant (DEX) (Ozurdex) injection technique is showed at the end of a 25G vitrectomy for epiretinal membrane peeling. After trocar removal, DEX injection is performed into the vitreous chamber filled of air. We are use checking the implantation trough indirect system. Using the optical fibre in one hand and the implant system into the other, DEX is gently injected and controlled (MP4 59662 kb)
Statistical analysis
Two-way ANOVA for repeated measurements for both groups was applied. Sidak test has been used as correction for multiple comparisons. To test the correlation between age, BCVA and CMT Pearson correlation test was used. A p value < 0.05 was considered statistically significant.
Results
Details of the two groups, “DEX YES” (n = 20) and “DEX NO” (n = 20), are shown in Table 1. Table 2 showed the differences during the follow-up. The two groups were comparable in terms of gender distribution (11 vs 12 women in the “DEX YES” and “DEX NO” groups, respectively) and age (mean age 70.2 ± 7.5 vs 70.9 ± 6.9, respectively).
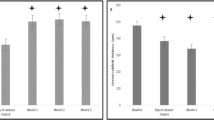
In the “DEX YES” group, a statistically significant improvement of visual acuity was observed after 15, 30 and 90 days (p < 0.001); in the “DEX NO” group, there was no statistically significant improvement of BCVA at 15 days (p = 0.63) observed, but it turned significant at 30 days (p < 0.001) and 90 days (p < 0.001). In both groups, CMT significantly decreased at each follow-up visit (p < 0.001). At the end of follow-up, four patients of the “DEX NO” group showed a slight, but not statistically significant, increase in CMT; additionally, BCVA of those eyes was not affected (Fig. 1). In both groups, a statistically significant increase of IOP was not detected at each follow-up visit, even if in the “DEX YES” group, three eyes developed high ocular pressure, which was controlled using topical ß-blockers. None of the eyes in both groups developed vitreous haemorrhage, retinal detachment, DEX migration into the anterior chamber and endophthalmitis.
a, b and c included eyes which received slow-release dexamethasone implant (DEX) during surgery. d, e and f grouped eyes that did not receive DEX during the surgery. From the top to the bottom, the differences between these two groups regarding best-corrected visual acuity (BCVA), central macular thickness (CMT) and intraocular pressure (IOP) are represented
Discussion
PPV with membrane peeling is the gold standard of care for patients with symptomatic ERM, and most patients have a favourable outcome and complete visual acuity recovers. However, persistent and/or residual intraretinal oedema can be sometimes present, limiting the possibility of complete visual function improvement. To date, the mechanism of MO, in idiopathic ERM, has not been fully understood. According to Miyazaki et al. and Bu et al., MO could be related to the breakdown of the blood-retinal barrier, which is caused by inflammatory cytokine release, growth factors from preoperative mechanical traction and intraoperative manipulation [2, 13].
Corticosteroids are strong anti-inflammatory agents that can block several pathological processes involved in MO development by inhibiting VEGF synthesis, prostaglandins and many pro-inflammatory cytokines. Topical or local administration usually leads to a suboptimal drug level in the vitreous; therefore, direct intravitreal injection seems to be most effective to achieve optimal drug level in the vitreous [24]. The development of DEX enabled improved drug delivery control, with a potentially lower rate of adverse events and a reduction of frequent intraocular injections in vasectomized eyes [22, 25].
In literature, several studies report the efficacy of DEX for MO after ERM peeling surgery [26, 27].
On the other hand, only few studies with controversial results evaluated the efficacy of intraoperative implant of DEX in patients undergoing ERM peeling.
In a pilot single-arm study by Hostovsky et al. on 12 eyes of 12 patients (seven phakic and five pseudophakic) treated with PPV and intraoperative DEX implantation, mean BCVA and CRT significantly improved 3 and 6 months following surgery, with no safety concerns.
Guidi et al. analysed 60 eyes of 60 pseudophakic patients with idiopathic ERM and intraoperative DEX implantation in one of the study arms. Their macular thickness at baseline was more than 250 mm as measured by SD-OCT and had the integrity of sub-foveal inner segment/outer segment (IS/OS) junction [28].. After 1, 3 and 6 months, BCVA and CMT significantly improved in each group, with no significant differences between the eyes with or without DEX implant. Therefore, authors suggested that the improvement of these parameters were mainly related to the surgical procedure rather than the anti-inflammatory action of DEX. Notably, patients included in this study had a wide variability in terms of ERM stage and presence or absence of intraretinal cysts.
Conversely, Iovino et al. analysed 40 pseudophakic eyes with idiopathic stages 3–4 ERM. In stage 4 ERM, the retinal layers are significantly compromised. In all patients, BCVA significantly increased at 1, 3 and 6 months after surgery compared with baseline, but at 3 and 6 months, the visual gain was higher in the DEX group. Similarly, CMT was significantly lower in the DEX group compared with the control group at 3 and 6 months after surgery [29]. All eyes included in this study had intraretinal cysts. In both these studies, authors did not report any statistically significant difference in IOP during the follow-up in both groups.
We prefer to perform phacoemulsification combined with PPV to increase patients’ adherence to surgical procedures, particularly considering that the majority of our patients are elderly, even though we know from literature that in an early postoperative phacoemulsification setting, MO can occur. In addition, risks of post-phacoemulsification oedema are particularly increased in patients with diabetes, which were excluded from our study [30].
According to our results, the use of DEX, after ERM peeling surgery and cataract phacoemulsification, showed a statistically significant improvement in BCVA at 15 days compared with PPV-treated patients (“NO DEX” group). Eyes, which underwent ERM peeling surgery and cataract phacoemulsification without using DEX, achieved the same values of BCVA at the second follow-up, i.e., 15 days later compared with patients treated with DEX implantation.
However, no significant differences were observed in BCVA, CMT and IOP between the two groups at 1 and 3 months after surgery. In addition, our study demonstrates that no ocular or systemic adverse events, related to the use of Ozurdex, were reported during the follow-up. Despite IOP increased in three patients at the second follow-up—controlled prescribing topical ß-blockers twice daily—none of the DEX-YES eyes showed adverse side effects.
Overall, our results showed no significant benefits of intraoperative DEX implantation in patients with mild ERM and with integrity of sub-foveal ellipsoid and myoid zone of the photoreceptors. This could be related to the fact that, in these patients, the increase of macular thickness is mostly due to primary tractional forces, which are exerted on the retinal structure by the epiretinal membrane, and to a lesser extent to the inflammatory component. Thus, it is likely that the role of corticosteroids is not essential in the recovery of a normal macular shape and visual acuity, as we initially expected [29].
In our opinion, MO could be traction-induced or neurosensory retina distortion by losing its elasticity; consequently, the affecting time of the ERM can play a very important role. In the light of our results, no major benefits in terms of visual acuity improvement and macular thickness reduction have been observed in eyes affected by mild ERM not associated with MO at the end of our follow-up; however, a slight improvement of BCVA was detected at 15 days in DEX YES group. Prospective randomized trials can be useful to understand the usefulness of intraoperative DEX in this kind of disease and in cases of eyes affected by advanced stages of ERM associated or not with MO.
Study limitation
Due to the retrospective nature of the study, the choice of using DEX or not at the end of vitrectomy was decided according to surgeons’ choice and experience, based on OCT images evaluation, whether possible inflammatory pathogenesis was suspected in each case.
We tried to make the two groups (A and B) as much equivalent as possible based on OCT evaluation (the integrity of the sub-foveal ellipsoid and myoid zone and absence of MO).
References
Joshi M, Agrawal S, Christoforidis JB (2013) Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediat Inflamm 192582. https://doi.org/10.1155/2013/192582
Cheung N, Tan SP, Lee SY, Cheung GCM, Tan G, Kumar N et al (2017) Prevalence and risk factors for epiretinal membrane: the Singapore Epidemiology of Eye Disease study. Br J Ophthalmol 101:371–376. https://doi.org/10.1136/bjophthalmol-2016-308563
Iwanoff A (1865) Beiträge zur normalen und pathologischen anatomie des auges. Graefes Arch Clin Exp Ophthalmol 11:135
Koizumi H, Spaide RF, Fisher YL, Freund KB, Klancnik JM Jr, Yannuzzi LA (2008) Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol 145:509–517. https://doi.org/10.1016/j.ajo.2007.10.014
Gass JDM (1997) Macular dysfunction caused by epiretinal membrane contraction. In: Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, vol 2, 4th edn. Mosby, St Louis, pp 938–590
Stevenson W, Prospero Ponce C, Agarwal D, Gelman R, Christoforidis J (2016) Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol 10:527–534. https://doi.org/10.2147/OPTH.S97722
Duker JS, Kaiser PK, Binder S et al (2013) The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 120:2611–2619. https://doi.org/10.1016/j.ophtha.2013.07.042
Kritzenberg M, Jungas B, Framme C, Helbig H, Gabel VP, Fuchshofer R et al (2011) Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 58:953–965. https://doi.org/10.1111/j.1365-2559.2011.03820
Kampik A (2012) Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome. Retina 32(Suppl 2):S194–S198;discussion S198–9. https://doi.org/10.1097/IAE.0b013e31825bc20a
Sebag J, Gupta P, Rosen RR et al (2007) Macular holes and macular pucker: the role of vitreoschisis as imaged by optical coherence tomography/scanning laser ophthalmoscopy. Trans Am Ophthalmol Soc 105:121–129
Romano MR, Comune C, Ferrara M, Cennamo G, De Cillà S, Toto L et al (2015) Retinal changes induced by epiretinal tangential forces. J Ophthalmol 2015:372564. https://doi.org/10.1155/2015/372564
Miyazaki M, Nakamura H, Kubo M, Kiyohara Y, Iida M, Ishibashi T et al (2003) Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayama study. Graefes Arch Clin Exp Ophthalmol 241:642–646. https://doi.org/10.1007/s00417-003-0723-8
Katira RC, Zamani M, Berinstein DM, Garfinkel RA (2008) Incidence and characteristics of macular pucker formation after primary retinal detachment repair by pars plana vitrectomy alone. Retina 28:744–748. https://doi.org/10.1097/IAE
Bu S, Kuijer R, Li X, Hooymans J, Los L (2014) Idiopathic epiretinal membrane. Retina 34:2317–2335. https://doi.org/10.1097/IAE.0000000000000349
Suh MH, Seo JM, Park KH, Yu HG (2009) Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol 147:473–480. https://doi.org/10.1016/j.ajo.2008.09.020
Miguel AI, Legris A (2017) Prognostic factors of epiretinal membranes: a systematic review. J Fr Ophtalmol 40:61–79. https://doi.org/10.1016/j.jfo.2016.12.001
Carpentier C, Zanolli M, Wu L et al (2013) Residual internal limiting membrane after epiretinal membrane peeling: results of the pan-American collaborative retina study group. Retina 33:2026–2031. https://doi.org/10.1097/IAE.0b013e31828e69c2
Ripandelli G, Scarinci F, Piaggi P et al (2015) Macular pucker: to peel or not to peel the internal limiting membrane? A microperimetric response. Retina 35:498–507. https://doi.org/10.1097/IAE.0000000000000330
Boyer D, Yoon Y, Belfort R, Bandello F, Ozurdex MEAD Study Group et al (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121:1904–1914. https://doi.org/10.1016/j.ophtha.2014.04.024
Massa H, Georgoudis P, Panos G (2019) Dexamethasone intravitreal implant (OZURDEX®) for macular edema secondary to noninfectious uveitis: a review of the literature. Ther Deliv 10:343–351. https://doi.org/10.4155/tde-2019-0024
Chang Y, Liu P, Kao T, Wu H et al (2018) Dexamethasone intravitreal implant (Ozurdex) for long-term macular edema after epiretinal membrane peeling surgery. Ophthalmol 2018:5832186. https://doi.org/10.1155/2018/5832186
Furino C, Boscia F, Recchimurzo N, Sborgia C, Alessio G (2014) Intravitreal dexamethasone implant for refractory macular edema secondary to vitrectomy for macular pucker. Retina 34:1612–1616. https://doi.org/10.1097/IAE.0000000000000105
Rizzo S, Pacini B, De Angelis L, Barca F et al (2020) Intrascleral hydration for 23-gauge pars plana vitrectomy sclerotomy closure. Retina. https://doi.org/10.1097/IAE.0000000000002703
Chang-Lin E, Burke JA, Peng Q et al (2011) Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci 52:4605–4609. https://doi.org/10.1167/iovs.10-6387
Hattenbach L, Springer-Wanner C, Hoerauf H, Callizo J et al (2017) Intravitreal sustained-release steroid implants for the treatment of macular edema following surgical removal of epiretinal membranes. Ophthalmologica 237:232–237. https://doi.org/10.1159/000464259
Chatziralli I, Dimitriou E, Theodossiadis G, Chatzirallis A, Kazantzis D, Theodossiadis P (2019) Treatment of macular edema after pars plana vitrectomy for idiopathic epiretinal membrane using intravitreal dexamethasone implant: long-term outcomes. Ophthalmologica 242:16–21. https://doi.org/10.1159/000496705
Taney LS, Baumal CR, Duker JS (2015) Sustained-release dexamethasone intravitreal implant for persistent macular edema after vitrectomy for epiretinal membrane. Ophthalmic Surg Lasers Imaging Retina 46:224–228. https://doi.org/10.3928/23258160-20150213-01
Guidi G, Casini G, Ripandelli G, Piaggi P et al (2018) Intraretinal edema after 25-gauge vitrectomy and macular pucker removal: is intraoperative sustained-release dexamethasone a real treatment option? Retina 38:993–999. https://doi.org/10.1097/IAE.0000000000001627
Iovino C, Giannaccare G, Pellegrini M, Bernabei F (2019) Efficacy and safety of combined vitrectomy with intravitreal dexamethasone implant for advanced stage epiretinal membrane. Drug Des Devel Ther 13:4107–4114. https://doi.org/10.2147/DDDT.S229031
Giansanti F, Bitossi A, Giacomelli G, Virgili G et al (2013) Evaluation of macular thickness after uncomplicated cataract surgery using optical coherence tomography. Eur J Ophthalmol 23:751–756. https://doi.org/10.5301/ejo.5000280
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Azienda Ospedaliero Universitaria Careggi, Florence, Italy, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Savastano, A., Bitossi, A., Giansanti, F. et al. Evaluation of intraoperative slow-release dexamethasone implant combined with idiopathic epiretinal membrane removal. Graefes Arch Clin Exp Ophthalmol 259, 379–385 (2021). https://doi.org/10.1007/s00417-020-04911-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04911-5