Abstract
Purpose
Only a few reports in the literature have investigated the presence of ocular abnormalities in neurofibromatosis type 1 (NF-1) patients. The aim of this study was to evaluate the prevalence of ocular abnormalities in a large population of NF1 patients, focusing on the choroidal changes.
Methods
This study was conducted on 160 consecutive patients with NF1 and 106 sex- and age-matched healthy subjects (control). Each patient underwent a complete ophthalmological examination inclusive of best-corrected visual acuity, intraocular pressure measurement, slit-lamp biomicroscopy, indirect fundus biomicroscopy, and near-infrared reflectance (NIR) retinography by using the spectral domain OCT. Moreover, all patients underwent complete dermatological exam and 1.5-Tesla MRI scan of the brain to assess the presence of optic nerve gliomas.
Results
Choroidal abnormalities were detected in 97% of patients, with a positive predictive value of 100% and a negative predictive value of 96.4%. Interestingly, a small number of patients (4/160; 2.5%) showed Lisch nodules without choroidal abnormalities, whereas a larger number of patents (22/160; 13.75%) presented choroidal lesions in absence of Lisch nodules. None of the patients showed the absence of both choroidal lesions and Lisch nodules. The number of choroidal lesions increased with age (r = 0.364, p = 0.0001) and with the severity of pathology (r = 0.23, p = 0.003). Any statistically significant correlation between choroidal lesions, visual acuity, and intraocular pressure was observed.
Conclusions
NIR imaging represents an in vivo, non-invasive, sensitive and reproducible exam to detect choroidal nodules in NF-1 patients, suggesting that choroidal changes may represent an additional diagnostic criteria for NF1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type I (NF1), also known as Von Recklinghausen’s disease, is one of the most common autosomal dominant disorders, occurring with an estimated incidence of 1:3000 and a prevalence of 1:4000 to 1:5000 [1]. Iris Lisch nodules, which are described as iris hamartomas, represent the most known ocular involvement in NF1 [2]. Further ocular manifestations include optic gliomas, orbital and eyelid neurofibromas, eyelid café-au-lait spots, choroidal nevi, congenital absence of the greater wing of sphenoid bone, and congenital glaucoma [3]. Another ocular feature of recent observation is represented by choroidal abnormalities, described as ovoidal bodies consisting of proliferating Schwann cells arranged in concentric rings around an axon [4]. They are fully asymptomatic and undetectable by using conventional ophthalmoscopy or fluorescein angiography [5]. These abnormalities were originally described as hypofluorescent patches in the early choroidal angiographic phases when performing indocyanine green angiography [6]. More recently, the spectral domain OCT in near-infrared (NIR-OCT) reflectance modality, a noninvasive tool, was claimed to provide superior visibility of these features. There are only a few reports in the literature, predominantly conducted on the pediatric population that analyze the presence of choroidal abnormalities in NF-1 patients [7, 8]. The aim of this study was to evaluate, in a large sample of consecutive patients with NF1, the prevalence, sensitivity, and specificity of NF1-related choroidal abnormalities.
Materials and methods
An observational, cross-sectional study was carried out on 160 patients (320 eyes) with a diagnosis of NF1 between October 2014 and January 2016 and on 106 healthy control subjects (212 eyes) matched for age, gender, and race at the University of Rome ‘Sapienza’, Umberto I Hospital, Italy.
We included patients diagnosed as having NF1 on the basis of the National Institutes of Health (NIH) criteria [9]. Exclusion criteria were:
-
poor-quality NIR reflectance images that prevented evaluation and quantification of data (i.e., media opacities, fixing defects in pediatric patients)
-
refractive defects greater than ±6 D (spherical equivalent);
-
any other previous or coexisting ocular disease that could affect choroidal or retinal appearance (i.e., uveitis, congenital ocular malformations, retinal hamartomas, maculopathy).
More in detail, nine patients were excluded because of severe ocular media opacities, three patients because of myopic chorioretinitis, four patients because of previous or coexisting choroidal e/or retinal diseases. In addition, one pediatric patient was judged not able to comply with study procedures.
The presence of gliomas and osseous lesions was previously assessed through appropriate diagnostic exams at the ‘Rare Diseases Centre’ of the Umberto I Hospital, Rome. NF-1 first-degree familiarity was evaluated in both patients and controls. The severity grading was evaluated according to the Riccardi Scale and the Ablon visibility index [10]. Each patient underwent dermatologic and ophthalmologic examination to evaluate the presence/absence of each NIH diagnostic criterion. All patients underwent a comprehensive ophthalmological examination including measurement of the best-corrected visual acuity, intraocular pressure, slit-lamp biomicroscopy to detect iris Lisch nodules, mydriatic indirect fundus biomicroscopy, and near-infrared (NIR) reflectance imaging with SD-OCT. NIR images (787 nm excitation) were obtained by using the spectral domain OCT (Spectralis Family Acquisition Module, V 5.1.3.0; Heidelberg Engineering) with Heidelberg Eye Explorer (V 1.6.2.0). All patients were dilated with tropicamide1% (Visufarma, Italy) and examined with NIR retinography scans by two experienced investigators.
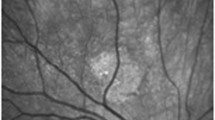
Ophthalmologic findings were masked to the dermatologists performing patients’ evaluation. Moreover, two investigators, masked to other patient characteristics, independently assessed Lisch nodules on the iris by slit-lamp biomicroscopic examination and choroidal abnormalities by using NIR-OCT. The presence of Lisch nodules as a diagnostic criterion was defined as the presence of at least two Lisch nodules (NIH criteria). Considering that choroidal abnormalities are found in all of the regions of the fundus but are more frequent at the posterior pole [11], we evaluated the total number of choroidal abnormalities by means of a 30° infra-red reflectance image centered on the fovea. Choroidal abnormalities appeared as bright, patchy regions in NIR images (Fig. 1). We defined their presence on the basis of at least two hyperreflective choroidal spots, as suggested by Viola et al. [12], and with accordance of both the masked investigators. Descriptive statistical analysis was performed in all collected data. Intraobserver and interobserver agreement was evaluated with Cohen’s kappa coefficient. To evaluate the association between categorical variables, we used the Pearson’s Chi-squared test. The correlations between the number of choroidal lesions, patient age, and grading of pathology were calculated by using the Spearman Rho index. To evaluate the predictability and the diagnostic accuracy of variables associated to the pathology, we calculated the sensitivity, specificity, positive and negative predictive values with corresponding 95% confidence intervals. P values of 0.05 or less were considered statistically significant. The study was approved by the Ethics Committee of the Sapienza University of Rome. The study followed the tenets of the Declaration of Helsinki.
Results
One-hundred sixty consecutive patients (74 males and 86 females; mean age 32 ± 17 years), and 106 healthy subjects (46 males and 60 females, mean age 31 ± 16 years) were included in the study.
Demographic and clinical characteristics of the 160 patients designed as having NF-1 are given in Table 1. Table 2 shows the frequency of the NIH diagnostic criteria and of the choroidal lesions. We detected choroidal abnormalities in 97% of patients, with a positive predictive value of 100% and a negative predictive value of 96.4%. Choroidal patchy lesions were present in both eyes of each positive patient, whereas no choroidal lesions were observed within our control group. The number of choroidal nodules detected by NIR-OCT showed a high intraobserver and interobserver agreement evaluated with Cohen’s kappa coefficient (results between 88 and 95).
The diagnostic indicators of choroidal abnormalities and of the NIH diagnostic criteria are compared in Table 3. Interestingly, a small number of patients (4/160; 2.5%) showed Lisch nodules without choroidal abnormalities, whereas a larger number of patents (22/160; 13.75%) presented choroidal lesions in absence of Lisch nodules. Moreover, nobody showed the absence of both choroidal lesions and Lisch nodules. The number of choroidal lesions increased with age (Spearman Rho Index, r = 0.364, p = 0.0001) and with the severity of pathology (Spearman Rho Index, r = 0.23, p = 0.003). We did not find any statistically significant correlation between choroidal lesions, visual acuity, and intraocular pressure (Table 4).
In addition, subgroup analysis in the pediatric population (19 children 10 M; 9F, under 12 years of age) showed that choroidal lesions were detected in 18 pediatric patients (18/19; 95%) whereas Iris Lisch nodules were observed in only ten children (10/19; 52%).
Discussion
This study, conducted on a large sample of consecutive NF-1 patients, confirms the diagnostic role of an in vivo, non-invasive tool represented by NIR imaging in the evaluation of NF1-related choroidal abnormalities [13]. The choroidal alterations detected by the NIR-OCT may represent hamartomatous lesions, referable to electron microscopic findings conducted by Kurosawa on an enucleated eye in 1982 [4]. Furthermore, choroidal lesions are similar to iris Lisch nodules, given the same embryological origin from the neural crest [10]. According to this assertion, melanocytes’ proliferation in NF1 patients causes a patchy choroidal thickening, resulting in a strong absorption and a subsequent backscattering of near-infrared light through the high content of melanin. We counted the total number of choroidal abnormalities within a 30° angle of view centered on the fovea, considering that the choroid of the major retinal vascular arcade has a great number of melanocytes and neural cells [14]. We did not find any relationship between choroidal lesions’ localization and the course of retinal vessels. In accordance, previous studies did not find any remarkable abnormalities by using fluorescein angiography, so it is questionable that the lesions are caused by retinal or choriocapillaris circulation failure [5, 15, 16]. Different authors previously studied the prevalence of choroidal abnormalities in small samples of NF1 patients, with similar results [7, 12]. Moreover, choroidal lesions showed the highest sensitivity among NIH diagnostic criteria (97.5%), second only to café-au-lait spots (98%). Particularly, we identified a higher sensitivity than iris Lisch nodules (86%), the most common type of ocular involvement in NF-1. Choroidal bright, patchy nodules under infrared monochromatic light have already been described as normal areas under conventional ophthalmoscopic examination, fluorescein angiograms, and autofluorescence [5, 17]. Conversely, indocyanine-green angiography, at a wavelength similar to that of NIR, is considered to be a useful, even though invasive, tool in the detection of these NF1 related choroidal lesions [6].
We evaluated the correlations between choroidal abnormalities, patient age, the severity of pathology, Lisch nodules, and the other NIH diagnostic criteria for NF-1 and interestingly found a statistically significant correlation between choroidal lesions and the severity of pathology (Spearman Rho Index, r = 0.23, p = 0.003). A strong, positive relationship between choroidal lesions and age (Spearman Rho Index, r = 0.364, p = 0.0001) was also observed, in line with previous studies [7, 8, 12, 18]. Choroidal nodules also seem to be highly associated with all NIH diagnostic criteria, except for osseous lesions, probably because of the rarity of this sign in our population. Choroidal lesions were detected in 18 of our 19 pediatric patients, whereas Iris Lisch nodules were observed in only ten children; supporting choroidal lesions’ role as potential predictors of pathology.
As a limit to the present study, evaluation was performed only within the 30° of the central retina, and consequently the presence of choroidal alterations in the peripheral retina of negative patients cannot be excluded. Nakakura et al. explored the whole detectable fundus under confocal scanning laser ophthalmscopy, concluding that choroidal lesions were more prominent within the vascular arcade than in the other four regions they studied (supero-temporal, infero-temporal, supero-nasal, and infero-nasal). The results of this study show that choroidal lesions rise in parallel with age, severity of pathology, Lisch nodules, neurofibromas, café-au-lait spots, familiarity, optic pathway gliomas, and freckling. However, further longitudinal, prospective studies are required to demonstrate that the number of choroidal lesions increases with patients’ aging.
In conclusion, our study supports NIR imaging as an in vivo, non-invasive, non-contact, fast, sensitive, and reproducible examination to detect choroidal nodules in NF-1 patients and suggests that choroidal lesions should be added to the actual diagnostic criteria for NF1.
References
Ratner N, Miller SJ (2015) A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer 15(5):290–301. https://doi.org/10.1038/nrc3911
Richetta A, Giustini S, Recupero SM, Pezza M, Calvieri S (2004) Lisch nodules of iris in Neurofibromatosis type 1. J Eur Dermatol Venerol 18(3):342–344. https://doi.org/10.1111/j.1468-3083.2004.00915.x
Hirbe AC, Gutmann DH (2014) Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 13(8):834–843. https://doi.org/10.1016/S1474-4422(14)70063-8
Kurosawa A, Kurosawa H (1982) Ovoid bodies in choroidal neurofibromatosis. Arch Ophthalmol 100(12):1939–1941
Yasunari T, Shiraki K, Hattori H, Miki T (2000) Frequency of choroidal abnormalities in neurofibromatosis type 1. Lancet 356(9234):988–992. https://doi.org/10.1016/S0140-6736(00)02716-1
Byun YS, Park YH (2012) Indocyanine green angiographic findings of obscure choroidal abnormalities in neurofibromatosis. Korean J Ophthalmol 26(3):230–234. https://doi.org/10.3341/kjo.2012.26.3.230
Parrozzani R, Clementi M, Frizziero L, Miglionico G, Perrini P, Cavarzeran F et al (2015) In vivo detection of choroidal abnormalities related to NF1: feasibility and comparison with standard NIH diagnostic criteria in pediatric patients. Invest Ophthalmol Vis Sci 56(10):6036–6042
Vagge A, Camicione P, Capris C, Sburlati C, Panarello S, Calevo MG et al (2015) Choroidal abnormalities in neurofibromatosis type 1 detected by near-infrared reflectance imaging in paediatric population. Acta Ophthalmol 93:e667–e671
National Institute of Health Consensus Development (1988) Neurofibromatosis. Conference statement. Arch Neurol 45:575–578
Wallace MR (2000) Neurofibromatosis: phenotype, natural history, and pathogenesis. Am J Hum Genet 67(1):264
Nakakura S, Shiraki K, Yasunari T, Hayashi Y, Ataka S, Kohno T et al (2005) Quantification and anatomic distribution of choroidal abnormalities in patients with type I neurofibromatosis. Graefes Arch Clin Exp Opthalmol 243(10):980–984
Viola F, Villani E, Natacci F, Selicorni A, Melloni G, Vezzola D et al (2012) Choroidal abnormalities detected by near-infrared reflectance imaging as a new diagnostic criterion for neurofibromatosis 1. Ophthalmology 119(2):369–375
Tadini G, Milani D, Menni F, Pezzani L, Sabatini C, Esposito S (2014) Is it time to change the neurofibromatosis 1 diagnostic criteria? Eur J Intern Med 25(6):506–510
Naumann GOH, Apple DJ (1980) Uvea, pathology of the eye. Springer-Verlag, New York
Makino S, Tampo H (2013) Optical coherence tomography imaging of choroidal abnormalities in neurofibromatosis type 1. Case Rep Ophthalmol Med 2013:292981
Ueda-Consolvo T, Miyakoshi A, Ozaki H, Houki S, Hayashi A (2012) Near-infrared fundus autofluorescence-visualized melanin in the choroidal abnormalities of neurofibromatosis type 1. Clin Ophthalmol 6:1191–1194
Ayata A, Unal M, Ersanli D, Tatlipinar S (2008) Near-infrared fluorescence and OCT features of choroidal abnormalities in type 1 neurofibromatosis. Clin Exp Ophthalmol 36(4):390–392
Makino S, Tampo H, Arai Y, Obata H (2014) Correlations between choroidal abnormalities, Lisch nodules, and age in patients with neurofibromatosis type 1. Clin Ophthalmol 8:165–168
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee o Sapienza University of Rome and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Moramarco, A., Giustini, S., Nofroni, I. et al. Near-infrared imaging: an in vivo, non-invasive diagnostic tool in neurofibromatosis type 1. Graefes Arch Clin Exp Ophthalmol 256, 307–311 (2018). https://doi.org/10.1007/s00417-017-3870-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3870-z