Abstract
Purpose
To investigate foveal avascular zone area, macular vascular density, choroidal thickness, and outer retina and choriocapillaris flow in myopic eyes by OCT angiography.
Methods
Automated macular maps and flow calculations were retrospectively evaluated in 42 myopic and in 40 control eyes.
Results
Myopic eyes presented lower whole superficial vessel density (46.4 ± 4.9 vs. 51.6 ± 3.6%, P < 0.0001) and higher flow area in the outer retina (1.3 ± 0.2 vs. 1.1 ± 0.3 mm2, P = 0.0012). Between the myopic and non-myopic eyes, no significant differences could be detected in the choriocapillaris perfusion area (1.9 ± 0.07 vs. 1.9 ± 0.05 mm2, respectively; P = 0.55) and in the foveal avascular zone area (0.23 ± 0.1 vs. 0.26 ± 0.1 mm2, respectively; P = 0.12). The spherical correction positively correlated with superficial vessel density and negatively correlated with outer retina perfusion (P ≤ 0.0021). The superficial vessel density and the local retinal thickness positively correlated at all macular locations (P < 0.005), especially in the foveal region (P < 0.0001).
Conclusions
Eyes with high myopia present reduced superficial vascular density and increased outer retina flow. Superficial vascular density and retinal thickness appear to be significantly correlated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few years, spectral domain (SD) optical coherence tomography (OCT) angiography devices have become commercially available and visualization of the superficial, deep macular vascular plexus and of the outer retinal layer is now commonly evaluated. Sequential acquisition of blood flow movement allows defined visualization of neovascular activity, but also parameters such as retinal vascular density maps and choriocapillary segmentation are gaining popularity among the researchers for the evaluation of pathophysiologic alterations in macular disease.
High myopia (HM) is one of the leading causes of visual impairment in many developed countries [1] because of its possible association with neovascularization, areas of macular chorioretinal atrophy, lacquer crack, and retinoschisis. Thus, to understand microvascular early, changes may be of clinical relevance. Although quantitative information from recent studies found that in HM the density of both the superficial and deep microvascular plexuses is significantly decreased and negatively correlated with the axial lengths [2, 3], less is known about choriocapillaris (CC). Thinner choroid is typically related to HM, and the presence of large areas of chorioretinal atrophy may further complicate choriocapillaris assessment, resulting in a challenge in some cases. Consequently, findings from some studies may be controversial. For example, the evaluation of choriocapillary flow is increased according to some researchers [4] but not according to others [5]. Moreover, although a reduction in macular vascular density has been described [2,3,4, 6], some clinical features such as the evaluation of the foveal avascular zone (FAZ) and outer retina flow variations have not been amply investigated to date.
It should be also considered that in most of the previous studies about HM, the microvascular characteristics were investigated separately, and that calculation software external to the OCT angiography device was used for quantitative measurement.
Therefore, the aim of the present study is to assess macular microvascular features more comprehensively, along with CC blood flow and retinal thickness correlations in a group of myopic eyes using an advanced OCT angiography.
Materials and methods
This study was conducted in accordance with the tenets of the Declaration of Helsinki, and informed consent was obtained from each individual. The eyes of the patients, all Caucasians, presenting in Retina 3000 Eye Center, Milano, Italy, in 2016 with HM (refractive error ≥ − 6D) and the eyes of the controls (refractive error 0 ± 2D) were enrolled. Refraction data were converted to the spherical equivalent, which was calculated as the spherical dioptric power plus one-half of the cylindrical dioptric power. The complete ophthalmic examinations of all subjects were unremarkable, and the best corrected visual acuity was ≥ 20/25. In particular, exclusion criteria were as follows: ocular hypertension, a history of any previous ocular disease or surgery, media opacities (corneal or lenticular opacities), and vitreoretinal disorders such as macular holes or macular puckers. Eyes with areas of chorioretinal or retinal pigment epithelium (RPE) atrophy affecting the macular region were also excluded because these conditions may frequently cause artifacts and alter quantitative analysis.
All participants underwent comprehensive ocular examination including autorefractometer, assessment, and slit-lamp biomicroscopy of the anterior and dilated posterior segment. Intraocular pressure was measured by applanation tonometry.
Optical coherence tomography angiography scans were obtained using the AngioVue, Angioanalytics, XR Avanti device (Software V.2016.1.0.26 Optovue Inc., Fremont, CA, USA) with the angio retina mode (3 × 3 mm). This OCT angiography is FDA approved and avails of the split-spectrum amplitude decorrelation algorithm (SSADA) that operates at 70,000 A scans per second to acquire volumes of 304 X 304 A-scans. Exclusion criteria for OCT angiography examinations were signal strength index < 55, low quality of images with severe artifacts because of poor fixation, and layer segmentation errors (provided automatically by the software).
The following parameters were evaluated: FAZ area, superficial vascular density, choroidal thickness, outer retina flow, and choriocapillaris flow.
Image selection and analysis
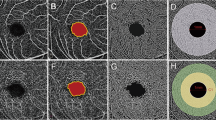
The AngioVue software included in the Avanti device can be used for several layouts with multiple retinal layers segmentations. In our study, the FAZ was calculated automatically in mm2 by the software using the “non-flow” measure function in correspondence to the macular superficial plexus visualization. The superficial vascular plexus is automatically segmented by the software from the inner limiting membrane (with an interface offset of 3 μm) to the inner plexiform layer (IPL) (with an interface offset of 16 μm). Using the “density” measure function, a map was automatically created that provided percentage of areas occupied by vessels in the whole macular area and in the fovea, parafovea, temporal, superior, nasal, and inferior macular areas (Fig. 1). The corresponding retinal thickness was also calculated automatically in micrometers. The blood flow was calculated in mm2 by the “flow” automatic analysis function in a selected area of 3144 mm2 centered on the fovea in the outer retina layer, segmented automatically from the IPL (with an interface offset of 72 μm) and the RPE (with an interface offset of 31) and in the choriocapillaris, segmented automatically from 31 to 59 μm inferiorly to the RPE (Fig. 2).
Map of the vessel density and retinal thickness in a myopic and non-myopic eye (left and right, respectively). Using the “density” measure function, the AngioVue software can provide an automatically elaborated map that calculates the percentage areas occupied by superficial vessels in the whole macular area and in the fovea, parafovea, temporal, superior, nasal, and inferior macular portions. The corresponding retinal thickness included between the inner limiting membrane and the retinal pigment epithelium is also measured in micrometers
Flow measurement in the outer retina and in the choriocapillaris of a myopic (top left and bottom left, respectively) and of a healthy individual (top right and bottom right, respectively). The circular area of 3144 mm2 centered on the fovea is automatically delimitated by the software that calculates in mm2 the total area of multiple bright areas corresponding to the choriocapillary lobular meshwork where the blood flow can be detected by the OCT angiography device
Statistical analysis
Effect magnitude and P values for factors and continuous variables were calculated using different linear mixed models. Random effects were used to account for correlations among clustered observations (two eyes from the same subject or multiple observations from the same eye). Two sets of multivariate linear models were used: the first set dealt with global variables, such as the global superficial vessel density, the choriocapillaris perfusion, and the outer retinal layer perfusion; the second set of models used topographical information to investigate any differential behavior in the superficial vessel density and retinal thickness at different locations of the retina (fovea, parafovea, superior, inferior, nasal, and temporal areas). In the latter, interactions were used to model and test different behaviors at the various locations. Only significant interactions (P < 0.05) were kept in the final models used for the analysis. All P values were calculated from the linear models using a t-type statistics on the effect estimates, as provided by the package Ime4 for R. We only reported effect magnitude since correlation coefficients and R2 indices are not univocally defined for mixed models. All calculations were performed in an R scripting environment (R Foundation for Statistical Computing, Vienna, Austria). Results are reported as mean ± SD (standard deviation) or estimate ± SE (standard error) according to whether they were raw calculations or marginal estimates from a model. In the topographical models, 95% confidence intervals are reported along with the estimates.
Results
A total of 42 myopic eyes from 37 individuals (12 men and 25 women, Caucasians) and 40 control eyes from 28 individuals (13 men and 15 women, Caucasians) were included. Demographics are reported in Table 1. Age was comparable between the two groups (51.85 ± 10.87 and 56.24 ± 16.64 years in the myopic and normal subjects, respectively; P = 0.19, mean ± SD). As expected, the spherical correction was significantly different between the two groups (− 10.26 ± 3.83 diopters and − 0.07 ± 1.44 diopters for the myopic and non-myopic eyes, respectively; P < 0.0001). Since there were more females in the myopia group, this is not a truly matched cohort.
Global analysis
We first analyzed the effect of myopia on the FAZ area, the whole superficial vessel density, the choriocapillaris, and the outer retina perfusion. The results are reported in Table 2. The effect of myopia was explored both in terms of differences between the two groups and in terms of correlation with the dioptric continuous value. The two analyses were performed separately to avoid correlation between the two predictor factors (group classification and spherical correction). Both analyses were corrected for age using a multivariate model. The effect of age as calculated in the analysis with the group classification was significant (negatively) for the whole superficial vessel density (P = 0.006), but not for the FAZ area, the choriocapillaris, and the outer retina perfusion (P = 0.17, P = 0.92, and P = 0.3, respectively). Myopic eyes presented a lower whole superficial vessel density (46.4 ± 4.9 vs.51.6 ± 3.6%, P < 0.0001) and higher flow area in the outer retina (1.3 ± 0.2 vs. 1.1 ± 0.3 mm2, P = 0.0012). Between the myopic and healthy eyes, no significant differences could be detected in the choriocapillaris perfusion area (1.9 ± 0.07 vs 1.9 ± 0.05 mm2, respectively; P = 0.55) or in the FAZ area (0.23 ± 0.1 vs 0.26 ± 0.1 mm2, P = 0.12). These findings were partially confirmed when the correlation with the spherical correction was explored, reporting a significant positive correlation with the superficial vessel density (P < 0.0001, meaning that vessel density decreased with more negative spherical values) and a significant negative correlation with the outer retina perfusion (P = 0.0021). Again, no significant correlation could be found between the spherical correction and the FAZ area or the choriocapillaris flow (P = 0.12 and P = 0.07, respectively).
Topographic analysis
We further characterized the differences in terms of retinal thickness and superficial vessel density at different retinal locations. The results are reported in Table 3. The retina was generally thicker in normal patients, but no statistically significant differences could be detected between the two groups at any location, except for the foveal region, where the myopic patients had a significantly thicker retinal thickness (273.62 ± 25.7 vs 257.87 ± 22.19 μm, P = 0.0025). On the other hand, we found a significantly reduced superficial vessel density in the myopic patients in all locations (P < 0.001), except in the foveal region, where no significant differences could be detected (P = 0.45).
As mentioned in the previous section, we analyzed the effect of myopia on retinal thickness and superficial vessel density by using the spherical correction as a continuous predictor (Table 4 and Fig. 3). Confirming the previous finding, the spherical correction was negatively correlated with the foveal thickness (P = 0.01) and positively correlated with the superficial vessel density at all locations (P < 0.001), except for the fovea (P = 0.27).
We then investigated the local correlation between the superficial vessel density and the local retinal thickness (Table 5 and Fig. 4). We found a significant positive correlation at all locations (P < 0.005), especially in the foveal region (P < 0.0001). We also explored interactions of the terms to investigate whether the thickness-density correlation could change between the myopic and normal patients, but no significant differences could be found (P > 0.2).
Discussion
The present study evidenced a reduced macular vascular density and a reduced outer retina flow in myopic eyes, but no significant differences between FAZ and CC flow areas measurements.
Consistently to previous reports [2,3,4, 6], in fact, we found that the whole macular vascular density is reduced in myopic eyes and it is correlated with the spherical equivalent (P < 0.0001, Table 2). This finding may be secondary to ocular elongation typically related to HM [2] or to reduced retinal blood flow as described by some authors using either the laser Doppler velocimetry technology [7,8,9] or the dynamic vessel analyzer software [10]. It has been also speculated that biomechanical stretching of the retina, the choroid, and the sclera can cause straightening and narrowing of the vessels and consequent capillary density reduction [4]. In our study, the macular vascular density was reduced in HM not only in the entire macular region but also in every portion of the macular area (topographic analysis, P < 0.0005, Table 3), except in the fovea (P = 0.45, Table 3) as confirmed by the analysis of the spherical correction used as a continuous predictor factor (Table 4). It is probable that the superficial vessel density in the foveal area is much lower in both myopic and non-myopic individuals for the presence of the FAZ (Table 3). Likely, no significant differences were disclosed in the FAZ area measurements in both groups; in fact, the FAZ area showed to vary greatly among normal individuals according to age and other factors [11,12,13,14] and, consistently with our findings, no significant association with axial length has been reported to date [11, 15, 16].
Despite other studies [17, 18], a mild retina thinning was present in all the sections of myopic eyes but not with statistical significance, whereas thickening of the foveal area was found with statistical significance (P = 0.0025, Table 3). The increased foveal thickness in myopic eyes has been already reported [19,20,21,22], and Wu with associates postulated that elevation of the fovea may be secondary to mechanical stretching of the eyeball with internal limiting membrane flattening resulting in foveal elevation and panretinal thinning [19]. This evidence is confirmed by the analysis of the spherical correction as continuous predictor factor (P = 0.011, Table 4 top).
One interesting and unreported finding of our study is the positive correlation between the retinal thickness and the superficial vessel density in every portion of the macular area and especially in the fovea (P < 0.005 and P < 0.0001, respectively). This relationship is not influenced by the refractive status, and, accordingly, no differences between the myopic and control groups could be found (P > 0.2). Although it is possible that increased vascular density may cause increased retinal thickness, this feature may need further investigation.
The V.2016.1.0.26 version of the AngioVue software can provide measurement of the blood flow in two automatically segmented retinal layers, the outer retina (between the IPL and the RPE), and the CC (31 μm posterior to the RPE). In both cases, the resulting elaboration is a granular image constituted by dark and bright adjacent areas corresponding to the absence or presence of blood flow, respectively, as detected by the tool. Flow measurement is expressed in terms of mm2 within a selected area of 3144 mm2 automatically centered on the fovea. Although this kind of flow evaluation is relatively new and therefore needs to be validated and compared, our study showed increased flow in the outer retina of myopic eyes, with a positive correlation to the degree of myopia and independent of age (Table 3). However, this interesting finding should be interpreted with caution because the automatic projection-artifact removal algorithm may be affected by minor superficial vessel density in myopic eyes, resulting in an augmented preservation of deeper vascular layer images. It is also inconsistent with the only two studies that investigated quantitative changes in both the outer retina density and flow: vessel density was found independent of axial length and to decrease with older age [15], and vascular flow showed no significant differences between myopic and healthy individuals [5]. In these studies, which included Chinese individuals instead of Caucasians, the measurements were evaluated in percentage maps and not in mm2 and were calculated using external software. These factors could explain the discrepancies. Mo and associates even found lower flow in myopic than in emmetropic eyes [5], but the presence of areas of chorioretinal atrophy in their series should have altered this measurement because of underestimation due to the shadowing effect caused by the lesions. We specifically excluded eyes with pathological fundus changes in our enrollment protocol.
On OCT angiography, the typical CC granular imaging reflects the anatomical meshwork of densely packed and interconnected capillaries that functions in a lobular mode in terms of blood flow [23]. Investigation of myopic CC is limited and complicated by extensive thinning of the choroid, typically related to this condition, progressing with age and the degree of myopia [4, 24,25,26]. This probably accounts for the different findings from other, previous studies where CC flow was found reduced [4, 5] or unchanged [15]. We were not able to identify significant measurable flow alteration in the CC associated with myopia (P = 0.55, Table 2) as enhanced by the spherical equivalent used as a continuous factor (P = 0.07, Table 2). The similar value in CC perfusion in both groups is a surprising finding, especially if considering the reduced retinal vascular density and retinal thickness. However, measurements may depend on the choroidal level of segmentation, on the SSADA technology limitations and may also differ if considering either flow-void number/size or density percentages. In fact, Al-Sheikh and associates found in myopic CC a decreased total number of flow voids, but an increased average total flow-void area versus the control group [4]. Similarly, Nicolo and associates showed a different spatial distribution of the vascular flow within the entire choroid in central serous chorioretinopathy, as the flow area at the level of CC was significantly smaller than at the deeper choroid, probably as a compensatory mechanism [27].
The impact of age on our series appears significantly inversely correlated only to the whole retinal vascular density, in agreement with other studies [14, 15], presumably because of physiologic age-related loss in vascularity. Aging does not seem to affect flow neither of the outer retina nor of the CC.
We acknowledge that some limitations affect this study: first, its retrospective design, single center, and monoracial background. Accuracy, sensitivity, and reproducibility of OCT angiography-derived maps are currently under investigation and continuously ameliorated, but artifacts may still alter measurements on some occasions, especially in HM [28]. Significant variability exists in FAZ and capillary density measurements among different machines and techniques [29]. The deep location of the choriocapillaris may have been associated with a loss in spatial resolution owing to the reduced penetration of the OCT signal beyond the RPE. Evaluation data of the CC might therefore have been less precise than those of the intraretinal vascular networks. Also, it could have been interesting to evaluate vessel density maps of the deep capillary plexus and of the choriocapillaris; however, these automatic elaborations were not available in the software at the time of the study and are particularly affected by artifacts and segmentation errors, resulting in poor reproducibility.
Overall, this paper presents some potential interesting points too. Using the device’s automated software provided by the company, it is the first study to describe quantitative features of the outer retina flow in Caucasians with HM and is presumably the first to assess the relationship between superficial vascular density and retinal thickness in a group of myopic and non-myopic individuals. Future studies may clarify this relationship and further investigate the role of choriocapillary flow.
References
Wong TY, Ferreira A, Hughes R et al (2014) Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 157:9–25
Li M, Yang Y, Jiang H et al (2017) Retinal microvascular network and microcirculation assessments in high myopia. Am J Ophthalmol 174:56–67
Yang Y, Wang J, Jiang H et al (2016) Retinal microvasculature alteration in high myopia. Invest Ophthalmol Vis Sci 57:6020–6030
Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R et al (2017) Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Invest Ophthalmol Vis Sci 58:2063–2069
Mo J, Duan A, Chan S, Wang X, Wei W (2017) Vascular flow density in pathological myopia: an optical coherence tomography angiography study. BMJ Open 7(2):e013571
Fan H, Chen HY, Ma HJ et al (2017) Reduced macular vascular density in myopic eyes. Chin Med J (Engl) 130:445–451
Shimada N, Ohno-Matsui K, Harino S et al (2004) Reduction of retinal blood flow in high myopia. Graefes Arch Clin Exp Ophthalmol 242:284–288
Akyol N, Kukner AS, Ozdemir T, Esmerligil S (1996) Choroidal and retinal blood flow changes in degenerative myopia. Can J Ophthalmol 31:113–119
Benavente-Perez A, Hosking SL, Logan NS, Broadway DC (2010) Ocular blood flow measurements in healthy human myopic eyes. Graefes Arch Clin Exp Ophthalmol 248:1587–1594
La Spina C, Corvi F, Bandello F, Querques G (2016) Static characteristics and dynamic functionality of retinal vessels in longer eyes with or without pathologic myopia. Graefes Arch Clin Exp Ophthalmol 254:827–834
Tan CS, Lim LW, Chow VS et al (2016) Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Invest Ophthalmol Vis Sci 57:224–234
Samara WA, Say EA, Khoo CT et al (2015) Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 35:2188–2195
Yu J, Jiang C, Wang X et al (2015) Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci 56:3212–3217
Iafe NA, Phasukkijwatana N, Chen X, Sarraf D (2016) Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Investig Ophthalmol Vis Sci 57:5780–5787
Wang Q, Chan S, Yang JY et al (2016) Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol 168:95–109
Chui TY, VanNasdale DA, Elsner AE, Burns SA (2014) The association between the foveal avascular zone and retinal thickness. Invest Ophthalmol Vis Sci 55:6870–6877
Myers CE, Klein BE, Meuer SM, Swift MK, Chandler CS, Huang Y et al (2015) Retinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: the beaver dam eye study. Am J Ophthalmol 159:445–456.e1
Song WK, Lee SC, Lee ES, Kim CY, Kim SS (2010) Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. Invest Ophthalmol Vis Sci 51:3913–3918
Wu PC, Chen YJ, Chen CH et al (2008) Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye (Lond) 22:551–555
Zhao Z, Zhou X, Jiang C, Sun X (2015) Effects of myopia on different areas and layers of the macula: a Fourier-domain optical coherence tomography study of a Chinese cohort. BMC Ophthalmol 15(1):90
Lam DS, Leung KS, Mohamed S et al (2007) Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci 48:376–382
Sato A, Fukui E, Ohta K (2010) Retinal thickness of myopic eyes determined by spectralis optical coherence tomography. Br J Ophthalmol 94(12):1624–1628
Spaide RF (2016) Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol 170:58–67
Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM (2013) The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol 155:314–319.e1
Ho M, Liu DT, Chan VC, Lam DS (2013) Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology 120:1909–1914
Ikuno Y, Fujimoto S, Jo Y, Asai T, Nishida K (2013) Choroidal thinning in high myopia measured by optical coherence tomography. Clin Ophthalmol 7:889–893
Nicolò M, Rosa R, Musetti D et al (2017) Choroidal vascular flow area in central serous chorioretinopathy using swept-source optical coherence tomography angiography. Invest Ophthalmol Vis Sci 58:2002–2010
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence tomography angiography. Retina 35:2163–2180
Magrath GN, Say EA, Sioufi K et al (2017) Variability in foveal avascular zone and capillary density using optical coherence tomography angiography machines in healthy eyes. Retina 37:2102–2111
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Milani, P., Montesano, G., Rossetti, L. et al. Vessel density, retinal thickness, and choriocapillaris vascular flow in myopic eyes on OCT angiography. Graefes Arch Clin Exp Ophthalmol 256, 1419–1427 (2018). https://doi.org/10.1007/s00417-018-4012-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4012-y