Abstract
Purpose
The purpose of our study was to describe ultra-widefield (UWF) imaging and optical coherence tomography angiography (OCT-A) findings in affected and fellow eyes of patients with Coats’ disease.
Methods
Consecutive patients affected by Coats’ disease were prospectively recruited at the Department of Ophthalmology, San Raffaele Hospital, Milan, Italy in this cross-sectional, observational study. Patients underwent UWF color fundus photographs, UWF green autofluorescence, UWF fluorescein angiography (FA), optical coherence tomography (OCT), with 3 × 3 mm and 6 × 6 mm OCT-A scans of the macula. Images were qualitatively evaluated by two independent operators for the presence of pathology.
Results
Eleven patients affected by Coats’ disease (eight males, mean age 17.1 ± 6.7 years). Nine and two patients had a clinical diagnosis of unilateral and bilateral disease, respectively. Five eyes had macular fibrosis. All clinically affected eyes exhibited retinal pathology at UWF imaging with the temporal sector most involved followed by the inferior, nasal, superior and macula. In all eyes with macular fibrosis, OCT-A revealed replacement of the foveal avascular zone with coarse vessels suggestive of vascularized fibrosis and flow void area in the choriocapillaris due to a masking effect; type 3 neovascularization was seen in 75% of cases. Seven out of nine clinically unaffected fellow eyes showed retinal pathology at UWF FA with the temporal quadrant most involved.
Conclusion
We demonstrated that Coats’ disease is a highly asymmetric bilateral disease and that UWF imaging is able to identify more retinal pathology than standard fundus imaging, thus guiding proper retinal photocoagulation. OCT-A allowed easy identification of type 3 neovascularization in a proportion of patients with macular fibrosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coats’ disease is a non-hereditary, idiopathic retinal vascular disease characterized by telangiectasia, intra- and sub-retinal exudation, capillary non-perfusion and artero-venous shunt [1]. Coats’ disease can be complicated by sight-threatening conditions, including exudative retinal detachment, macular fibrosis, epiretinal neovascularization, cataract, neovascular glaucoma, and phithis bulbi [1]. The disease usually affects young males with a median age of 5-years-old, and it is unilateral in 95% of eyes [2]. The largest clinical study about Coats’ disease (150 cases), published about 15 years ago by Shields and colleagues [2], was based mainly on traditional retinal imaging techniques, including standard color fundus photography and fluorescein angiography (FA).
Recent technological advances, together with the opportunity to combine different imaging modalities (multimodal imaging), have revolutionized the approach to many retinal diseases. For instance, widefield and ultra-widefield (UWF) fundus photography and FA allow greater exploration of the mid-to-extreme retinal periphery, overcoming the pitfalls of standard fundus camera (e.g., the need of mydriasis, patient collaboration, visualization of 50° at once, and inability to image all retina at the same) [3]. The Optos camera (Optos, PLC, Scotland) is a confocal selective laser ophthalmoscope instrument equipped with an ellipsoid mirror with two focal points, which image 82% of human retina (200°) in a single shot, with no need of mydriasis or contact lenses. Field of view can be further widened by merging images taken under a different steering position, as recently described [4]. UWF is more informative about retinal pathology than the traditional fundus camera and has been used to guide selected laser photocoagulation [5, 6].
Optical coherence tomography angiography (OCT-A) is a novel technology which visualizes retinal and choroidal vasculature in a rapid, non-invasive and dye-less fashion [7]. Unlike FA, OCT-A allows distinguishing superficial from deep capillary plexus and to study them individually. Only small case series and a case report have been published so far concerning OCT-A in Coats’ disease, highlighting non-specific abnormalities in the foveal avascular zone (FAZ), capillary non-perfusion, dilated capillary network, and aneurysmal outpouchings in macular capillary bed. To our knowledge, the literature lacks a systematic description of Coats’ disease features imaged by means of multimodal imaging, including UWF and OCT-A. Moreover, as pointed out by a recent narrative review, several questions are still unresolved regarding Coats’ disease, including the unilateral vs. asymmetric bilateral involvement and the pathogenesis of macular fibrosis [8].
The aim of this study is to describe UWF imaging and OCT-A findings in affected and fellow eyes of patients with Coats’ disease.
Materials and methods
Consecutive patients affected by Coats’ disease presenting between February 2016 and January 2017 at the Medical Retina & Imaging Unit of the Department of Ophthalmology, University Vita-Salute, San Raffaele Hospital were prospectively recruited in this cross-sectional observational study. The study was conducted in compliance with the Declaration of Helsinki and all patients signed a written consent to participate in observational studies approved by the ethics committee of San Raffaele Hospital.
Diagnosis of Coats’ disease relied on the presence of idiopathic retinal telangiectasia with intraretinal and/or subretinal exudation without retinal or vitreal traction [2]. Patients were excluded in case of any other retinal disease or positive family history for congenital retinal diseases. The disease was graded using the classification proposed by Shields and colleagues [9].
All patients underwent a complete ophthalmic examination, including indirect fundus exam, UWF fundus photograph, UWF green autofluorescence, UWF FA, structural spectral-domain (SD)-OCT (Spectralis, HRA Heidelberg, Heidelberg, Germany), and 3 × 3 mm and 6 × 6 mm OCT-A (Angioplex™, CIRRUS HD-OCT models 5000, Carl Zeiss Meditec, Inc., Dublin, CA, USA) scans of the macula. Medical history and demographic data were also recorded.
UWF imaging was acquired using the Optos California UWF retinal imaging system (Optos PLC, Dunfermline, Scotland, UK). Pupils were dilated with tropicamide 1% and UWF fundus photography and autofluorescence centered on the macula were performed before FA execution. After intravenous injection of fluorescein dye (5 mL of 20% sodium fluorescein), UWF FA was obtained starting from the unaffected eye in the early (< 60 s), middle (2 min and 30 s) and late phases (4–5 min). All UWF images were acquired centered in the macula. Moreover, UWF FA shots were also obtained in the four steering positions (i.e., left, right, up and down) in order to expose the maximum amount of peripheral retina. UWF were graded for the presence of retinal pathology including telangiectasia, vessel beading, exudation, capillary non-perfusion, vessel sheathing, artero-venous anastomosis, capillary network rarefaction, microaneurysm, light bulb aneurysm, macular fibrosis, retinal detachment, and chorioretinal atrophy due to laser photocoagulation and perivascular leakage.
The structural SD-OCT acquisition protocol (Spectralis, HRA Heidelberg, Heidelberg, Germany) included: 19 horizontal raster linear B-scans, each composed of nine averaged OCT B-scans (1024 A-scans per line) at 240 μm intervals, covering an area of 20 degrees by 15 degrees; six radial linear B-scans, each composed of 25 averaged OCT B-scans (768 A-scans per line) at 30 degrees centered on the fovea; 49 horizontal raster dense linear B-scans, each constisting of 16 averaged OCT B-scans (384 A-scans per line) at 30 μm intervals, covering an area of 15 degrees by 5 degrees. The latter two scan sequences were acquired in enhanced depth imaging (EDI) mode.
OCT-A 3 × 3 mm and 6 × 6 mm macula-centered images were acquired with the Cirrus HD-OCT (Angioplex™, CIRRUS HD-OCT models 5000, Carl Zeiss Meditec, Inc., Dublin, CA, USA). Only images with signal quality ≥6 were evaluated. Automatic segmentation of full-thickness retinal (inner limiting membrane to retinal pigment epithelium), superficial, deep and choriocapillary vascular layers was checked and boundaries were manually adjusted in case of segmentation errors.
All images were evaluated by two operators (AR, RS). In the case of discordance, an agreement was reached through an open adjudication. In situations where graders could not reach a consensus, a third senior adjudicator (GQ) made the final determination.
Variables included in this analysis were: age, sex, race, affected eye(s) (right/left), disease stage, age at diagnosis, previous treatments, presence and location of abnormalities at UWF, OCT and OCT-A.
Results
Eleven patients affected by Coats’ disease (eight males/three females, mean age 17.1 ± 6.7 years) were enrolled in this study. Nine patients had a clinical diagnosis of unilateral Coats’ disease, while both eyes of two patients had clinically evidenced disease. Demographic features and main clinical data are displayed in Table 1. Two patients (#5, #8) had end-stage disease in the clinically affected eye (i.e., stage 5 according to Shields’ classification [9]), and thus multimodal imaging was performed only in the fellow eyes. One patient (#9) experienced nausea and vomiting following UWF FA and examination was stopped prior to OCT and OCT-A execution. An autofluorescence UWF image of patient #6 was not acquired. Figures 1 and 2 show UWF color fundus images and FA of affected and clinically unaffected eye of two different patients, respectively.
Ultra-widefield (UWF) color image and UWF fluorescein angiography (FA) of an eye affected by Coats’ disease. (Top row, right panel) UWF color image showing the presence of retinal telangiectasia, vascular beading, extrafoveal retinal exudates, intra-retinal hemorrhages and peripheral laser photocoagulation. (Bottom row, right panel) UWF FA confirming the presence of retinal telangiectasia and disclosing perivascular leakage, microaneurysms, capillary bed abnormalities with dilated intercapillary space, non-perfusion and capillary telangiectasia, all better visualized on magnified FA image (left panel)
Ultra-widefield (UWF) color image and UWF fluorescein angiography (FA) of a clinically unaffected fellow eye. (Top row, right panel) UWF color image and (bottom row, right panel) UWF FA showing dilated intercapillary spaces with non-perfusion and telangiectatic capillaries in temporal quadrant, all better visualized on magnified FA image (left panel)
On color UWF images, all clinically affected eyes exhibited retinal pathology. All eyes had retinal telangiectasia and vascular beading. Vascular sheathing was the second most common abnormality, being found in ten out of 11 eyes (91.0%). In five out of 11 affected eyes (45.5%) extrafoveal retinal exudates were clearly visible, and in three of them (27.3%), foveal exudation was also present. Other manifestations included macular fibrosis in five eyes (45.5%), light bulb aneurysms in three eyes (27.3%), dilated intercapillary spaces in one eye (9.1%), retinal hemorrhages in one eye (9.1%) and peripheral fibrosis in one eye (9.1%). Ten eyes (90.1%) had chorioretinal scars secondary to previous laser photocoagulation (nine eyes) or cryotherapy (one eye). Among treated eyes, ghost vessels were detected in nine eyes. Figure 3 shows main abnormalities observed in Coats’ disease as seen at fundus color and FA. Unaffected fellow eyes (n = 9) disclosed normal UWF color images in all cases.
Main abnormalities observed in Coats’ disease as seen at fundus color and fluorescein angiography (FA). (Top row) Fundus color and (bottom row) FA image showing the presence of (first panel) macular fibrosis with retinal exudates, (second panel) light bulb aneurysms with extra-foveal retinal exudates, (third panel) retinal telangiectasia, (fourth panel) vascular beading associated with microaneurysms and intraretinal hemorrhages, (fifth panel) vessel sheathing and (sixth panel) vascular beading associated with microaneurysms and capillary rarefaction
On UWF autofluorescence, four affected eyes exhibited a diffuse background hypo-autofluorescence compared to the fellow eye, as shown in Fig. 4. Extrafoveal and foveal exudates were seen in four and three eyes, respectively. In one eye (#4) extrafoveal exudation observed at UWF color did not have any correspondent change at autofluorescence. Smaller exudates exhibited a stardust hyper-autofluorescence, whereas broad and confluent areas of retinal exudation were characterized by hyper-autofluorescent plaques. Macular fibrosis was seen in four out of ten affected eyes (40%) as a well circumscribed hypo-autofluorescent area surrounded by a variable hyper-autofluorescent halo. Chorioretinal atrophy secondary to laser photocoagulation appeared as discrete hypo-autofluorescence; in case of cryotherapy, scars were bigger and, sometimes, with interposed hyper-autofluorescent areas, related to scleral exposure. In one case, UWF autofluorescence showed a light bulb aneurysm as an area of hyper-autofluorescent roundness nearby one of the major retinal arcades. Retinal hemorrhages were seen as focal hypo-autofluorescence masking underneath retinal autofluorescence. A broad area of peripheral hypo-autofluorescence in the temporal retina was imaged in one eye consistent with peripheral fibrosis. Unlike UWF color images, UWF autofluorescence did not allow proper identification of telangiectasia, vascular sheathing, ghost vessels and dilated intercapillary spaces. As for the color photograph, all clinically unaffected fellow eyes did not exhibit abnormalities.
On UWF FA, all clinically affected eyes and seven out of nine fellow eyes disclosed vascular abnormalities. In affected eyes, temporal and inferior sectors were involved in 11 eyes (100%), followed by nasal (ten out of 11, 90.1%), superior (nine out of 11, 81.9%) and macular area (six out of 11, 54.6%). In these eyes, retinal telangiectasia was the most common abnormality, and it was present in all the retinal quadrants investigated (100%, 90.1%, 63.6% and 45.5% in the temporal, nasal, inferior and superior sectors, respectively). Conversely, retinal telangiectasia was not appreciated in the macular area.
Capillary bed abnormalities with dilated intercapillary space, non-perfusion, and telangiectasic capillaries were found in temporal and nasal sectors in nine eyes (90.1%), followed by inferior in eight (72.7%), superior in five (45.5%) and macula in one (9.1%). Perivascular leakage was recorded in five eyes (45.5%) in the temporal sector and two eyes (18.2%) superiorly, nasally and inferiorly. Macular fibrosis, imaged as a heterogeneous, bride, hyper-fluorescent staining, was present in five eyes, surrounded by a halo of faint hyper-fluorescence in three eyes. Transmission hyperfluorescence was seen in ten eyes due to previous laser treatment (nine eyes) and cryotherapy (one eye). Other retinal abnormalities seen at UWF FA included light bulb aneurysms, exudation, microhemorrhages, artero-venous shunts, peripheral ischemia, peripheral leakage and peripheral fibrosis.
Among clinically unaffected fellow eyes, pathological changes were seen in the extreme peripheral retina, with selective sparing of the macular region. In particular, seven out of nine eyes (77.8%) showed one or more pathological changes in at least one quadrant. Such findings were seen mostly in temporal (seven eyes; 77.8%), followed by nasal and superior (five; 55.6%), and inferior (four; 36.4%) quadrants. The macular area was always spared and pathological changes were seen in the extreme peripheral retina. Dilated intercapillary spaces with non-perfusion and telangiectatic capillaries were the most common abnormality and were seen in seven eyes (77.8%) in the temporal sector, followed by nasal in two eyes (22.2%), inferior and superior in one eye (11.1%). Microaneurysms were seen in two eyes (22.2%) in the temporal sector and one eye (11.1%) in the nasal and inferior quadrants. Other abnormalities were perivascular leakage (one eye), artero-venous shunt (one eye), aneurysm (one eye) and peripheral ischemia (one eye).
OCT showed abnormal findings in seven out of nine eyes (77.8%). Specifically, five eyes presented a hyper-reflective lesion extending from Bruch membrane (BM)/retinal pigment epithelium (RPE) to inner retina associated with profound disruption of foveal anatomy, resultant from macular fibrosis. Among these cases, three eyes featured wrinkling and folding of the inner limiting membrane (ILM), suggestive of fibrosis-induced retinal traction. In the same eyes, exudates and intraretinal cysts were also detected. One eye with macular fibrosis exhibited a sharp hyporeflective defect in the choroidal layer with BM/RPE interruption, interpreted as a focal choroidal excavation. In another eye out of those featuring fibrosis, macular atrophy was also present. Finally, one eye without macular fibrosis who underwent prior vitrectomy and epiretinal/ILM peeling presented dissociated outer nerve fiber layer appearance. Two affected eyes and all unaffected eyes disclosed normal OCT features.
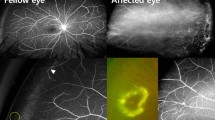
All the study eyes underwent OCT-A; images of two patients (#3, #7) were excluded from the analysis because of artifacts and poor signal quality. Five out of seven affected eyes (71.4%) exhibited qualitative changes. One eye without macular fibrosis had dilated intercapillary spaces with non-perfusion and telengectasic capillaries. Among patients with macular fibrosis, four eyes disclosed FAZ obliterations by irregular, coarse vessels in retinal plexuses (suggestive of vascularized fibrosis), and flow void area in the choriocapillaris due to masking effect. OCT-A revealed the presence of chorioretinal anastomosis in three eyes, as shown in Fig. 5. In two out of seven affected eyes (without macular fibrosis) and in all clinically unaffected fellow eyes, FAZ and macular vascular network at every plexus did not exhibit any qualitative change. Multimodal imaging findings are illustrated in Table 2.
Infrared reflectance (IR), optical coherence tomography (OCT) and OCT-angiography (OCT-A) of an eye affected by Coats’ disease. (First panel) IR and structural OCT B-scan passing through the fovea show a hyper-reflective lesion extending from Bruch’s membrane to inner retina associated with profound disruption of foveal anatomy, resultant from macular fibrosis. (Second and third panel) Full-thickness retina segmentation on 3 × 3 OCT-A, corresponding en-face OCT and B-scan with flow showing disruption of the normal vascular network with absence of the FAZ and the presence of a chorioretinal anastomosis (arrows)
Discussion
In the present study, we examined patients affected by Coats’ disease both in affected and fellow unaffected eye using multimodal imaging, including UWF imaging and OCT-A. UWF color fundus photograph revealed to be the best imaging modality (among those performed in the current study) to reveal specific peripheral fundus clinical signs (i.e., vascular sheathing, ghost vessels, and light bulb aneurysm.) In our study, all the affected eyes exhibited some signs of peripheral pathology; on the other hand, clinically unaffected fellow eyes did not reveal any objective retinal alteration on retinal photography.
To the best of our knowledge, no study dealt with fundus autofluorescence in Coats’ disease. Interestingly, we found diffuse background hypo-fluorescence of affected eyes compared to unaffected fellow eyes in four patients. This finding would suggest diffuse RPE alteration, which should not be primarily affected by the disease. Macular fibrosis appeared as an irregular hypo-autofluorescent area surrounded by a variable hyper-autofluorescent halo, reflecting the heterogeneous components of such lesions, including proteinaceous material, fibrin, macrophages, spindle cells, cholesterol crystals, vessels, pigmented cells, and calcium [10]. Fundus autofluorescence was able to properly identify retinal exudates and chorioretinal atrophy as hyper- and hypo-fluorescent lesions, respectively; however, several other important features of Coats’ disease were missed by this technique, including telangiectasia, vascular sheathing, ghost vessels, and dilated intercapillary spaces.
UWF FA revealed more information than color fundus photography and autofluorescence, both in affected and in fellow eyes. Using FA, Shields et al. [2] reported the presence of retinal telangiectasia in the temporal (42%), the inferior (26%), the superior (5%), and in the nasal sectors (4%), as well as at the posterior pole (1%) in the affected eyes. Moreover, a diffuse involvement was seen in 22% of cases. In our study, we found a similar distribution for telangiectasia with the temporal quadrant being the most involved; conversely, we did not appreciate any telangiectasia in the macular area. The disparity between our study and the one by Shields et al. [2] could be explained by the fact that they used a narrow-field imaging technique and, thus, could have missed very peripheral retinal changes. Other frequent features found only on UWF FA with respect to other imaging modalities were telangiectatic capillary bed, dilated intercapillary space, focal or diffuse retinal non-perfusion, and perivascular leakage.
Since more than 50% of retinal lesions were located in the equator area of the retina, imaging of peripheral retina by means of UWF imaging was revealed to be of great importance in these patients. The use of UWF imaging in the evaluation of Coats’ disease patients would allow the identification of more retinal pathology than traditional imaging and would represent a guide for laser photocoagulation. As highlighted by other studies, Optos UWF FA has other practical advantages over standard FA since it allows the evaluation of retinal periphery with less need for cooperation, with no need for general anesthesia, in the outpatient setting [5, 6]. This comes up to be particularly useful in pediatric patients suffering from Coats’ disease.
As far as it regarded the fellow eyes, seven out nine eyes judged normal at clinical examination and with other imaging modalities, exhibited retinal abnormalities in the extreme periphery only using UWF FA. These findings are in accordance with the study by Blair et al. [11], who retrospectively evaluated the clinically unaffected fellow eyes of patients with unilateral Coats’ disease by means of a RetCam device (Clarity Medical Systems, Pleasanton, CA, USA), displaying 130° of retinal surface. His group found peripheral retinal non-perfusion and microaneurysms in 2/3 of the eyes investigated. However, that study had several limitations including its retrospective design, lack of FA images in one-third of patients and the use of a Retcam device, which is not able to image the very far peripheral retina. In our cohort of patients, retinal lesions followed a similar topographic distribution, with the temporal sector as the most involved, followed by nasal, superior and inferior quadrants, with the macular area always spared. The most common abnormality was the dilation of intercapillary spaces with non-perfusion and telangiectatic capillaries, which is usually found in affected eyes, and it resembles an abortive development of retinal vasculature.
By displaying almost the entire retina surface in a single shot, UWF FA led to the recognition of peripheral retinal abnormalities in other conditions, such as pathologic myopia [12] and branch retinal vein occlusion [13]. Moreover, UWF FA permitted identifying novel angiographic entities, including peripheral vessel leakage [14] and white dots [15]. Interestingly, some subtle peripheral vascular anatomic variations have been detected in eyes without peripheral disease, eyes with floaters, and fellow eyes of patients suffering from retinal vein occlusion or central serous chorioretinopathy [16, 17]. Surprisingly, in a proportion of eyes from patients complaining of floaters but with no other signs of disease, UWF FA disclosed late leakage in the far peripheral retina permitting uncovering mild, intermediate uveitis [16]. Singer and colleagues [4] were the only ones to study the peripheral retina vasculature in a cohort of normal patients. In their study, they confirmed that retinal capillaries do not reach the ora serrata and quantified such nonperfused areas [4]. However, they did not describe any signs of peripheral retinal vascular involvement other than peripheral nonperfusion [4]. In the present study, we demonstrated retinal abnormalities even in the fellow eyes of patients suffering from Coats’ disease. Since these anomalies were mild and clinically silent, we are convinced that they do not represent a relevant clinical risk for patient vision, and they should not modify the management, therapy, monitoring, and prognosis of these patients.
According to these observations, however, Coats’ disease should be considered an asymmetric bilateral disease rather than a unilateral one associated with poor vascular development, similarly to other vitreoretinopathies such as familial exudative vitreoretinopathy (FEVR) or Norrie disease. FEVR is a bilateral, although often asymmetric, exudative vitreoretinopathy linked to mutations in three genes: FZZD4, LRP5, TSPAN12. Norrie disease is an x-linked congenital retinal dysplasia characterized by grayish-yellow mass, termed "pseudoglioma”, due to mutations in the NDP gene. All these genes encode for proteins involved in the Norrin/Fz4/Wnt signaling pathway, which is fundamental for the proper vascular development in the retina and in the inner ear. Black et al. [18] hypothesized that also Coats’ disease derives from somatic mutation in NDP gene. One may ask why a genetically determined condition manifests itself in such an asymmetric way. Other genetic eye diseases in the family of vitreoretinopathies (e.g., FEVR [19] and incontinentia pigmenti [20]) have a marked asymmetric course. A 2-hit model has been advocated to explain the unilateral/asymmetric involvement of Coats’ disease [18]. However, a genetic contribution underlying Coats’ disease is still unknown and further studies are warranted.
In the current literature, only a few reports have inquired about macular microvascular features at OCT-A. Specifically, Yonekawa et al. [21] demonstrated the possibility to individuate signs of Coats’ disease (i.e., capillary non-perfusion, dilated capillary network, aneurysmal outpouchings) using widefield OCT-A thorough images photomontage. Hautz et al. [22] illustrated that OCT-A might be a useful tool in the follow-up period, but it was not able to substitute FA as a sole diagnostic imaging modality. Muakkassa and colleagues [23] reported the absence of FAZ due to capillaries crossing the fovea in all affected and in half of unaffected fellow eyes. This condition has been referred to as macular-foveal capillaries, and it has been found in healthy eyes and in different retinal diseases [24, 25]. It has been hypothesized that this could be a coincident finding rather than a hallmark of disease, and it does not seem to be related to visual acuity. We did not find macular-foveal capillaries in any eye and FAZ was clearly recognizable in all the unaffected eyes and in the affected eyes without macular fibrosis. Conversely, eyes with macular fibrosis exhibited a disrupted vascular network in the macular area with coarse vessels, possibly representing neovascular tissue within fibrosis or vascularized fibrotic tissue. This finding is not surprising, as, a vascular nature in macular fibrosis has been previously described. Jumper and colleagues [26] have found networks of intra-retinal neovascularization in patients with macular fibrosis and have hypothesized that blood vessel formation is stimulated by the lipid exudate accumulation and inflammation. Using Doppler OCT and widefield FA, Sigler and Calzada [27] found a type 3 neovascularization/retinal angiomatous proliferation (RAP) in all patients featuring macular fibrosis. However, they were not able to unequivocally demonstrate the choroidal component of type 3 neovascularization lesion since indocyanine green angiography (ICG) was not performed. In our study, OCT-A revealed type 3 neovascularization in three out of four eyes featuring macular fibrosis. Since ICG is an invasive test that is not routinely performed in patients with Coats’ disease, OCT-A can be a useful tool to identify cases of RAP and to study both the retinal and the choroidal component of these lesions in a non-invasive fashion.
Longitudinal studies are warranted to clarify whether macular fibrosis derives from neovascular activity or chronic lipid exudation; if the first hypothesis is confirmed, it could represent a solid rationale for early anti-VEGF therapy, in conjunction with previous experimental and clinical studies [28].
The main limitation of this study relies on its small sample size, as Coats’ disease is a relatively rare condition. Moreover, we were not able to provide all imaging modalities in all patients due to poor image quality or insufficient patient collaboration. Most patients were not enrolled at the disease onset, but they had rather received a diagnosis of Coats’ disease many years before, the mean age of our population was frankly older than that expect for this condition. Finally, most patients were not naïve but had undergone previous treatment in the affected eyes.
Several conclusions can be drawn from the present study. First, UWF imaging is able to identify more retinal pathology than standard fundus imaging and could guide proper retinal photocoagulation. Furthermore, we demonstrated that Coats’ disease is a highly asymmetric bilateral disease rather than a unilateral affection, and could be pathologically related to other vitreoretinopathies (i.e., FEVR and Norrie disease). Since abnormalities involving the fellow eyes were mild and clinically silent, we are convinced that they do not represent a relevant clinical risk for the patient vision, and they should not modify the management, therapy, monitoring, and prognosis of these patients. Finally, a proportion of patients featured a type 3 neovascularization, which can be easily identified with OCT-A.
References
Shields JA, Shields CL (2002) Review: coats disease: the 2001 LuEsther T. Mertz lecture. Retina 22:80–91
Shields JA, Shields CL, Honavar SG, Demirci H (2001) Clinical variations and complications of coats disease in 150 cases: the 2000 Sanford Gifford memorial lecture. Am J Ophthalmol 131:561–571
Witmer MT, Kiss S (2013) Wide-field imaging of the retina. Surv Ophthalmol 58:143–154. https://doi.org/10.1016/j.survophthal.2012.07.003
Singer M, Sagong M, van Hemert J, Kuehlewein L, Bell D, Sadda SR (2016) Ultra-widefield imaging of the peripheral retinal vasculature in normal subjects. Ophthalmology 123:1053–1059. https://doi.org/10.1016/j.ophtha.2016.01.022
Kang KB, Wessel MM, Tong J, D'Amico DJ, Chan RV (2013) Ultra-widefield imaging for the management of pediatric retinal diseases. J Pediatr Ophthalmol Strabismus 50:282–288. https://doi.org/10.3928/01913913-20130528-04
Kumar V, Chandra P, Kumar A (2017) Ultra-wide field imaging in the diagnosis and management of adult-onset Coats' disease. Clin Exp Optom 100:79–82. https://doi.org/10.1111/cxo.12418
Rabiolo A, Carnevali A, Bandello F, Querques G (2016) Optical coherence tomography angiography: evolution or revolution? Expert Rev Ophthalmol 11:243–245
Grosso A, Pellegrini M, Cereda MG, Panico C, Staurenghi G, Sigler EJ (2015) Pearls and pitfalls in diagnosis and management of coats disease. Retina 35:614–623. https://doi.org/10.1097/IAE.0000000000000485
Shields JA, Shields CL, Honavar SG, Demirci H, Cater J (2001) Classification and management of coats disease: the 2000 proctor lecture. Am J Ophthalmol 131:572–583
Daruich AL, Moulin AL, Tran HV, Matet A, Munier FL (2016) SUBFOVEAL NODULE IN COATS' DISEASE: toward an updated classification predicting visual prognosis. Retina. https://doi.org/10.1097/IAE.0000000000001399
Blair MP, Ulrich JN, Elizabeth Hartnett M, Shapiro MJ (2013) Peripheral retinal nonperfusion in fellow eyes in coats disease. Retina 33:1694–1699. https://doi.org/10.1097/IAE.0b013e318285cb86
Kaneko Y, Moriyama M, Hirahara S, Ogura Y, Ohno-Matsui K (2014) Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. Invest Ophthalmol Vis Sci 55:1432–1439. https://doi.org/10.1167/iovs.13-13706
Tsui I, Bajwa A, Franco-Cardenas V, Pan CK, Kim HY, Schwartz SD (2013) Peripheral fluorescein angiographic findings in fellow eyes of patients with branch retinal vein occlusion. Int J Inflamm 2013:464127. https://doi.org/10.1155/2013/464127
Oliver SC, Schwartz SD (2010) Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol 25:27–33. https://doi.org/10.3109/08820538.2010.481239
Dodo Y, Murakami T, Unoki N, Ogino K, Uji A, Yoshitake S, Yoshimura N (2016) White dots as a novel marker of diabetic retinopathy severity in ultrawide field imaging. PLoS One 11:e0165906. https://doi.org/10.1371/journal.pone.0165906
Lu J, Mai G, Luo Y, Li M, Cao D, Wang X, Yan H, Sadda SR, Lu L (2017) Appearance of far peripheral retina in normal eyes by ultra-widefield fluorescein angiography. Am J Ophthalmol 173:84–90. https://doi.org/10.1016/j.ajo.2016.09.024
Shah AR, Abbey AM, Yonekawa Y, Khandan S, Wolfe JD, Trese MT, Williams GA, Capone A Jr (2016) Widefield fluorescein angiography in patients without peripheral disease: a study of normal peripheral findings. Retina 36:1087–1092. https://doi.org/10.1097/IAE.0000000000000878
Black GC, Perveen R, Bonshek R, Cahill M, Clayton-Smith J, Lloyd IC, McLeod D (1999) Coats' disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet 8:2031–2035
Gilmour DF (2015) Familial exudative vitreoretinopathy and related retinopathies. Eye (Lond) 29:1–14. https://doi.org/10.1038/eye.2014.70
Holmstrom G, Thoren K (2000) Ocular manifestations of incontinentia pigmenti. Acta Ophthalmol Scand 78:348–353
Yonekawa Y, Todorich B, Trese MT (2016) Optical coherence tomography angiography findings in Coats' disease. Ophthalmology 123:1964. https://doi.org/10.1016/j.ophtha.2016.05.004
Hautz W, Golebiewska J, Kocyla-Karczmarewicz B (2017) Optical coherence tomography and optical coherence tomography angiography in monitoring Coats' disease. J Ophthalmol 2017:7849243. https://doi.org/10.1155/2017/7849243
Muakkassa NW, de Carlo TE, Choudhry N, Duker JS, Baumal CR (2016) Optical coherence tomography angiography findings in Coats' disease. Ophthalmic Surg Lasers Imaging Retina 47:632–635. https://doi.org/10.3928/23258160-20160707-04
Cicinelli MV, Carnevali A, Rabiolo A, Querques L, Zucchiatti I, Scorcia V, Bandello F, Querques G (2017) Clinical spectrum of macular-foveal capillaries evaluated with optical coherence tomography angiography. Retina 37:436–443. https://doi.org/10.1097/IAE.0000000000001199
Yeung J, Crock G, Cairns J, Heinze J, Troski S, Billson F (1973) Macular-foveal capillaries in human retina. Aust J Ophthalmol 1:17–23
Jumper JM, Pomerleau D, McDonald HR, Johnson RN, Fu AD, Cunningham ET Jr (2010) Macular fibrosis in coats disease. Retina 30:S9–14
Sigler EJ, Calzada JI (2015) Retinal angiomatous proliferation with chorioretinal anastomosis in childhood coats disease: a reappraisal of macular fibrosis using multimodal imaging. Retina 35:537–546. https://doi.org/10.1097/IAE.0000000000000341
Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG (2014) Current management of coats disease. Surv Ophthalmol 59:30–46. https://doi.org/10.1016/j.survophthal.2013.03.007
Acknowledgments
Prof Giuseppe Querques and Prof Francesco Bandello have contributed equally to this study and should be considered as equivalent authors.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alessandro Rabiolo, Alessandro Marchese, Riccardo Sacconi, Maria Vittoria Cicinelli, Andrea Grosso and Lea Querques have no disclosures. Giuseppe Querques is a consultant for: Alimera Sciences (Alpharetta, GA, USA), Allergan Inc. (Irvine, CA, USA), Bayer Schering-Pharma (Berlin, Germany), Heidelberg (Germany), Novartis (Basel, Switzerland), Sandoz (Berlin, Germany), Zeiss (Dublin, CA, USA). Francesco Bandello has the following disclosures: ALLERGAN (S), ALIMERA (S), BAYER (S), FARMILA-THEA (S), SCHERING PHARMA (S), SANOFI-AVENTIS (S), NOVAGALI (S), PHARMA (S), HOFFMANN-LA ROCHE (S), GENENTECH (S), and NOVARTIS (S).
Rights and permissions
About this article
Cite this article
Rabiolo, A., Marchese, A., Sacconi, R. et al. Refining Coats’ disease by ultra-widefield imaging and optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 255, 1881–1890 (2017). https://doi.org/10.1007/s00417-017-3794-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3794-7